Chromosomal Evolution in Lower Vertebrates: Sex Chromosomes in Neotropical Fishes
Abstract
:1. Introduction
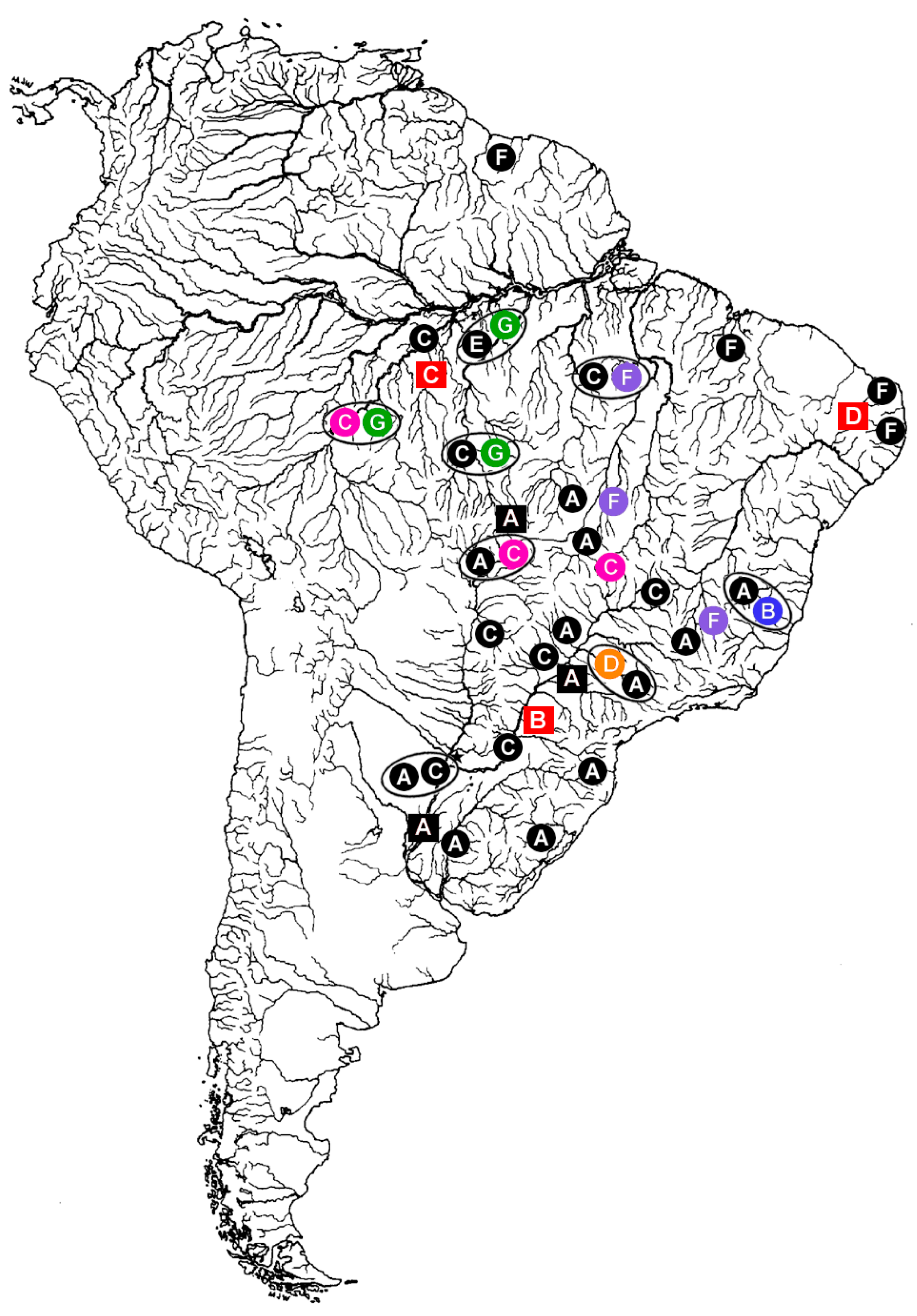
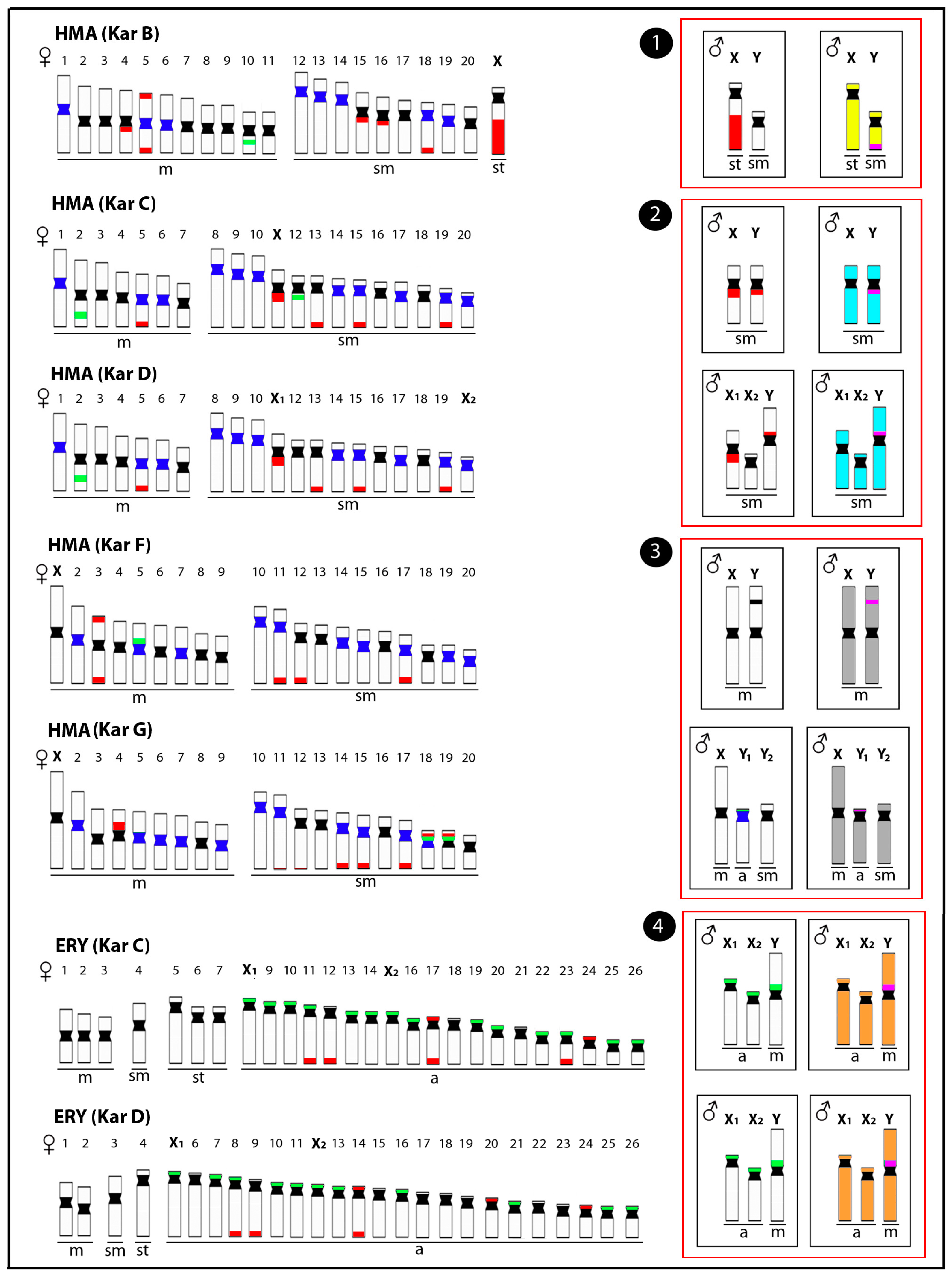
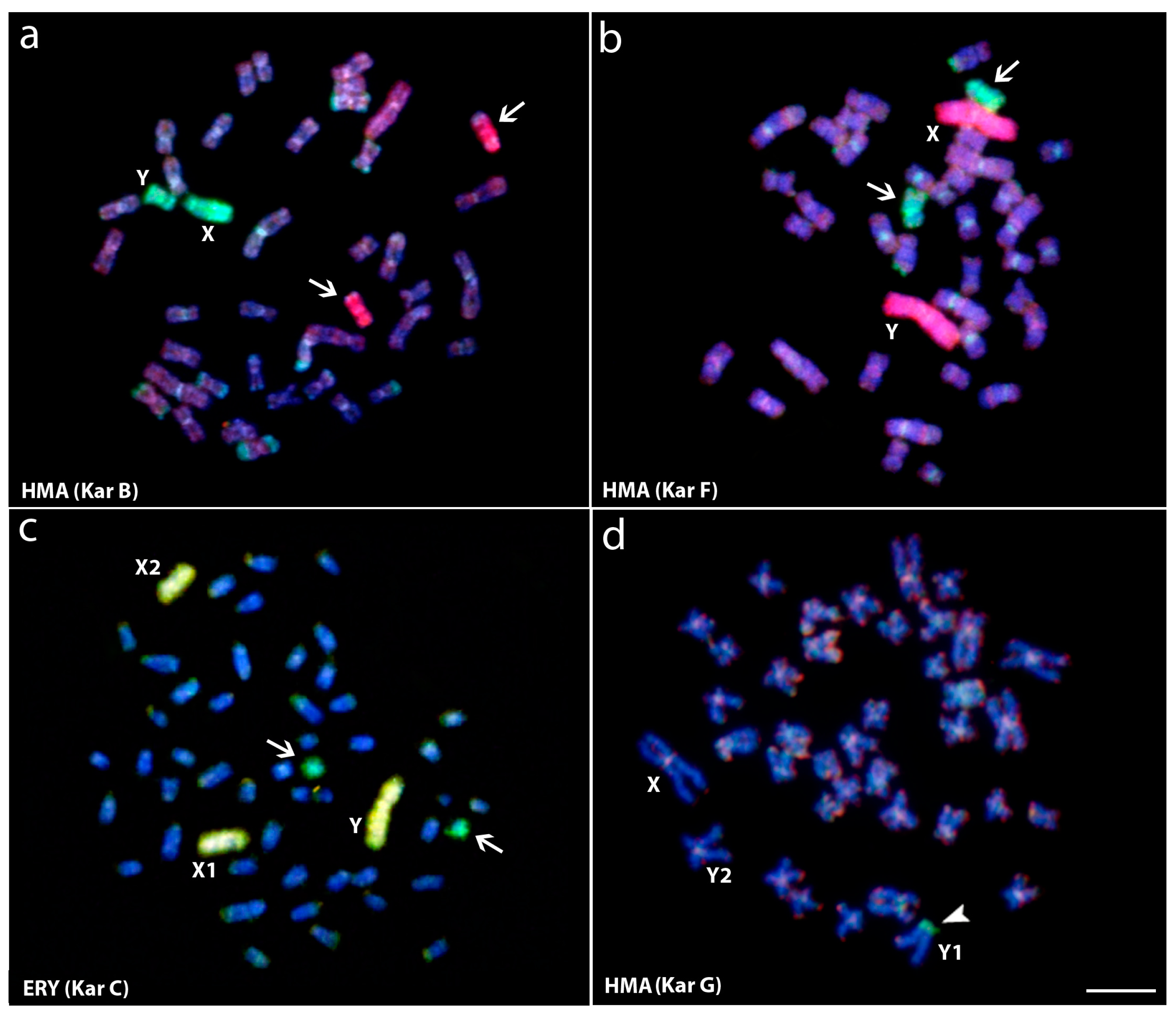
2. The Erythrinidae Family: A Broad Scenario on Fish Sex Chromosomes Evolution
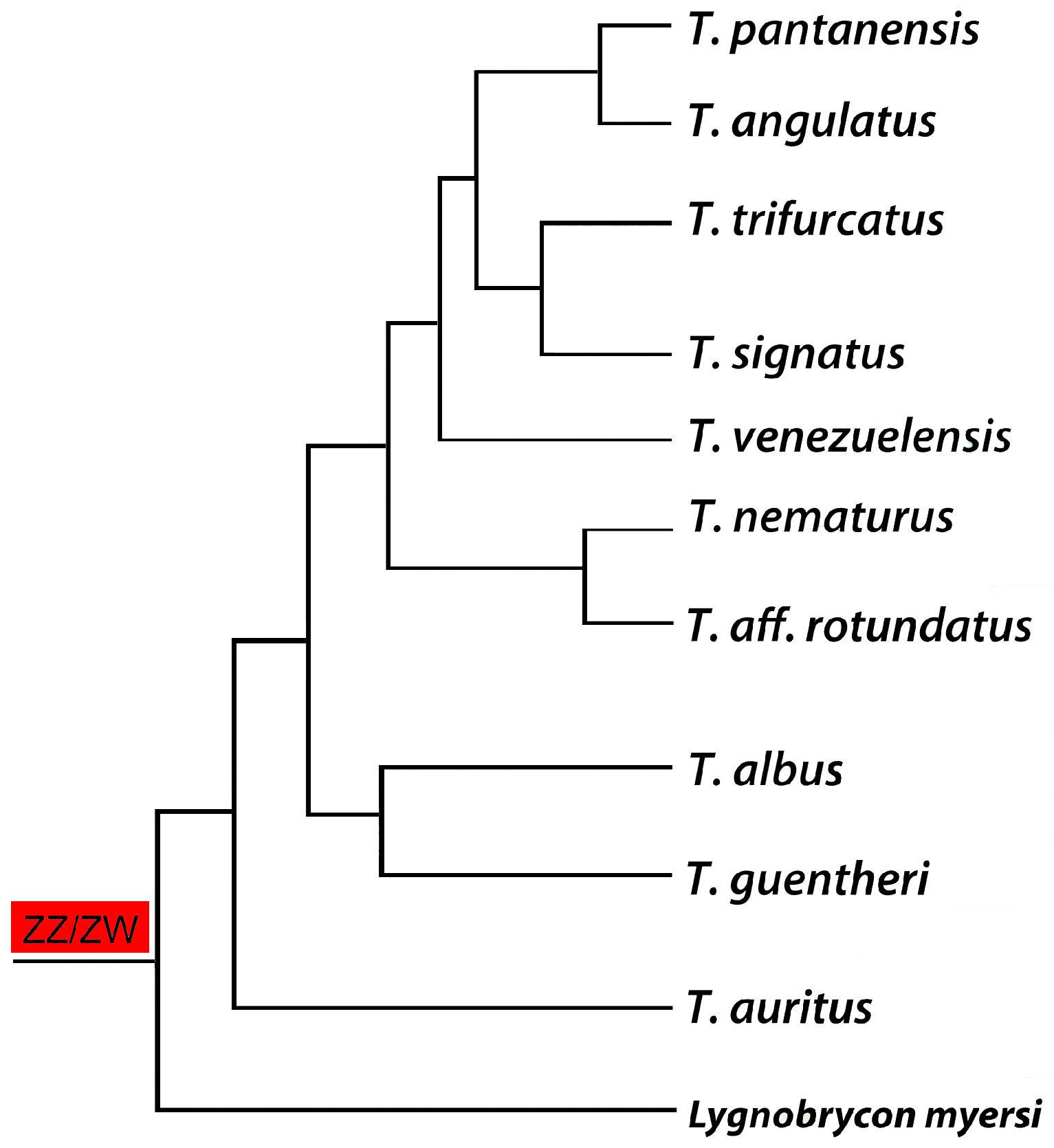
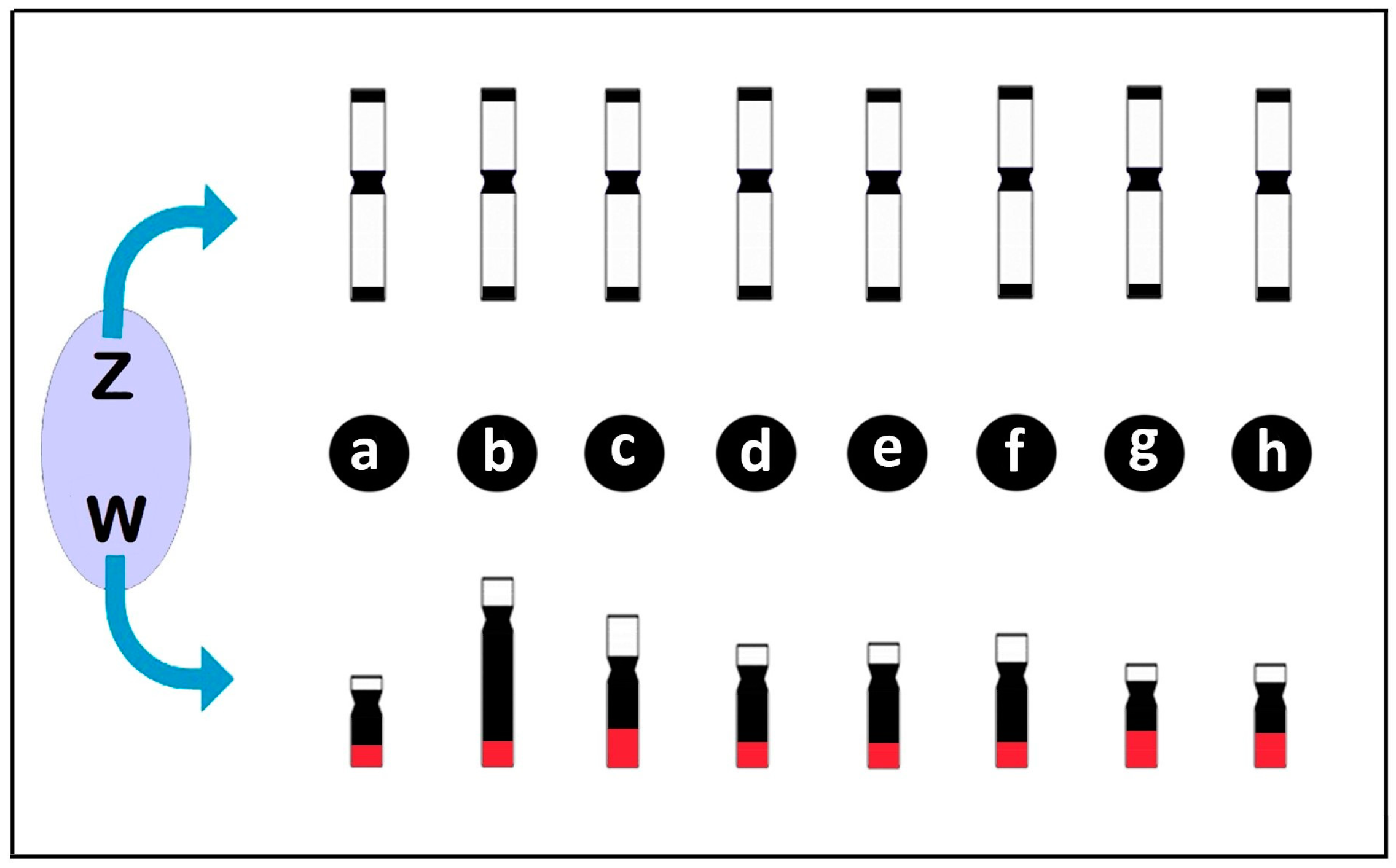
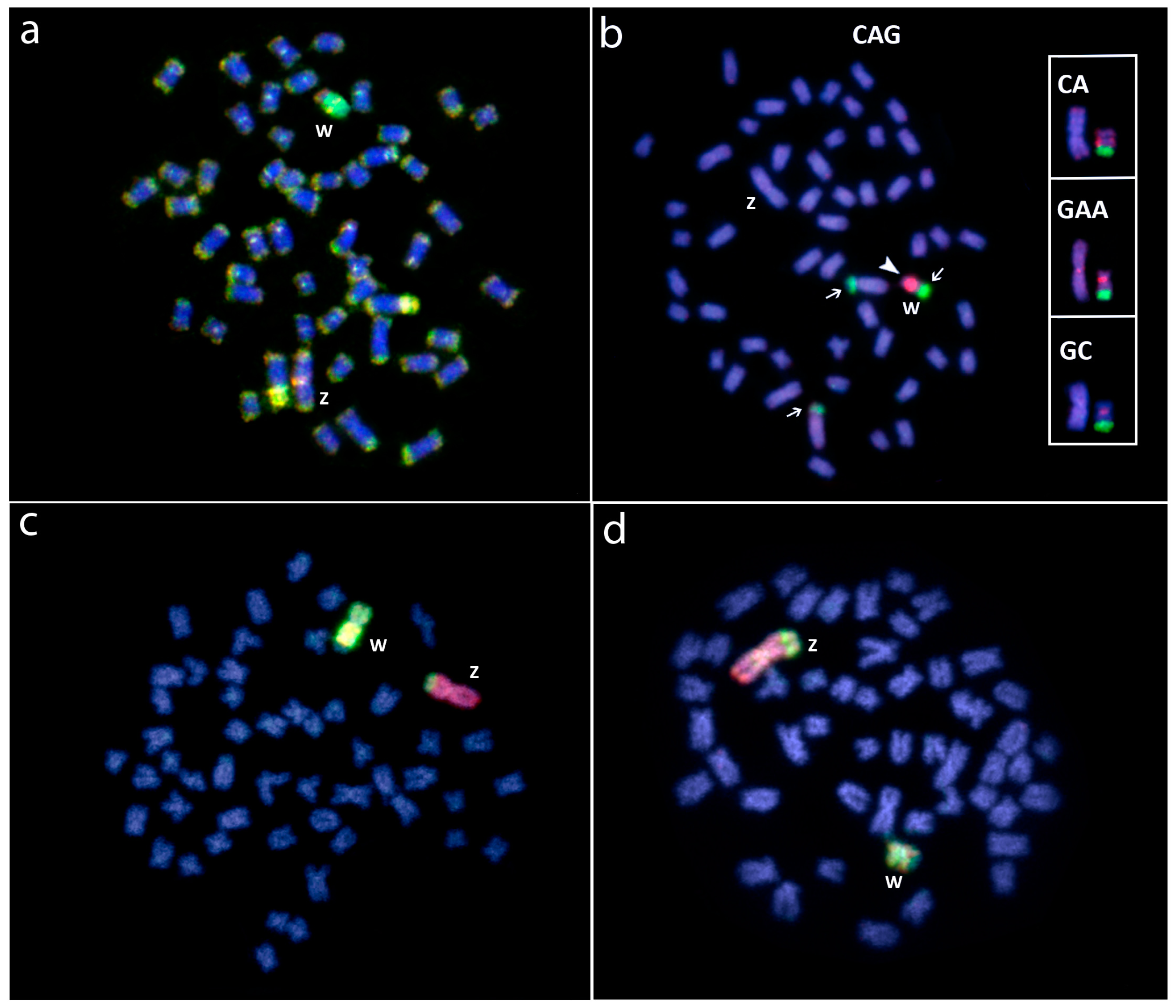
3. The Triportheus Genus: A Particular Pathway on Fish Sex Chromosomes Evolution
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohno, S. Sex Chromosomes and Sex-Linked Genes; Springer-Verlag: New York, NY, USA, 1967; p. 185. ISBN 978-3-642-88180-0. [Google Scholar]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, S.; Steinemann, M. Retroelements: Tools for sex chromosome evolution. Cytogenet. Genome Res. 2005, 110, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bachtrog, D. A dynamic view of sex chromosome evolution. Curr. Opin. Genet. Dev. 2006, 16, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.A.M. Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annu. Rev. Genet. 2008, 42, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.E.; Dean, R.; Zimmer, F.; Mank, J.E. How to make a sex chromosome. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Toledo, L.F.; Foresti, F. Morphologically differentiated sex chromosomes in neotropical freshwater fish. Genetica 2001, 111, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Camacho, J.P.M.; Bertollo, L.A.C. Repetitive DNAs and differentiation of sex chromosomes in neotropical fishes. Cytogenet. Genome Res. 2011, 132, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Moreira-Filho, O.; Almeida-Toledo, L.F.; Bertollo, L.A.C. The contrasting role of heterochromatin in the differentiation of sex chromosomes: An overview from Neotropical fishes. J. Fish Biol. 2012, 80, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Mank, J.E.; Avise, J.C. Evolutionary diversity and turn-over of sex determination in teleost fishes. Sex. Dev. 2009, 3, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Oliveira, C.; Almeida-Toledo, L.F.; Foresti, F. Karyotypic evolution in Neotropical fishes. In Fish Cytogenetics; Pisano, E., Ozouf-Costaz, C., Foresti, F., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2007; pp. 111–164. ISBN 978-1-57808-330-5. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 2 February 2017).
- Kitano, J.; Peichel, C.L. Turnover of sex chromosomes and speciation in fishes. Environ. Biol. Fishes 2012, 94, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Garcia, C.; Matoso, D.A.; de Jesus, I.S.; Feldberg, E. A new multiple sex chromosome system X1X1X2X2/X1Y1X2Y2 in Siluriformes: Cytogenetic characterization of Bunocephalus coracoideus (Aspredinidae). Genetica 2016, 144, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Foresti, F.; Hilsdorf, A.W.S. Genetics of neotropical fish: From chromosomes to populations. Fish Physiol. Biochem. 2009, 35, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Arai, R. Fish Karyotypes: A Check List, 1st ed.; Springer: Tokyo, Japan, 2011; p. 340. ISBN 978-4-431-53877. [Google Scholar]
- Parise-Maltempi, P.P.; Martins, C.; Oliveira, C.; Foresti, F. Identification of a new repetitive element in the sex chromosomes of Leporinus elongatus (Teleostei: Characiformes: Anostomidae): New insights into the sex chromosomes of Leporinus Cytogenet. Genome Res. 2007, 116, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Feldberg, E.; dos Anjos, M.B.; Zuanon, J. Occurrence of multiple sexual chromosomes (XX/XY1Y2 and Z1Z1Z2Z2/Z1Z2W1W2) in catfishes of the genus Ancistrus (Siluriformes: Loricariidae) from the Amazon basin. Genetica 2008, 134, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Araya-Jaime, C.; Mateussi, N.T.B.; Utsunomia, R.; Costa-Silva, G.J.; Oliveira, C.; Foresti, F. ZZ/Z0: The new system of sex chromosomes in Eigenmannia aff. trilineata (Teleostei: Gymnotiformes: Sternopygidae) characterized by molecular cytogenetics and DNA barcoding. Zebrafish 2017. [Google Scholar] [CrossRef] [PubMed]
- Woram, R.A.; Gharbi, K.; Sakamoto, T.; Hoyheim, B.; Holm, L.-E.; Naish, K.; McGowan, C.; Ferguson, M.M.; Phillips, R.B.; Stein, J.; et al. Comparative genome analysis of the primary sex-determining locus in salmonid fishes. Genome Res. 2003, 13, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mank, J.E.; Promislow, D.E.L.; Avise, J.C. Evolution of alternative sex-determining mechanisms in teleost fishes. Biol. J. Linn. Soc. 2006, 87, 83–93. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Molina, W.F.; Artoni, R.F.; Bertollo, L.A.C. Chromosomes as tools for discovering biodiversity—The case of Erythrinidae fish family. In Recent Trends in Cytogenetic Studies—Methodologies and Applications; Tirunilai, P., Ed.; InTech Publisher: Rijeka, Croatia, 2012; ISBN 978-953-51-0178-9. [Google Scholar]
- Cioffi, M.B.; Liehr, T.; Trifonov, V.; Molina, W.F.; Bertollo, L.A.C. Independent sex chromosome evolution in lower vertebrates: A molecular cytogenetic overview in the Erythrinidae fish family. Cytogenet. Genome Res. 2013, 141, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Artoni, R.F.; Falcão, J.N.; Moreira-Filho, O.; Bertollo, L.A.C. An uncommon condition for a sex chromosome system in Characidae fish. Distribution and differentiation of the ZZ/ZW system in Triportheus. Chromosome Res. 2001, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Bertollo, L.A.C.; Liehr, T.; Troy, W.P.; Cioffi, M.B. W Chromosome dynamics in Triportheus species (Characiformes, Triportheidae): An ongoing process narrated by repetitive sequences. J. Hered. 2016, 107, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Galetti, P.M., Jr.; Lima, N.R.W.; Venere, P.C. A monophyletic ZW sex chromosome system in Leporinus (Anostomidae, Characiformes). Cytologia 1995, 60, 375–382. [Google Scholar] [CrossRef]
- Vicente, V.E.; Bertollo, L.A.C.; Valentini, S.R.; Moreira-Filho, O. Origin and differentiation of sex chromosome system in Parodon hilarii (Pisces, Parodontidae). Satellite DNA, G and C-banding. Genetica 2003, 119, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Manchado, M.; Zuasti, E.; Cross, I.; Merlo, A.; Infante, C.; Rebordinos, L. Molecular characterization and chromosomal mapping of the 5S rRNA gene in Solea senegalensis: A new linkage to the U1, U2, and U5 small nuclear RNA genes. Genome 2006, 49, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pansonato-Alves, J.C.; Serrano, É.A.; Utsunomia, R.; Scacchetti, P.C.; Oliveira, C.; Foresti, F. Single origin of sex chromosomes and multiple origins of B chromosomes in fish genus Characidium. PLoS ONE 2014, 9, e107169. [Google Scholar] [CrossRef] [PubMed]
- Pennell, M.W.; Kirkpatrick, M.; Otto, S.P.; Vamosi, J.C.; Peichel, C.L.; Valenzuela, N.; Kitano, J. Y fuse? Sex chromosome fusions in fishes and reptiles. PLoS Genet. 2015, 11, e1005237. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.K.; Nordén, A.K.; Hansson, B. Sex chromosome evolution: Historical insights and future perspectives. Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, G.S.; Kirkpatrick, M. Turnover of sex chromosomes induced by sexual conflict. Nature 2007, 449, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, M.; Altmanová, M.; Kratochvíl, L. Multiple sex chromosomes in the light of female meiotic drive in amniote vertebrates. Chromosome Res. 2014, 22, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Brykov, V.A. Mechanisms of sex determination in fish: Evolutionary and practical aspects. Russ. J. Mar. Biol. 2014, 40, 407–417. [Google Scholar] [CrossRef]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Sex determination: Why so many ways of doing it? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, P.; Viñas, A.M.; Sánchez, L.; Díaz, N.; Ribas, L.; Piferrer, F. Genetic architecture of sex determination in fish: Applications to sex ratio control in aquaculture. Front. Genet. 2014, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Henning, F.; Trifonov, V.; Ferguson-Smith, M.A.; Almeida-Toledo, L.F. Non-homologous sex chromosomes in two species of the genus Eigenmannia (Teleostei: Gymnotiformes). Cytogenet. Genome Res. 2008, 121, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Pazian, M.F.; Shimabukuro-Dias, C.K.; Pansonato-Alves, J.C.; Oliveira, C.; Foresti, F. Chromosome painting of Z and W sex chromosomes in Characidium (Characiformes, Crenuchidae). Genetica 2013, 141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Bertollo, L.A.C.; Ezaz, T.; Trifonov, V.; Sember, A.; Liehr, T.; Cioffi, M.B. Highly conserved Z and molecularly diverged W chromosomes in the fish genus Triportheus (Characiformes, Triportheidae). Heredity 2017, 118, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C.; Born, G.G.; Dergam, J.A.; Fenocchio, A.S.; Moreira-Filho, O. A biodiversity approach in the neotropical Erythrinidae fish, Hoplias malabaricus. Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic considerations. Chromosome Res. 2000, 8, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C. Chromosome evolution in the Neotropical Erythrinidae fish family: An overview. In Fish Cytogenetics; Pizano, E., Ozouf-Costaz, C., Foresti, F., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2007; pp. 195–211. [Google Scholar]
- Freitas, N.L.; Al-Rikabi, A.B.H.; Bertollo, L.A.C.; Ezaz, T.; Yano, C.F.; Oliveira, E.A.; Hatanaka, T.; Cioffi, M.B. Early stages of XY sex chromosomes differentiation in the fish Hoplias malabaricus (Characiformes, Erythrinidae) revealed by DNA repeats accumulation. Curr. Genomics 2017, 18. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Sember, A.; Bertollo, L.A.C.; Yano, C.F.; Ezaz, T.; Moreira-Filho, O.; Hatanaka, T.; Trifonov, V.; Liehr, T.; Ráb, P.; et al. Tracking the evolutionary pathway of sex chromosomes among fishes: Characterizing the unique XX/XY1Y2 system in Hoplias malabaricus (Teleostei, Characiformes). Chromosoma 2017. Submitted. [Google Scholar]
- Sember, A.; Bertollo, L.A.C.; Yano, C.F.; Hatanaka, T.; Ráb, P.; de Oliveira, E.A.; Cioffi, M.B. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front. Genet. 2017. Submitted. [Google Scholar]
- Born, G.G.; Bertollo, L.A.C. An XX/XY sex chromosome system in a fish species, Hoplias malabaricus, with a polymorphic NOR-bearing X chromosome. Chromosome Res. 2000, 8, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Martins, C.; Vicari, M.R.; Rebordinos, L.; Bertollo, L.A.C. Differentiation of the XY sex chromosomes in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Unusual accumulation of repetitive sequences on the X chromosome. Sex. Dev. 2010, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Martins, C.; Bertollo, L.A.C. Comparative chromosome mapping of repetitive sequences. Implications for genomic evolution in the fish, Hoplias malabaricus. BMC Genet. 2009, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Kejnovský, E.; Bertollo, L.A.C. The chromosomal distribution of microsatellite repeats in the genome of the wolf fish Hoplias malabaricus, focusing on the sex chromosomes. Cytogenet. Genome Res. 2011, 132, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Sánchez, A.; Marchal, J.A.; Kosyakova, N.; Liehr, T.; Trifonov, V.; Bertollo, L.A.C. Whole chromosome painting reveals independent origin of sex chromosomes in closely related forms of a fish species. Genetica 2011, 139, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Bertollo, L.A.C. Initial steps in XY chromosome differentiation in Hoplias. malabaricus and the origin of an X1X2Y sex chromosome system in this fish group. Heredity 2010, 105, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C.; Fontes, M.S.; Fenocchio, A.S.; Cano, J. The X1X2Y sex chromosome system in the fish Hoplias. malabaricus. I. G-, C- and chromosome replication banding. Chromosome Res. 1997, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C.; Mestriner, C.A. The X1X2Y sex chromosome system in the fish Hoplias. malabaricus (Pisces, Erythrinidae). II. Meiotic analyses. Chromosome Res. 1998, 6, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Laforga Vanzela, A.L.; Rubert, M.; Martins-Santos, I.C.; Giuliano-Caetano, L. Differentiation of Y chromosome in the X1X1X2X2/X1X2Y sex chromosome system of Hoplias malabaricus (Characiformes, Erythrinidae). Cytogenet. Genome Res. 2009, 127, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Green, J.E.; Dalíková, M.; Sahara, K.; Marec, F.; Akam, M. XX/XY system of sex determination in the geophilomorph centipede Strigamia maritima. PLoS ONE 2016, 11, e0150292. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C.; Oliveira, C.; Molina, W.F.; Margarido, V.P.; Fontes, M.S.; Pastori, M.C.; Falcão, J.N.; Fenocchio, A.S. Chromosome evolution in the erythrinid fish, Erythrinus erythrinus (Teleostei: Characiformes). Heredity 2004, 93, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Martins, C.; Bertollo, L.A.C. Chromosome spreading of associated transposable elements and ribosomal DNA in the fish Erythrinus erythrinus. Implications for genome change and karyoevolution in fish. BMC Evol. Biol. 2010, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.F.; Bertollo, L.A.C.; Troy, W.P.; Feldberg, E.; Valentin, F.C.S.; Cioffi, M.B. Differentiation and evolutionary relationships in Erythrinus erythrinus (Characiformes, Erythrinidae): Comparative chromosome mapping of repetitive sequences. Rev. Fish Biol. Fish. 2013, 23, 261–269. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Sánchez, A.; Marchal, J.A.; Kosyakova, N.; Liehr, T.; Trifonov, V.; Bertollo, L.A.C. Cross-species chromosome painting tracks the independent origin of multiple sex chromosomes in two cofamiliar Erythrinidae fishes. BMC Evol. Biol. 2011, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Avelino, G.S.; Abe, K.T.; Mariguela, T.C.; Benine, R.C.; Ortí, G.; Vari, R.P.; Corrêa e Castro, R.M. Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling. BMC Evol. Biol. 2011, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Mariguela, T.C.; Roxo, F.F.; Foresti, F.; Oliveira, C. Phylogeny and biogeography of Triportheidae (Teleostei: Characiformes) based on molecular data. Mol. Phylogenet. Evol. 2016, 96, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Malabarba, M.C.S.L. Revision of the Neotropical genus Triportheus. Cope, 1872 (Characiformes: Characidae). Neotrop. Ichthyol. 2004, 2, 167–204. [Google Scholar] [CrossRef]
- Prestes, L.; Mota Soares, M.G.; Silva, F.R.; Bittencourt, M.M. Dynamic population from Triportheus albus, T. angulatus and T. auritus (Characiformes: Characidae) in Amazonian Central lakes. Biota Neotrop. 2010, 10, 177–181. [Google Scholar] [CrossRef]
- Falcão, J.N. Caracterização Cariotípica em Peixes do Gênero Triportheus (Teleostei, Characiformes, Characidae). Ph.D. Thesis, Universidade de São Paulo, Ribeirão Preto, SP, Brazil, 1988. [Google Scholar]
- Bertollo, L.A.C.; Cavallaro, Z.I.A. Highly differentiated ZZ/ZW sex chromosome system in a Characidae fish, Triportheus guentheri. Cytogenet. Genome Res. 1992, 60, 60–63. [Google Scholar] [CrossRef]
- Sanchez, S.; Jorge, L.C. A new report of the ZZ/ZW sex chromosome system in the genus Triportheus. (Pisces, Triportheinae). Cytologia 1999, 64, 395–400. [Google Scholar] [CrossRef]
- Artoni, R.F.; Bertollo, L.A.C. Evolutionary aspects of the ZZ/ZW sex chromosome system in the Characidae fish, genus Triportheus. A monophyletic state and NOR location on the W chromosome. Heredity 2002, 89, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Nirchio, M.; Oliveira, C.; Ferreira, I.A.; Granado, A.; Ron, E. Extensive polymorphism and chromosomal characteristics of ribosomal DNA in the characid fish Triportheus venezuelensis (Characiformes, Characidae). Genet. Mol. Biol. 2007, 30, 25–30. [Google Scholar] [CrossRef]
- Diniz, D.; Laudicina, A.; Cioffi, M.B.; Bertollo, L.A.C. Microdissection and whole chromosome painting. Improving sex chromosome analysis in Triportheus (Teleostei, Characiformes). Cytogenet. Genome Res. 2008, 122, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.; Moreira-Filho, O.; Bertollo, L.A.C. Molecular cytogenetics and characterization of a ZZ/ZW sex chromosome system in Triportheus nematurus (Characiformes, Characidae). Genetica 2008, 133, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.; Laudicina, A.; Bertollo, L.A.C. Chromosomal location of 18S and 5S rDNA sites in Triportheus fish species (Characiformes, Characidae). Genet. Mol. Biol. 2009, 32, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Poltronieri, J.; Bertollo, L.A.C.; Artoni, R.F.; Liehr, T.; Cioffi, M.B. Chromosomal mapping of repetitive DNAs in Triportheus trifurcatus (Characidae, Characiformes): Insights into the differentiation of the Z and W chromosomes. PLoS ONE 2014, 9, e90946. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Bertollo, L.A.C.; Rebordinos, L.; Merlo, M.A.; Liehr, T.; Portela-Bens, S. Evolutionary dynamics of rDNAs and U2 small nuclear DNAs in Triportheus (Characiformes, Triportheidae): High variability and particular syntenic organization. Zebrafish 2017, 14, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Marquioni, V.; Bertollo, L.A.C.; Diniz, D.; Cioffi, M.B. Comparative chromosomal mapping in Triportheus fish species. Analysis of synteny between ribosomal genes. Micron 2013, 45, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.M.; Phillips, R.B. Polymorphism of the nucleolar organizer region (NOR) on the putative sex chromosomes of Arctic char (Salvelinus alpinus) is not sex related. Chromosome Res. 1997, 5, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Nishida-Umehara, C.; Ishijima, J.; Tsuda, Y.; Ota, H.; Matsuda, Y. Different origins of bird and reptile sex chromosomes inferred from comparative mapping of chicken Z-linked genes. Cytogenet. Genome Res. 2007, 117, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, D.; Stanyon, R.; Engstrom, T.; Valenzuela, N. A ZZ/ZW microchromosome system in the spiny softshell turtle, Apalone spinifera, reveals an intriguing sex chromosome conservation in Trionychidae. Chromosome Res. 2013, 21, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gatto, K.P.; Busin, C.S.; Lourenço, L.B. Unraveling the sex chromosome heteromorphism of the paradoxical frog Pseudis tocantins. PLoS ONE 2016, 11, e0156176. [Google Scholar] [CrossRef] [PubMed]
- Kejnovský, E.; Michalovová, M.; Šteflová, P.; Kejnovská, I.; Manzano, S.; Hobza, R.; Kubát, Z.; Kovařík, J.; Jamilena, M.; Vyskot, B. Expansion of microsatellites on evolutionary young Y chromosome. PLoS ONE 2013, 8, e45519. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Kejnovský, E.; Marquioni, V.; Poltronieri, J.; Molina, W.F.; Diniz, D.; Bertollo, L.A.C. The key role of repeated DNAs in sex chromosome evolution in two fish species with ZW sex chromosome system. Mol. Cytogenet. 2012, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Aline Souza Medrado, A.S.; Diniz, D.; Oliveira, C.; Affonso, P.R.A.M. ZZ/ZW sex chromosome system in the endangered fish Lignobrycon myersi Miranda-Ribeiro, 1956 (Teleostei, Characiformes, Triportheidae). Comp. Cytogenet. 2016, 10, 245–254. [Google Scholar] [CrossRef]
- Terencio, M.L.; Schneider, C.H.; Gross, M.C.; Silva, A.M.; Feldberg, E.; Porto, J.I.R. Comparative cytogenetics of Carnegiella marthae and Carnegiella strigata (Characiformes, Gasteropelecidae) and description of a ZZ/ZW sex chromosome system. Genet. Mol. Biol. 2008, 31, 231–234. [Google Scholar] [CrossRef]
- Soares, R.X.; Bertollo, L.A.C.; Cioffi, M.B.; Costa, G.W.W.F.; Molina, W.F. Chromosomal distribution of two multigene families and the unusual occurrence of an X1X1X2X2/X1X2Y sex chromosome system in the dolphinfish (Coryphaenidae): An evolutionary perspective. Genet. Mol. Res. 2014, 13, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, J.A.; Sampaio, I.; Ramos, R.T.C.; Vicari, M.R.; Affonso, P.R.A.M. First report of sex chromosomes in Achiridae (Teleostei: Pleuronectiformes) with inferences about the origin of the multiple X1X1X2X2/X1X2Y system and dispersal of ribosomal genes in Achirus achirus. Zebrafish 2016, 14, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Filho, O.; Bertollo, L.A.C.; Galetti, P.M., Jr. Distribution of sex chromosome mechanisms in neotropical fish and description of a ZZ/ZW system in Parodon hilarii (Parodontidae). Caryologia 1993, 46, 115–125. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioffi, M.d.B.; Yano, C.F.; Sember, A.; Bertollo, L.A.C. Chromosomal Evolution in Lower Vertebrates: Sex Chromosomes in Neotropical Fishes. Genes 2017, 8, 258. https://doi.org/10.3390/genes8100258
Cioffi MdB, Yano CF, Sember A, Bertollo LAC. Chromosomal Evolution in Lower Vertebrates: Sex Chromosomes in Neotropical Fishes. Genes. 2017; 8(10):258. https://doi.org/10.3390/genes8100258
Chicago/Turabian StyleCioffi, Marcelo de Bello, Cassia Fernanda Yano, Alexandr Sember, and Luiz Antônio Carlos Bertollo. 2017. "Chromosomal Evolution in Lower Vertebrates: Sex Chromosomes in Neotropical Fishes" Genes 8, no. 10: 258. https://doi.org/10.3390/genes8100258




