Differential Expression Patterns of Pleurotus ostreatus Catalase Genes during Developmental Stages and under Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Culture Conditions and Genome Sequence Information
2.2. Catalase Identification, Gene Cloning and Sequence Analysis
2.3. Fruiting-Body Growth and Sample Collection
2.4. Heat Stress Treatment
2.5. Protein Extraction and Catalase Activity Assay
2.6. Western Blot Analysis
2.7. RNA Extraction, Reverse Transcription and Quantitative PCR (qPCR)
2.8 Liquid Chromatogram-Tandem Mass Spectrometry Analysis
2.9. Data Analysis
3. Results
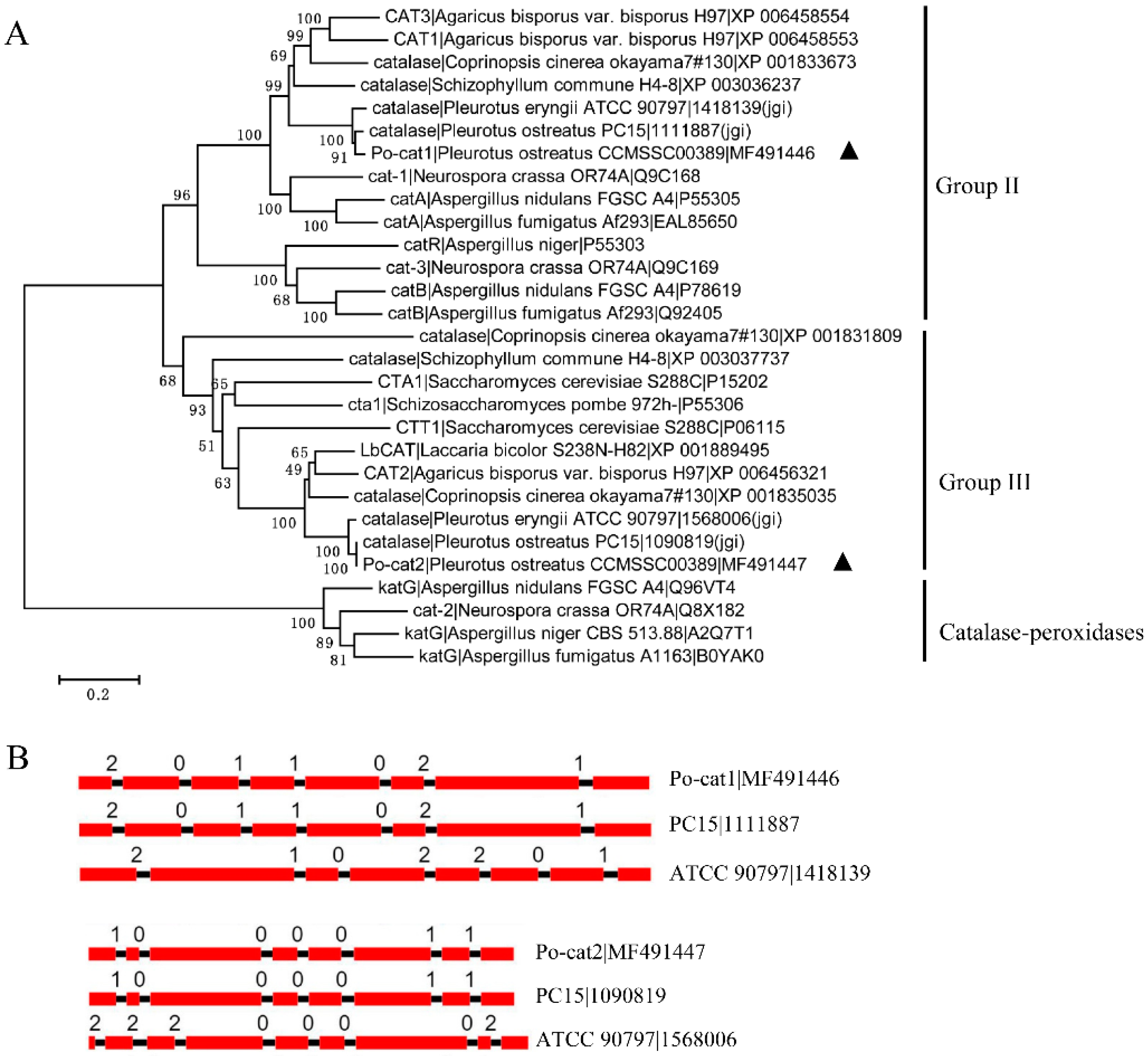
3.1. Cloning and Analysis of Catalase Encoding Genes
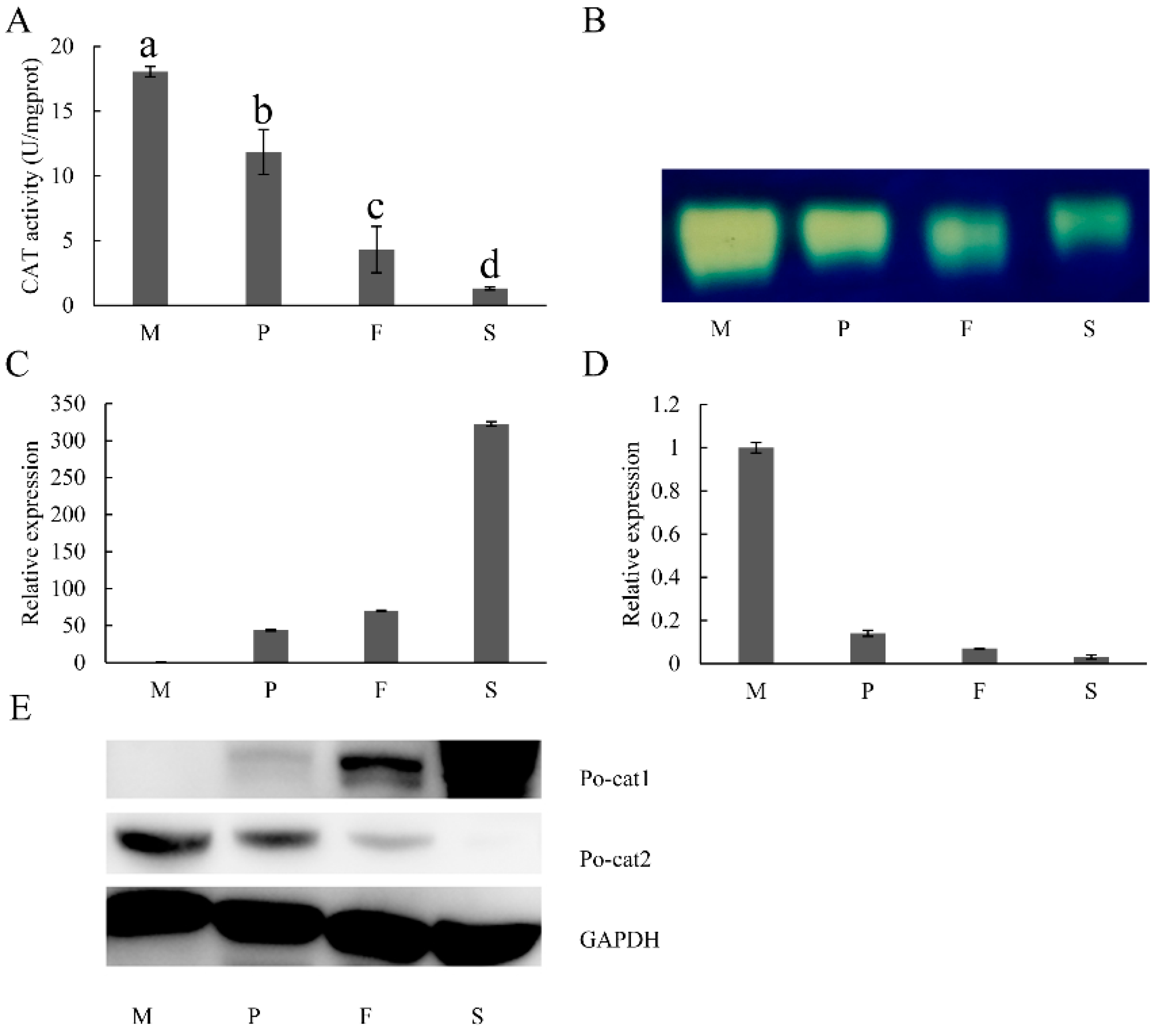
3.2. Expression Patterns of Catalases during Different Developmental Stages of P. ostreatus
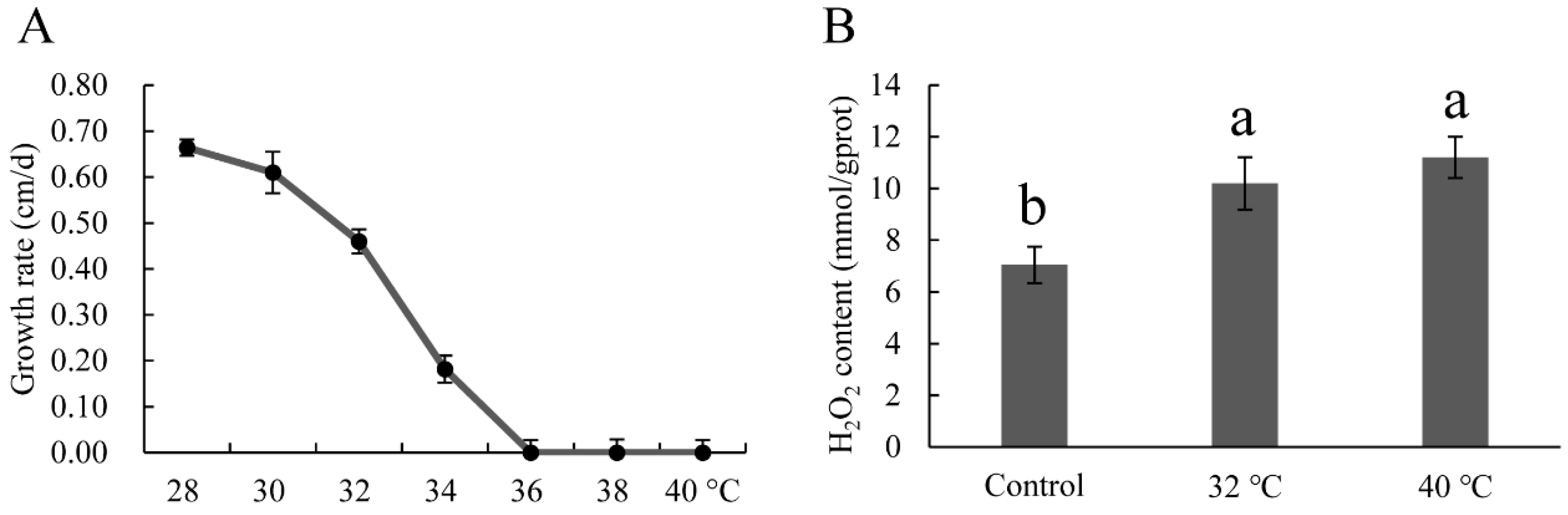
3.3. Selection of Heat Stress Conditions
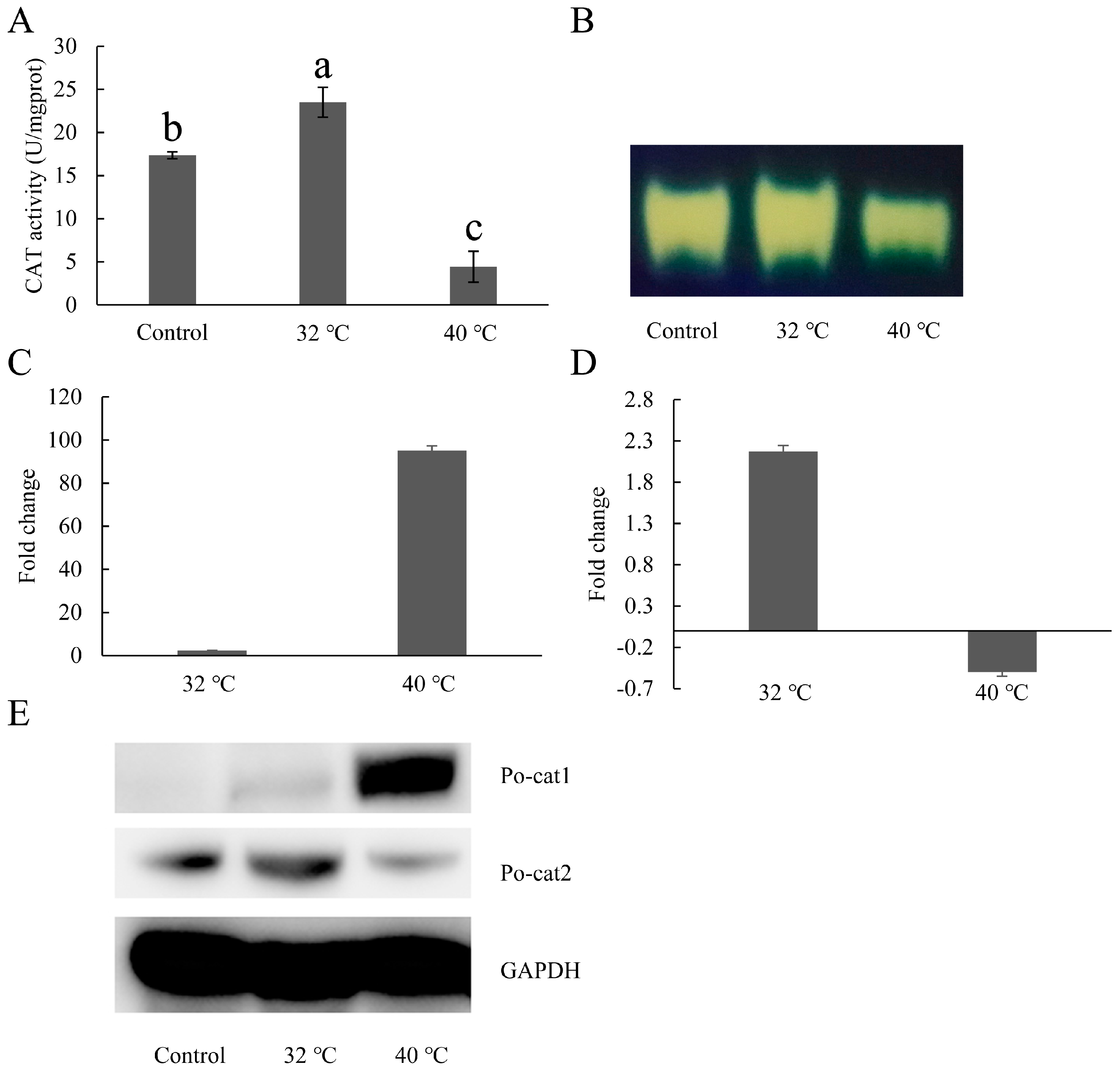
3.4. Expression Patterns of Catalases under Heat Stress
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Willekens, H.; Inzé, D.; Van Montagu, M.; van Camp, W. Catalases in plants. Mol. Breed. 1995, 1, 207–228. [Google Scholar] [CrossRef]
- Ding, M.; Kwok, L.Y.; Schluter, D.; Clayton, C.; Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Zámocký, M.; Gasselhuber, B.; Furtmüller, P.G.; Obinger, C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 2012, 525, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Chagas, R.F.; Bailao, A.M.; Pereira, M.; Winters, M.S.; Smullian, A.G.; Deepe, G.S., Jr.; de Almeida Soares, C.M. The catalases of Paracoccidioides brasiliensis are differentially regulated: Protein activity and transcript analysis. Fungal Genet. Biol. 2008, 45, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Blackman, L.M.; Hardham, A.R. Regulation of catalase activity and gene expression during Phytophthora nicotianae development and infection of tobacco. Mol. Plant Pathol. 2008, 9, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Wei, H.; Liese, R.; Fischer, R. Aspergillus nidulans catalase-peroxidase gene (cpeA) is transcriptionally induced during sexual development through the transcription factor StuA. Eukaryot. Cell 2002, 1, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Bussink, H.-J.; Oliver, R. Identification of two highly divergent catalase genes in the fungal tomato pathogen, Cladosporium fulvum. Eur. J. Biochem. 2001, 268, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Shiozawa, A.; Banno, S.; Fukumori, F.; Ichiishi, A.; Kimura, M.; Fujimura, M. Involvement of OS-2 MAP kinase in regulation of the large-subunit catalases CAT-1 and CAT-3 in Neurospora crassa. Genes Genet. Syst. 2007, 82, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, R.; Banno, S.; Ichikawa, R.; Fukumori, F.; Ichiishi, A.; Kimura, M.; Yamaguchi, I.; Fujimura, M. Identification of OS-2 MAP kinase-dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa. Fungal Genet. Biol. 2007, 44, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, L.; Wysong, D.; Diamond, R.; Aguirre, J. Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J. Bacteriol. 1997, 179, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.E.; Stringer, M.A.; Hansberg, W.; Timberlake, W.E.; Aguirre, J. CatA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr. Genet. 1996, 29, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Zhang, L.B.; Ying, S.H.; Feng, M.G. Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ. Microbiol. 2013, 15, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, A.; Chen, H.; Zhao, M.; Shi, L.; Chen, M.; Wang, H.; Feng, Z. Transcriptome analysis and its application in identifying genes associated with fruiting body development in basidiomycete Hypsizygus marmoreus. PLoS ONE 2015, 10, e0123025. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.H.; Lee, S.H.; Lee, J.S.; Kim, K.H.; Jeong, S.J.; Youn, W.H.; Kim, N.K.; Son, E.S.; Cho, Y.S.; Yoo, Y.B.; et al. Isolation of genes expressed during the developmental stages of the oyster mushroom, Pleurotus ostreatus, using expressed sequence tags. FEMS Microbiol. Lett. 2007, 276, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Au, C.H.; Wilke, S.K.; Stajich, J.E.; Zolan, M.E.; Pukkila, P.J.; Kwan, H.S. 5′-Serial Analysis of Gene Expression studies reveal a transcriptomic switch during fruiting body development in Coprinopsis cinerea. BMC Genomics 2013, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Li, C.; Zhang, X.; Li, X.; Shi, L.; Ren, A.; Zhao, M. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: An essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ. Microbiol. 2014, 16, 1709–1728. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.H.; Lee, Y.R.; Lin, Y.L.; Chu, F.H. Cytochrome P450 genes in medicinal mushroom Antrodia cinnamomea T.T. Chang et W.N. Chou (higher Basidiomycetes) are strongly expressed during fruiting body formation. Int. J. Med. Mushrooms 2011, 13, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Zhang, L.; Wang, R.Q.; Xie, B.; Li, X.; Chen, R.L.; Guo, L.X.; Xie, B.G. The sequence characteristics and expression models reveal superoxide dismutase involved in cold response and fruiting body development in Volvariella volvacea. Int. J. Mol. Sci. 2016, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.E.; Horton, J.S. Mushrooms by magic: Making connections between signal transduction and fruiting body development in the basidiomycete fungus Schizophyllum commune. FEMS Microbiol. Lett. 2006, 262, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chen, Q.; Wu, X.; Zhang, J.; Huang, C. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr. Microbiol. 2014, 69, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 451. ISBN 9780849310430. [Google Scholar]
- Lu, Z.; Kong, X.; Lu, Z.; Xiao, M.; Chen, M.; Zhu, L.; Shen, Y.; Hu, X.; Song, S. Para-Aminobenzoic Acid (PABA) synthase enhances thermotolerance of mushroom Agaricus bisporus. PLoS ONE 2014, 9, e91298. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Kong, W.; Wu, X.; XM, L.; Huang, C.; Zhang, J. Biochemical pathway analysis of exogenous NO improving heat-tolerance of Pleurotus eryngii var. tuoliensis. Mycosystema 2015, 34, 632–639. [Google Scholar] [CrossRef]
- China Edible Fungi Association. Available online: http://mushroomsci.org/html/001/c0f6c4f4-a.html (accessed on 28 September 2017).
- Qu, J.B.; Zhao, M.R.; Hsiang, T.; Feng, X.X.; Zhang, J.X.; Huang, C.Y. Identification and characterization of small noncoding RNAs in genome sequences of the edible fungus Pleurotus ostreatus. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 28 September 2017).
- JGI. Available online: http://genome.jgi.doe.gov/PleosPC15_2/PleosPC15_2.home.html (accessed on 28 September 2017).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- ExPASy. Available online: http://web.expasy.org/compute_pi/ (accessed on 28 September 2017).
- PROMO. Available online: http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3 (accessed on 28 September 2017).
- SignalP 4.1 Server. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 28 September 2017).
- Conserved Domains and Protein Classification. Available online: https://www.ncbi.nlm.nih.gov/Structure/cdd/docs/cdd_search.html (accessed on 28 September 2017).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- PIECE. Available online: https://wheat.pw.usda.gov/piece/GSDraw.php (accessed on 28 September 2017).
- Zhang, M.J.; Liu, X.M.; Zou, Y.J.; Huang, C.Y.; Liu, B.; Zhang, J.X. Optimization of heat stress for Pleurotus spp. cultivation. Mycosystema 2015, 34, 662–669. [Google Scholar] [CrossRef]
- Lledías, F.; Rangel, P.; Hansberg, W. Oxidation of catalase by singlet oxygen. J. Biol. Chem. 1998, 273, 10630–10637. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, O.M.; Rouxhet, P.G. Characterization of hydrogen peroxide and superoxide degrading pathways of Aspergillus niger catalase: A steady-state analysis. Free Radic. Res. 1994, 20, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Q.; Ding, Z.; Gai, K.; Han, X.; Kaleri, F.N.; He, Q.; Wang, Y. Regulation of Neurospora Catalase-3 by global heterochromatin formation and its proximal heterochromatin region. Free Radic. Biol. Med. 2016, 99, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhou, Z.; Liu, X.; Gai, K.; Liu, Q.; Cha, J.; Kaleri, F.N.; Wang, Y.; He, Q. Suppression of WHITE COLLAR-independent frequency transcription by histone H3 lysine 36 methyltransferase SET-2 is necessary for clock function in Neurospora. J. Biol. Chem. 2016, 291, 11055–11063. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Cheng, C.Y.; Tang, P.C.; Chen, C.F.; Chen, H.H.; Lee, Y.P.; Huang, S.Y. Acute heat stress induces differential gene expressions in the testes of a broiler-type strain of Taiwan country chickens. PLoS ONE 2015, 10, e0125816. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Nierman, W.C.; Pain, A.; Anderson, M.J.; Wortman, J.R.; Kim, H.S.; Arroyo, J.; Berriman, M.; Abe, K.; Archer, D.B.; Bermejo, C.; et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 2005, 438, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.S.; Stajich, J.E.; Nichols, C.; Gerrald, Q.D.; Alspaugh, J.A.; Dietrich, F.; Perfect, J.R. The Cryptococcus neoformans catalase gene family and its role in antioxidant defense. Eukaryot. cell 2006, 5, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Chary, P.; Natvig, D.O. Evidence for three differentially regulated catalase genes in Neurospora crassa: Effects of oxidative stress, heat shock, and development. J. Bacteriol. 1989, 171, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Barajas, J.A.; Montoya-Perez, R.; Salgado-Garciglia, R.; Aguilera-Aguirre, L.; Cortes-Rojo, C.; Mejia-Zepeda, R.; Arellano-Plaza, M.; Saavedra-Molina, A. Oxidative stress and antioxidant response in a thermotolerant yeast. Braz. J. Microbiol. 2017, 48, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chi, M.; Li, G.; Chen, H.; Sui, Y.; Sun, H.; Wisniewski, M.; Liu, Y.; Liu, J. Heat shock improves stress tolerance and biocontrol performance of Rhodotorula mucilaginosa. Biol. Control 2016, 95, 49–56. [Google Scholar] [CrossRef]
- Pongpom, P.; Cooper, C.R., Jr.; Vanittanakom, N. Isolation and characterization of a catalase-peroxidase gene from the pathogenic fungus, Penicillium marneffei. Med. Mycol. 2005, 43, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gómez, M.; Hernández, J.A.; Luis, A. Metabolism of activated oxygen in peroxisomes from two Pisum sativum L. cultivars with different sensitivity to sodium chloride. J. Plant Physiol. 1993, 141, 160–165. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P.; Hui, Z.; Li, F.; Zhao, M.R.; Zhang, J.; Wang, W. Improvement of heat and drought photosynthetic tolerance in wheat by overaccumulation of glycinebetaine. Plant Biotechnol. Rep. 2010, 4, 213–222. [Google Scholar] [CrossRef]
- Ramírez-Quijas, M.D.; Zazueta-Sandoval, R.; Obregón-Herrera, A.; López-Romero, E.; Cuéllar-Cruz, M. Effect of oxidative stress on cell wall morphology in four pathogenic Candida species. Mycol. Prog. 2015, 14, 8. [Google Scholar] [CrossRef]
- Sundaram, A.; Grant, C.M. Oxidant-specific regulation of protein synthesis in Candida albicans. Fungal Genet. Biol. 2014, 67, 15–23. [Google Scholar] [CrossRef] [PubMed]
| Name | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| Po-cat1 | ATGTCGTCCATCACAGCTG | TCAATACGCAATCCTCGC | 2238 |
| Po-cat2 | ATGCCCACTCAAGAAGTC | TCAGTGGGCGGTGGACTT | 1587 |
| gapdh | GTGTTAACCTCGAGACTTACG | TGGTGGCGTGGATTGTGCTC | 144 |
| Po-cat1_1 | TGTGCATTGGTTGAGAGAGG | TACGACGCTACAACTTCCG | 147 |
| Po-cat2_1 | CGGACTTTCTTGCCCACAG | GACTTGCTCGCCCATTTCG | 149 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wu, X.; Gao, W.; Zhao, M.; Zhang, J.; Huang, C. Differential Expression Patterns of Pleurotus ostreatus Catalase Genes during Developmental Stages and under Heat Stress. Genes 2017, 8, 335. https://doi.org/10.3390/genes8110335
Wang L, Wu X, Gao W, Zhao M, Zhang J, Huang C. Differential Expression Patterns of Pleurotus ostreatus Catalase Genes during Developmental Stages and under Heat Stress. Genes. 2017; 8(11):335. https://doi.org/10.3390/genes8110335
Chicago/Turabian StyleWang, Lining, Xiangli Wu, Wei Gao, Mengran Zhao, Jinxia Zhang, and Chenyang Huang. 2017. "Differential Expression Patterns of Pleurotus ostreatus Catalase Genes during Developmental Stages and under Heat Stress" Genes 8, no. 11: 335. https://doi.org/10.3390/genes8110335






