Synthesis of Rhizobial Exopolysaccharides and Their Importance for Symbiosis with Legume Plants
Abstract
:1. Introduction
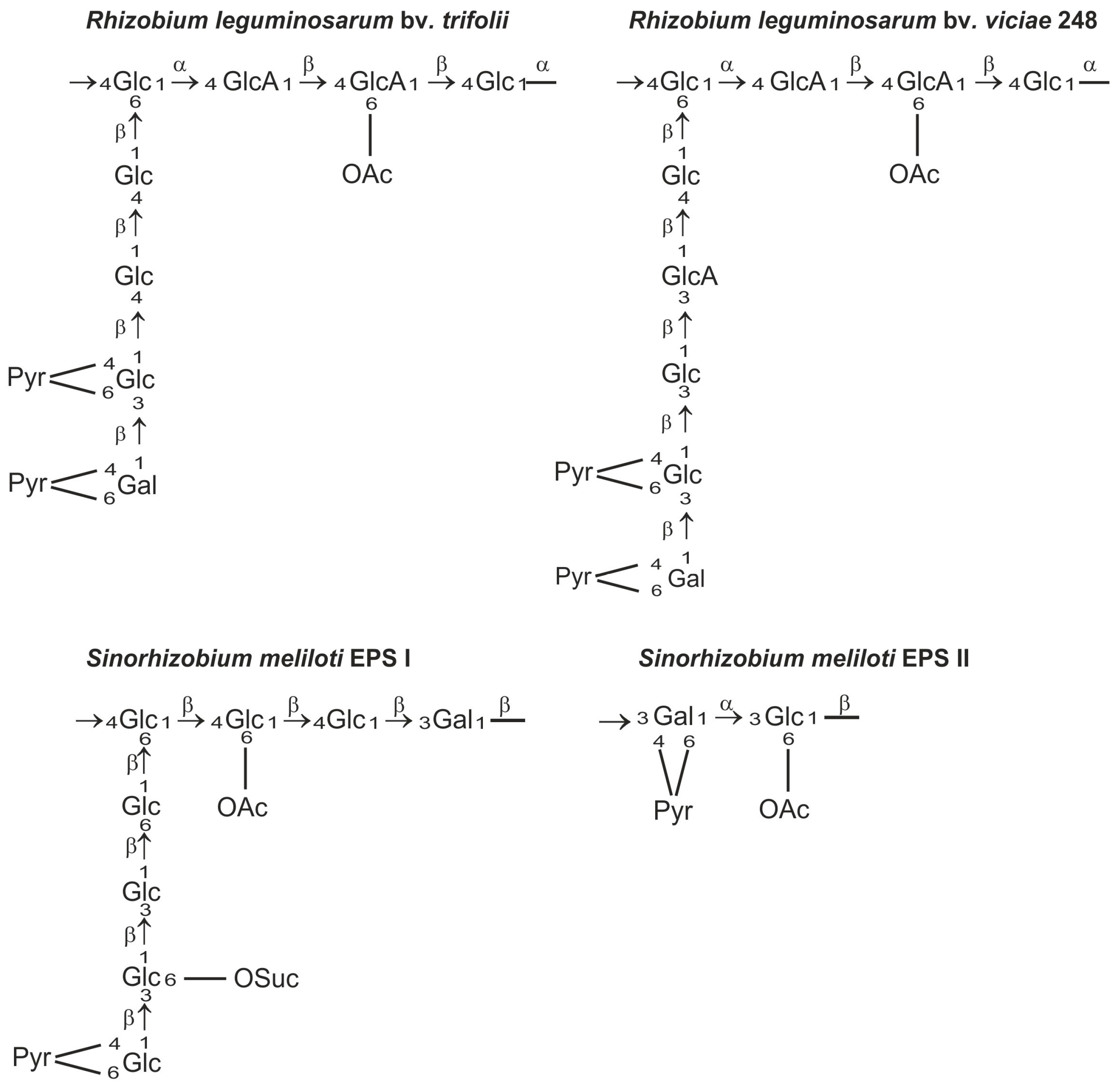
2. Rhizobial Exopolysaccharides Play Diverse Roles in Symbiotic Interaction with Legume Plants
3. Wzx/Wzy-Dependent Synthesis of Exopolysaccharide in Rhizobium leguminosarum bv. trifolii
3.1. Basic EPS Subunits Are Synthesized by Glycosyltransferases
3.2. EPS Subunits Are Transferred Across the Inner Membrane by the Action of the Wzx Flippase
3.3. Wzy Protein Is Responsible for Polymerization of the EPS Subunits
3.4. Polysaccharide Co-Polymerases Determine the Length of Exopolysaccharide Chains
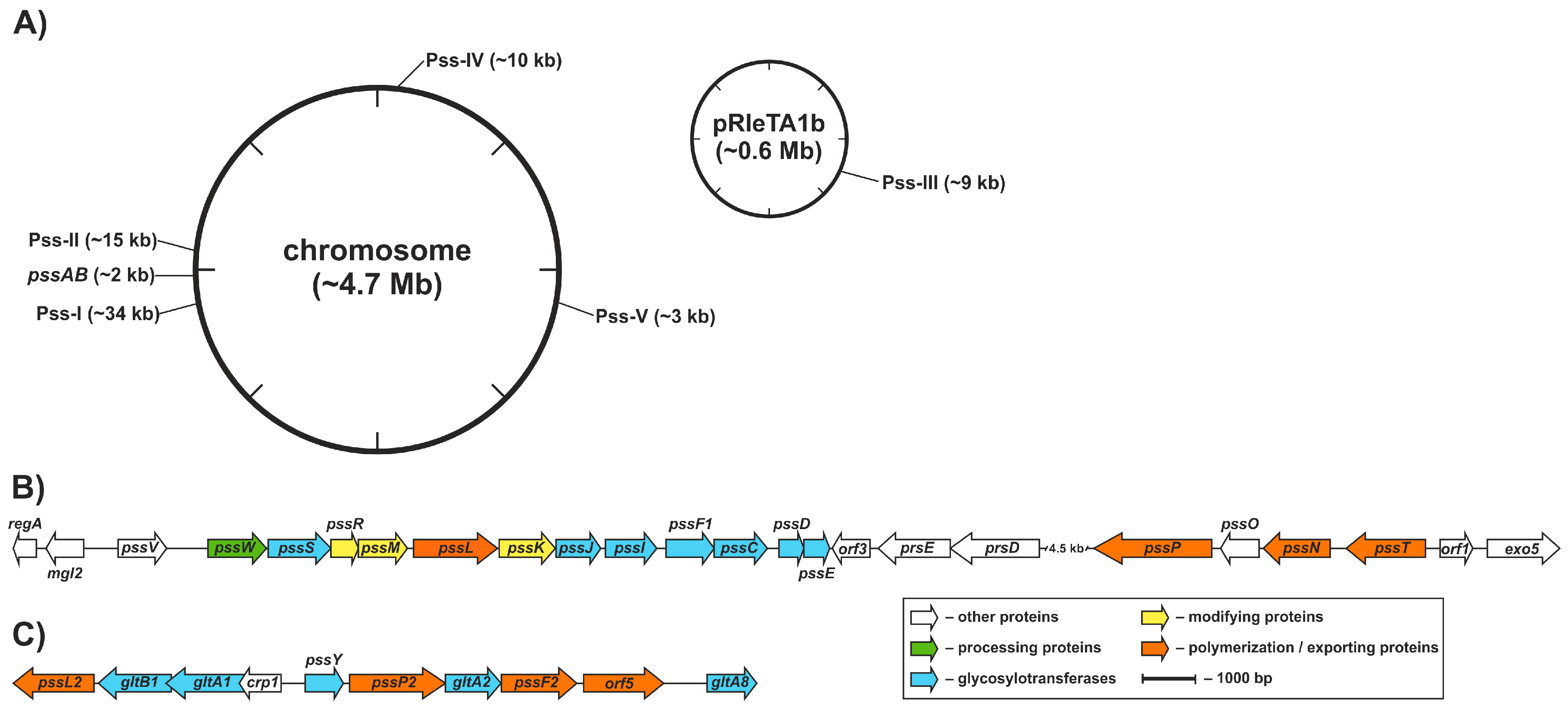
3.5. Other Genomic Regions that Contribute to (Exo)Polysaccharide Synthesis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Long, S.R. Rhizobium symbiosis: Nod factors in perspective. Plant Cell 1996, 8, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing nitrogen-fixing symbiosis with legumes: How many rhizobium recipes? Trends Microbiol. 2009, 17, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.A. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 2010, 34, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.E.; Kobayashi, H.; Walker, G.C. Molecular determinants of a symbiotic chronic infection. Annu. Rev. Genet. 2008, 42, 413–441. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Guerrero, M.G.; Ormeno-Orrillo, E.; Acosta, J.L.; Mendoza-Vargas, A.; Rogel, M.A.; Ramirez, M.A.; Rosenblueth, M.; Martinez-Romero, J.; Martinez-Romero, E. Rhizobial extrachromosomal replicon variability, stability and expression in natural niches. Plasmid 2012, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, A.; Janczarek, M.; Marczak, M.; Mazur, A.; Król, J. Rhizobial exopolysaccharides: Genetic control and symbiotic functions. Microb. Cell Fact. 2006, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Marek-Kozaczuk, M.; Mazur, A.; Kubik-Komar, A.; Skorupska, A. Genetic and metabolic divergence within a Rhizobium leguminosarum bv. trifolii population recovered from clover nodules. Appl. Environ. Microbiol. 2010, 76, 4593–4600. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Marek-Kozaczuk, M.; Kubik-Komar, A.; Skorupska, A. Increased metabolic potential of Rhizobium spp. is associated with bacterial competitiveness. Can. J. Microbiol. 2007, 53, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Multiple responses of rhizobia to flavonoids during legume root infection. Adv. Bot. Res. 2004, 41, 1–62. [Google Scholar]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The Sinorhizobium-Medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant. Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Denarie, J.; Cullimore, J. Lipo-oligosaccharide nodulation factors: A minireview new class of signaling molecules mediating recognition and morphogenesis. Cell 1993, 74, 951–954. [Google Scholar] [CrossRef]
- Fisher, R.F.; Long, S.R. Rhizobium–plant signal exchange. Nature 1992, 357, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; et al. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 2003, 425, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Broghammer, A.; Krusell, L.; Blaise, M.; Sauer, J.; Sullivan, J.T.; Maolanon, N.; Vinther, M.; Lorentzen, A.; Madsen, E.B.; Jensen, K.J.; et al. Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc. Natl. Acad. Sci. USA 2012, 109, 13859–13864. [Google Scholar] [CrossRef] [PubMed]
- Gage, D.J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 2004, 68, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, A.; Liu, C.; Roy, S.; Stacey, N.; Rogers, C.; Trick, M.; Morieri, G.; Mysore, K.S.; Wen, J.; Oldroyd, G.E.; et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 2014, 26, 4680–4701. [Google Scholar] [CrossRef] [PubMed]
- Timmers, A.C.J.; Auriac, M.C.; Truchet, G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 1999, 126, 3617–3628. [Google Scholar] [PubMed]
- Jones, K.M.; Sharopova, N.; Lohar, D.P.; Zhang, J.Q.; VandenBosch, K.A.; Walker, G.C. Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. Proc. Natl. Acad. Sci. USA 2008, 105, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.H.; Lin, Y.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Gage, D.J.; Margolin, W. Hanging by a thread: Invasion of legume plants by rhizobia. Curr. Opin. Microbiol. 2000, 3, 613–617. [Google Scholar] [CrossRef]
- Heidstra, R.; Bisseling, T. Nod factor-induced host responses and mechanisms of Nod factor perception. New Phytol. 1996, 133, 25–43. [Google Scholar] [CrossRef]
- Prell, J.; Poole, P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006, 14, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin biosynthesis promotes cortical cell responses during nodule development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Maroti, G.; Kondorosi, E. Nitrogen-fixing Rhizobium-legume symbiosis: Are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front. Microbiol. 2014, 5, 326. [Google Scholar] [PubMed]
- Vasse, J.; Debilly, F.; Camut, S.; Truchet, G. Correlation between ultrastructural differentiation of bacteroids and nitrogen-fixation in alfalfa nodules. J. Bacteriol. 1990, 172, 4295–4306. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, A.; Vinardell, J.M.; Kiss, E.; Oláh, B.; Roudier, F.; Kondorosi, A.; Kondorosi, E. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 1999, 18, 4476–4484. [Google Scholar] [CrossRef] [PubMed]
- Kondorosi, E.; Mergaert, P.; Kereszt, A. A paradigm for endosymbiotic life: Cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu. Rev. Microbiol. 2013, 67, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Zehirov, G.; Szatmari, A.; Debreczeny, M.; Ishihara, H.; Kevei, Z.; Farkas, A.; Mikulass, K.; Nagy, A.; Tiricz, H.; et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 2010, 327, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Mergaert, P.; Kondorosi, E. Bacteroid development in legume nodules: Evolution of mutual benefit or of sacrificial victims? Mol. Plant Microbe Interact. 2011, 24, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Uchiumi, T.; Alunni, B.; Evanno, G.; Cheron, A.; Catrice, O.; Mausset, A.E.; Barloy-Hubler, F.; Galibert, F.; Kondorosi, A.; et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5230–5235. [Google Scholar] [CrossRef] [PubMed]
- Timmers, A.C.; Soupene, E.; Auriac, M.C.; de Billy, F.; Vasse, J.; Boistard, P.; Truchet, G. Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant Microbe Interact. 2000, 13, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Golus, J.; Marek-Kozaczuk, M.; Skorupska, A. Symbiosis-stage associated alterations in quorum sensing autoinducer molecules biosynthesis in Sinorhizobium meliloti. Plant Soil 2010, 329, 399–410. [Google Scholar] [CrossRef]
- Wielbo, J.; Kuske, J.; Marek-Kozaczuk, M.; Skorupska, A. The competition between Rhizobium leguminosarum bv. viciae strains progresses until late stages of symbiosis. Plant Soil 2010, 337, 125–135. [Google Scholar] [CrossRef]
- Maroti, G.; Downie, J.A.; Kondorosi, E. Plant cysteine-rich peptides that inhibit pathogen growth and control rhizobial differentiation in legume nodules. Curr. Opin. Plant Biol. 2015, 26, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Haag, A.F.; Arnold, M.F.F.; Myka, K.K.; Kerscher, B.; Dall’Angelo, S.; Zanda, M.; Mergaert, P.; Ferguson, G.P. Molecular insights into bacteroid development during Rhizobium-legume symbiosis. FEMS Microbiol. Rev. 2013, 37, 364–383. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.K.; Tabata, S.; Parniske, M.; Stougaard, J. Lotus japonicus: Legume research in the fast lane. Trends Plant Sci. 2005, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M. Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int. J. Mol. Sci. 2011, 12, 7898–7933. [Google Scholar] [CrossRef] [PubMed]
- Laus, M.C.; Logman, T.J.; Lamers, G.E.; Van Brussel, A.A.; Carlson, R.W.; Kijne, J.W. A novel polar surface polysaccharide from Rhizobium leguminosarum binds host plant lectin. Mol. Microbiol. 2006, 59, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, N.; Couderc, F.; Poinsot, V. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 2003, 270, 1365–1380. [Google Scholar] [CrossRef] [PubMed]
- Zevenhuizen, L.P.T.M. Selective synthesis of polysaccharides by Rhizobium Trifolii, strain TA1. FEMS Microbiol. Lett. 1986, 35, 43–47. [Google Scholar] [CrossRef]
- Breedveld, M.W.; Miller, K.J. Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 1994, 58, 145–161. [Google Scholar] [PubMed]
- Becker, A.; Fraysse, N.; Sharypova, L. Recent advances in studies on structure and symbiosis-related function of rhizobial K-antigens and lipopolysaccharides. Mol. Plant Microbe Interact. 2005, 18, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Wilkinson, A.; Krehenbrink, M.; Russo, D.M.; Zorreguieta, A.; Downie, J.A. Glucomannan-mediated attachment of Rhizobium leguminosarum to pea root hairs is required for competitive nodule infection. J. Bacteriol. 2008, 190, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Canter Cremers, H.C.; Stevens, K.; Lugtenberg, B.J.; Wijffelman, C.A.; Batley, M.; Redmond, J.W.; Breedveld, M.W.; Zevenhuizen, L.P. Unusual structure of the exopolysaccharide of Rhizobium leguminosarum bv. viciae strain 248. Carbohydr. Res. 1991, 218, 185–200. [Google Scholar] [CrossRef]
- Laus, M.C.; van Brussel, A.A.; Kijne, J.W. Exopolysaccharide structure is not a determinant of host-plant specificity in nodulation of Vicia sativa roots. Mol. Plant Microbe Interact. 2005, 18, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.A.; Darvill, A.G.; Albersheim, P. The degree of esterification and points of substitution by O-acetyl and O-(3-hydroxybutanoyl) groups in the acidic extracellular polysaccharides secreted by Rhizobium leguminosarum biovars viciae, trifolii, and phaseoli are not related to host range. J. Biol. Chem. 1991, 266, 9549–9555. [Google Scholar] [PubMed]
- Robertsen, B.K.; Ăman, P.; Darvill, A.G.; McNeil, M.; Albersheim, P. Host-symbiont interactions: V. The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii. Plant Physiol. 1981, 67, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, B.B.; Chan, S.Y.; Reuber, T.L.; Marra, A.; Walker, G.C.; Reinhold, V.N. Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J. Bacteriol. 1994, 176, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Zevenhuizen, L.P.T.M. Succinoglycan and galactoglucan. Carbohydr. Polym. 1997, 33, 139–144. [Google Scholar] [CrossRef]
- Kelly, S.J.; Muszyński, A.; Kawaharada, Y.; Hubber, A.M.; Sullivan, J.T.; Sandal, N.; Carlson, R.W.; Stougaard, J.; Ronson, C.W. Conditional requirement for exopolysaccharide in the Mesorhizobium–Lotus symbiosis. Mol. Plant Microbe Interact. 2013, 26, 319–329. [Google Scholar] [CrossRef] [PubMed]
- López-Baena, F.J.; Ruiz-Sainz, J.E.; Rodríguez-Carvajal, M.A.; Vinardell, J.M. Bacterial molecular signals in the Sinorhizobium fredii-soybean symbiosis. Int. J. Mol. Sci. 2016, 17, 755. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, K.; Becker, A. The role of microbial surface polysaccharides in the Rhizobium-legume interaction. In Sub-Cellular Biochemistry, Plant-Microbe Interactions; Biswas, B.B., Das, H.K., Eds.; Springer Science and Business Media: Boston, MA, USA, 1998; Volume 29, pp. 73–116. [Google Scholar]
- Cheng, H.P.; Walker, G.C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar] [PubMed]
- Leigh, J.A.; Signer, E.R.; Walker, G.C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 1985, 82, 6231–6235. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, B.G.; Carlson, R.W.; Ridge, R.W.; Dazzo, F.B.; Mateos, P.F.; Pankhurst, C.E. Defective infection and nodulation of clovers by exopolysaccharide mutants of Rhizobium leguminosarum bv. trifolii. Aust. J. Plant Physiol. 1996, 23, 285–303. [Google Scholar] [CrossRef]
- Her, G.R.; Glazebrook, J.; Walker, G.C.; Reinhold, V.N. Structural studies of a novel exopolysaccharide produced by a mutant of Rhizobium meliloti strain Rm1021. Carbohydr. Res. 1990, 198, 305–312. [Google Scholar] [CrossRef]
- Gonzalez, J.E.; Reuhs, B.L.; Walker, G.C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc. Natl. Acad. Sci. USA 1996, 93, 8636–8641. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, S.P.; Chen, H.; Batley, M.; Redmond, J.W.; Rolfe, B.G. Nitrogen-fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J. Bacteriol. 1987, 169, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Król, J.E.; Marczak, M.; Skorupska, A. Membrane topology of PssT, the transmembrane protein component of the type I exopolysaccharide transport system in Rhizobium leguminosarum bv. trifolii strain TA1. J. Bacteriol. 2003, 185, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Król, J.E.; Wielbo, J.; Urbanik-Sypniewska, T.; Skorupska, A. Rhizobium leguminosarum bv. trifolii PssP protein is required for exopolysaccharide biosynthesis and polymerization. Mol. Plant Microbe Interact. 2002, 15, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Battisti, L.; Lara, J.C.; Leigh, J.A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc. Natl. Acad. Sci. USA 1992, 89, 5625–5629. [Google Scholar] [CrossRef] [PubMed]
- Pellock, B.J.; Cheng, H.P.; Walker, G.C. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 2000, 182, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Wang, Y.; Pellock, B.; Walker, G.C. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 1999, 181, 6788–6796. [Google Scholar] [PubMed]
- Mendis, H.C.; Madzima, T.F.; Queiroux, C.; Jones, K.M. Function of succinoglycan polysaccharide in Sinorhizobium meliloti host plant invasion depends on succinylation, not molecular weight. MBio 2016, 7, e00606-16. [Google Scholar] [CrossRef] [PubMed]
- González, J.E.; Semino, C.E.; Wang, L.X.; Castellano-Torres, L.E.; Walker, G.C. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 1998, 95, 13477–13482. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.A.; Reed, J.W.; Hanks, J.F.; Hirsch, A.M.; Walker, G.C. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 1987, 51, 579–587. [Google Scholar] [CrossRef]
- York, G.M.; Walker, G.C. The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J. Bacteriol. 1998, 180, 4184–4191. [Google Scholar] [PubMed]
- Gharzouli, R.; Carpéné, M.A.; Couderc, F.; Benguedouar, A.; Poinsot, V. Relevance of fucose-rich extracellular polysaccharides produced by Rhizobium sullae strains nodulating Hedysarum coronarium l. legumes. Appl. Environ. Microbiol. 2013, 79, 1764–1776. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Wood, K.; Reuhs, B.L. Structural analysis of succinoglycan oligosaccharides from Sinorhizobium meliloti strains with different host compatibility phenotypes. J. Bacteriol. 2013, 195, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.J.; Brownsey, G.J.; York, G.M.; Walker, G.C.; Morris, V.J. Effect of O-acyl substituents on the functional behaviour of Rhizobium meliloti succinoglycan. Int. J. Biol. Macromol. 1997, 20, 1–7. [Google Scholar] [CrossRef]
- Lehman, A.P.; Long, S.R. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J. Bacteriol. 2013, 195, 5362–5369. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M. Increased production of the exopolysaccharide succinoglycan enhances Sinorhizobium meliloti 1021 symbiosis with the host plant Medicago truncatula. J. Bacteriol. 2012, 194, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Marczak, M.; Dzwierzyńska, M.; Skorupska, A. Homo- and heterotypic interactions between Pss proteins involved in the exopolysaccharide transport system in Rhizobium leguminosarum bv. trifolii. Biol. Chem. 2013, 394, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, L.; Manning, P.A.; Morona, R. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol. Microbiol. 1997, 23, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Payne, S.M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol. Microbiol. 1997, 24, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszynski, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Grønlund, M.; Sato, S.; Nakamura, Y.; Tabata, S.; Sandal, N.; et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Muszyński, A.; Heiss, C.; Hjuler, C.T.; Sullivan, J.T.; Kelly, S.J.; Thygesen, M.B.; Stougaard, J.; Azadi, P.; Carlson, R.W.; Ronson, C.W. Structures of exopolysaccharides involved in receptor-mediated perception of Mesorhizobium loti by Lotus japonicus. J. Biol. Chem. 2016, 291, 20946–20961. [Google Scholar] [CrossRef] [PubMed]
- Kawaharada, Y.; Nielsen, M.W.; Kelly, S.; James, E.K.; Andersen, K.R.; Rasmussen, S.R.; Füchtbauer, W.; Madsen, L.H.; Heckmann, A.B.; Radutoiu, S.; et al. Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat. Commun. 2017, 8, 14534. [Google Scholar] [CrossRef] [PubMed]
- Ivashina, T.V.; Ksenzenko, V.N. Exopolysaccharide Biosynthesis in Rhizobium leguminosarum: From Genes to Functions. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 99–126. [Google Scholar]
- Finan, T.M.; Weidner, S.; Wong, K.; Buhrmester, J.; Chain, P.; Vorhölter, F.J.; Hernandez-Lucas, I.; Becker, A.; Cowie, A.; Gouzy, J.; et al. The complete sequence of the 1,683-kb pSymB megaplasmid from the N2-fixing endosymbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 2001, 98, 9889–9894. [Google Scholar] [CrossRef] [PubMed]
- González, V.; Santamaria, R.I.; Bustos, P.; Hernández-González, I.; Medrano-Soto, A.; Moreno-Hagelsieb, G.; Janga, S.C.; Ramirez, M.A.; Jiménez-Jacinto, V.; Collado-Vides, J.; et al. The partitioned Rhizobium etli genome: Genetic and metabolic redundancy in seven interacting replicons. Proc. Natl. Acad. Sci. USA 2006, 103, 3834–3839. [Google Scholar] [CrossRef] [PubMed]
- Król, J.E.; Mazur, A.; Marczak, M.; Skorupska, A. Syntenic arrangements of the surface polysaccharide biosynthesis genes in Rhizobium leguminosarum. Genomics 2007, 89, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Young, J.P.W.; Crossman, L.C.; Johnston, A.W.B.; Thomson, N.R.; Ghazoui, Z.F.; Hull, K.H.; Wexler, M.; Curson, A.R.J.; Todd, J.D.; Poole, P.S.; et al. The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol. 2006, 7, R34. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Kleickmann, A.; Keller, M.; Arnold, W.; Pühler, A. Identification and analysis of the Rhizobium meliloti exoAMONP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol. Gen. Genet. 1993, 241, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Kleickmann, A.; Kuster, H.; Keller, M.; Arnold, W.; Pühler, A. Analysis of the Rhizobium meliloti genes exoU, exoV, exoW, exoT, and exoI involved in exopolysaccharide biosynthesis and nodule invasion—ExoU and exoW probably encode glucosyltransferases. Mol. Plant Microbe Interact. 1993, 6, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Kleickmann, A.; Arnold, W.; Puhler, A. Analysis of the Rhizobium meliloti exoH/exoK/exoL fragment: ExoK shows homology to excreted endo-β-1,3-1,4-glucanases and ExoH resembles membrane proteins. Mol. Gen. Genet. 1993, 238, 145–154. [Google Scholar] [PubMed]
- Becker, A.; Niehaus, K.; Pühler, A. Low-molecular-weight succinoglycan is predominantly produced by Rhizobium meliloti strains carrying a mutated ExoP protein characterized by a periplasmic N-terminal domain and a missing C-terminal domain. Mol. Microbiol. 1995, 16, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Glucksmann, M.A.; Reuber, T.L.; Walker, G.C. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J. Bacteriol. 1993, 175, 7033–7044. [Google Scholar] [CrossRef] [PubMed]
- Glucksmann, M.A.; Reuber, T.L.; Walker, G.C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: A model for succinoglycan biosynthesis. J. Bacteriol. 1993, 175, 7045–7055. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Roxlau, A.; Weng, W.M.; Schmidt, M.; Quandt, J.; Niehaus, K.; Jording, D.; Arnold, W.; Puhler, A. Molecular analysis of the Rhizobium meliloti mucR gene regulating the biosynthesis of the exopolysaccharides succinoglycan and galactoglucan. Mol. Plant Microbe Interact. 1995, 8, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.W.; Glazebrook, J.; Walker, G.C. The exoR gene of Rhizobium meliloti affects RNA levels of other exo genes but lacks homology to known transcriptional regulators. J. Bacteriol. 1991, 173, 3789–3794. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.K.; Whitfield, C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr. Res. 2012, 356, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.C.; Howell, P.L. Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 2013, 21, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Eckford, P.D.; Jones, M.L.; Nugent, T.; Bear, C.E.; Vogel, C.; Lam, J.S. Proton-dependent gating and proton uptake by Wzx support O-antigen-subunit antiport across the bacterial inner membrane. MBio 2013, 4, e00678-13. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Rachwał, K. Mutation in the pssA gene involved in exopolysaccharide synthesis leads to several physiological and symbiotic defects in Rhizobium leguminosarum bv. trifolii. Int. J. Mol. Sci. 2013, 14, 23711–23735. [Google Scholar] [CrossRef] [PubMed]
- Król, J.; Wielbo, J.; Mazur, A.; Kopcińska, J.; Lotocka, B.; Golinowski, W.; Skorupska, A. Molecular characterization of pssCDE genes of Rhizobium leguminosarum bv. trifolii strain TA1: PssD mutant is affected in exopolysaccharide synthesis and endocytosis of bacteria. Mol. Plant Microbe Interact 1998, 11, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Marczak, M.; Król, J.E.; Skorupska, A. Topological and transcriptional analysis of pssL gene product: A putative Wzx-like exopolysaccharide translocase in Rhizobium leguminosarum bv. trifolii TA1. Arch. Microbiol. 2005, 184, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marczak, M.; Mazur, A.; Król, J.E.; Gruszecki, W.I.; Skorupska, A. Lipoprotein PssN of Rhizobium leguminosarum bv. trifolii: Subcellular localization and possible involvement in exopolysaccharide export. J. Bacteriol. 2006, 188, 6943–6952. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, G.; Peralta, H.; Aguilar, A.; Díaz, R.; Villalobos, M.A.; Medrano-Soto, A.; Mora, J. Evolutionary, structural and functional relationships revealed by comparative analysis of syntenic genes in Rhizobiales. BMC Evol. Biol. 2005, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, H.R.; Posadas, D.M.; Paris, G.; Carrica, M.D.; Frederickson, M.; Pietrasanta, L.I.; Bogomolni, R.A.; Zorreguieta, A.; Goldbaum, F.A. Light regulates attachment, exopolysaccharide production, and nodulation in Rhizobium leguminosarum through a LOV-histidine kinase photoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 12135–12140. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, J.; Kopycińska, M.; Łukowska, M.; Bieganowski, A.; Janczarek, M. Surface properties of wild-type Rhizobium leguminosarum bv. trifolii strain 24.2 and its derivatives with different extracellular polysaccharide content. PLoS ONE 2016, 11, e0165080. [Google Scholar] [CrossRef] [PubMed]
- Mendrygal, K.E.; González, J.E. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 2000, 182, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, M.W.; Zevenhuizen, L.P.T.M.; Cremers, H.C.J.C.; Zehnder, A.J.B. Influence of growth conditions on production of capsular and extracellular polysaccharides by Rhizobium leguminosarum. Anton. Leeuw. 1993, 64, 1–8. [Google Scholar] [CrossRef]
- Mimmack, M.L.; Borthakur, D.; Jones, M.A.; Downie, J.A.; Johnston, A.W. The psi operon of Rhizobium leguminosarum biovar phaseoli: Identification of two genes whose products are located at the bacterial cell surface. Microbiology 1994, 140 Pt 5, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Skorupska, A. The Rhizobium leguminosarum bv. trifolii RosR: Transcriptional regulator involved in exopolysaccharide production. Mol. Plant Microbe Interact. 2007, 20, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Reeve, W.G.; Dilworth, M.J.; Tiwari, R.P.; Glenn, A.R. Regulation of exopolysaccharide production in Rhizobium leguminosarum biovar viciae WSM710 involves exoR. Microbiology 1997, 143 Pt 6, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Król, J.; Skorupska, A. The pssB gene product of Rhizobium leguminosarum bv. trifolii is homologous to a family of inositol monophosphatases. FEMS Microbiol. Lett. 1999, 173, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Becker, A. Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front. Microbiol. 2015, 6, 687. [Google Scholar] [CrossRef] [PubMed]
- Reuber, T.L.; Walker, G.C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 1993, 74, 269–280. [Google Scholar] [CrossRef]
- Janczarek, M.; Skorupska, A. Exopolysaccharide synthesis in Rhizobium leguminosarum bv. trifolii is related to various metabolic pathways. Res. Microbiol. 2003, 154, 433–442. [Google Scholar] [CrossRef]
- Janczarek, M.; Rachwal, K.; Turska-Szewczuk, A. A mutation in pssE affects exopolysaccharide synthesis by Rhizobium leguminosarum bv. trifolii, its surface properties, and symbiosis with clover. Plant Soil 2017, 417, 331–347. [Google Scholar] [CrossRef]
- Pollock, T.J.; van Workum, W.A.T.; Thorne, L.; Mikolajczak, M.J.; Yamazaki, M.; Kijne, J.W.; Armentrout, R.W. Assignment of biochemical functions to glycosyl transferase genes which are essential for biosynthesis of exopolysaccharides in Sphingomonas strain S88 and Rhizobium leguminosarum. J. Bacteriol. 1998, 180, 586–593. [Google Scholar] [PubMed]
- Guerreiro, N.; Ksenzenko, V.N.; Djordjevic, M.A.; Ivashina, T.V.; Rolfe, B.G. Elevated levels of synthesis of over 20 proteins results after mutation of the Rhizobium leguminosarum exopolysaccharide synthesis gene pssA. J. Bacteriol. 2000, 182, 4521–4532. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Rachwał, K.; Kopcińska, J. Genetic characterization of the Pss region and the role of PssS in exopolysaccharide production and symbiosis of Rhizobium leguminosarum bv. trifolii with clover. Plant Soil 2015, 396, 257–275. [Google Scholar] [CrossRef]
- Hvorup, R.; Chang, A.B.; Saier, M.H., Jr. Bioinformatic analyses of the bacterial l-ascorbate phosphotransferase system permease family. J. Mol. Microbiol. Biotechnol. 2003, 6, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Reeves, P.R. Diversity of O-antigen repeat unit structures can account for the substantial sequence variation of Wzx translocases. J. Bacteriol. 2014, 196, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Cunneen, M.M.; Reeves, P.R. Membrane topology of the Salmonella enterica serovar Typhimurium Group B O-antigen translocase Wzx. FEMS Microbiol. Lett. 2008, 287, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Taylor, V.L.; Qi, M.; Lam, J.S. Membrane topology mapping of the O-antigen flippase (Wzx), polymerase (Wzy), and ligase (WaaL) from Pseudomonas aeruginosa PAO1 reveals novel domain architectures. MBio 2010, 1, e00189-10. [Google Scholar] [CrossRef] [PubMed]
- Marolda, C.L.; Li, B.; Lung, M.; Yang, M.; Hanuszkiewicz, A.; Rosales, A.R.; Valvano, M.A. Membrane topology and identification of critical amino acid residues in the Wzx O-antigen translocase from Escherichia coli O157:H4. J. Bacteriol. 2010, 192, 6160–6171. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Fieldhouse, R.J.; Anderson, E.M.; Taylor, V.L.; Keates, R.A.; Ford, R.C.; Lam, J.S. A cationic lumen in the Wzx flippase mediates anionic O-antigen subunit translocation in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 2012, 84, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Lam, J.S. Wzx flippase-mediated membrane translocation of sugar polymer precursors in bacteria. Environ. Microbiol. 2013, 15, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.; Yi, W.; Li, L.; Zhao, G.; Eguchi, H.; Sridhar, P.R.; Guo, H.; Song, J.K.; Motari, E.; Cai, L.; et al. In vitro bacterial polysaccharide biosynthesis: Defining the functions of Wzy and Wzz. Nat. Chem. Biol. 2010, 6, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.; Vindurampulle, C.; Morona, R. Overexpression and topology of the Shigella flexneri O-antigen polymerase (Rfc/Wzy). Mol. Microbiol. 1998, 28, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Gold, A.C.; Taylor, V.L.; Anderson, E.M.; Ford, R.C.; Lam, J.S. Dual conserved periplasmic loops possess essential charge characteristics that support a catch-and-release mechanism of O-antigen polymerization by Wzy in Pseudomonas aeruginosa PAO1. J. Biol. Chem. 2011, 286, 20600–20605. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Morona, R. Mutational analysis of the major periplasmic loops of Shigella flexneri Wzy: Identification of the residues affecting O-antigen modal chain length control, and Wzz-dependent polymerization activity. Microbiology 2015, 161, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Huszczynski, S.M.; Nugent, T.; Gold, A.C.; Lam, J.S. Conserved-residue mutations in Wzy affect O-antigen polymerization and Wzz-mediated chain-length regulation in Pseudomonas aeruginosa PAO1. Sci. Rep. 2013, 3, 3441. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Morcilla, V.A.; Liu, M.A.; Russell, E.L.; Reeves, P.R. Three Wzy polymerases are specific for particular forms of an internal linkage in otherwise identical O units. Microbiology 2015, 161, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Bastin, D.A.; Stevenson, G.; Brown, P.K.; Haase, A.; Reeves, P.R. Repeat unit polysaccharides of bacteria—A model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain-length. Mol. Microbiol. 1993, 7, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Beness, A.M.; Saier, M.H., Jr. Computer-based analyses of the protein constituents of transport systems catalysing export of complex carbohydrates in bacteria. Microbiology 1997, 143 Pt 8, 2685–2699. [Google Scholar] [CrossRef] [PubMed]
- Morona, R.; Van Den Bosch, L.; Daniels, C. Evaluation of Wzz/MPA1/MPA2 proteins based on the presence of coiled-coil regions. Microbiology 2000, 146 Pt 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Grangeasse, C.; Terreux, R.; Nessler, S. Bacterial tyrosine-kinases: Structure-function analysis and therapeutic potential. Biochim. Biophys. Acta 2010, 1804, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Jadeau, F.; Bechet, E.; Cozzone, A.J.; Deléage, G.; Grangeasse, C.; Combet, C. Identification of the idiosyncratic bacterial protein tyrosine kinase (BY-kinase) family signature. Bioinformatics 2008, 24, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Tocilj, A.; Munger, C.; Proteau, A.; Morona, R.; Purins, L.; Ajamian, E.; Wagner, J.; Papadopoulos, M.; Van Den Bosch, L.; Rubinstein, J.L.; et al. Bacterial polysaccharide co-polymerases share a common framework for control of polymer length. Nat. Struct. Mol. Biol. 2008, 15, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.N.; Morona, R. Residues located inside the Escherichia coli FepE protein oligomer are essential for lipopolysaccharide O-antigen modal chain length regulation. Microbiology 2013, 159, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Kintz, E.N.; Goldberg, J.B. Site-directed mutagenesis reveals key residue for O-antigen chain length regulation and protein stability in Pseudomonas aeruginosa Wzz2. J. Biol. Chem. 2011, 286, 44277–44284. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.; Morona, R. Mutagenesis and chemical cross-linking suggest that Wzz dimer stability and oligomerization affect lipopolysaccharide O-antigen modal chain length control. J. Bacteriol. 2010, 192, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Tran, E.N.; Ericsson, D.J.; Casey, L.W.; Lonhienne, T.; Benning, F.; Morona, R.; Kobe, B. Structural and biochemical analysis of a single amino-acid mutant of WzzBSF that alters lipopolysaccharide O-antigen chain length in Shigella flexneri. PLoS ONE 2015, 10, e0138266. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.; Tran, E.N.; Murray, G.L.; Morona, R. Conserved transmembrane glycine residues in the Shigella flexneri polysaccharide co-polymerase protein WzzB influence protein-protein interactions. Microbiology 2016, 162, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, D.; Becker, A. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J. Bacteriol. 2001, 183, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Beis, K.; Collins, R.F.; Ford, R.C.; Kamis, A.B.; Whitfield, C.; Naismith, J.H. Three-dimensional structure of Wza, the protein required for translocation of group 1 capsular polysaccharide across the outer membrane of Escherichia coli. J. Biol. Chem. 2004, 279, 28227–28232. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.F.; Derrick, J.P. Wza: A new structural paradigm for outer membrane secretory proteins? Trends Microbiol. 2007, 15, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Beis, K.; Nesper, J.; Brunkan-Lamontagne, A.L.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. Wza the translocon for Escherichia coli capsular polysaccharides defines a new class of membrane protein. Nature 2006, 444, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Nesper, J.; Hill, C.M.; Paiment, A.; Harauz, G.; Beis, K.; Naismith, J.H.; Whitfield, C. Translocation of group 1 capsular polysaccharide in Escherichia coli serotype K30. Structural and functional analysis of the outer membrane lipoprotein Wza. J. Biol. Chem. 2003, 278, 49763–49772. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, N.N.; Mainprize, I.L.; Hampton, L.; Jones, M.L.; Naismith, J.H.; Whitfield, C. Trapped translocation intermediates establish the route for export of capsular polysaccharides across Escherichia coli outer membranes. Proc. Natl. Acad. Sci. USA 2014, 111, 8203–8208. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.F.; Beis, K.; Clarke, B.R.; Ford, R.C.; Hulley, M.; Naismith, J.H.; Whitfield, C. Periplasmic protein-protein contacts in the inner membrane protein Wzc form a tetrameric complex required for the assembly of Escherichia coli group 1 capsules. J. Biol. Chem. 2006, 281, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.F.; Beis, K.; Dong, C.; Botting, C.H.; McDonnell, C.; Ford, R.C.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. The 3D structure of a periplasm-spanning platform required for assembly of group 1 capsular polysaccharides in Escherichia coli. Proc. Natl. Acad. Sci. USA 2007, 104, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis of lipopolysaccharide O-antigens. Trends Microbiol. 1995, 3, 178–185. [Google Scholar] [CrossRef]
- Daniels, C.; Morona, R. Analysis of Shigella flexneri Wzz (Rol) function by mutagenesis and cross-linking: Wzz is able to oligomerize. Mol. Microbiol. 1999, 34, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Kalynych, S.; Cherney, M.; Bostina, M.; Rouiller, I.; Cygler, M. Quaternary structure of WzzB and WzzE polysaccharide copolymerases. Protein Sci. 2015, 24, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Kalynych, S.; Yao, D.; Magee, J.; Cygler, M. Structural characterization of closely related O-antigen lipopolysaccharide (LPS) chain length regulators. J. Biol. Chem. 2012, 287, 15696–15705. [Google Scholar] [CrossRef] [PubMed]
- Larue, K.; Kimber, M.S.; Ford, R.; Whitfield, C. Biochemical and structural analysis of bacterial O-antigen chain length regulator proteins reveals a conserved quaternary structure. J. Biol. Chem. 2009, 284, 7395–7403. [Google Scholar] [CrossRef] [PubMed]
- Marolda, C.L.; Tatar, L.D.; Alaimo, C.; Aebi, M.; Valvano, M.A. Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide O-antigen. J. Bacteriol. 2006, 188, 5124–5135. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.L.; Udaskin, M.L.; Islam, S.T.; Lam, J.S. The D3 bacteriophage α-polymerase inhibitor (Iap) peptide disrupts O-antigen biosynthesis through mimicry of the chain length regulator Wzz in Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Marczak, M.; Matysiak, P.; Kutkowska, J.; Skorupska, A. PssP2 is a polysaccharide co-polymerase involved in exopolysaccharide chain-length determination in Rhizobium leguminosarum. PLoS ONE 2014, 9, e109106. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Morona, R. Detection of Wzy/Wzz interaction in Shigella flexneri. Microbiology 2015, 161, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Allaway, D.; Jeyaretnam, B.; Carlson, R.W.; Poole, P.S. Genetic and chemical characterization of a mutant that disrupts synthesis of the lipopolysaccharide core tetrasaccharide in Rhizobium leguminosarum. J. Bacteriol. 1996, 178, 6403–6406. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W.; Reuhs, B.; Chen, T.B.; Bhat, U.R.; Noel, K.D. Lipopolysaccharide core structures in Rhizobium etli and mutants deficient in O-antigen. J. Biol. Chem. 1995, 270, 11783–11788. [Google Scholar] [CrossRef] [PubMed]
- Cava, J.R.; Elias, P.M.; Turowski, D.A.; Noel, K.D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J. Bacteriol. 1989, 171, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Nakamura, Y.; Sato, S.; Asamizu, E.; Kato, T.; Sasamoto, S.; Watanabe, A.; Idesawa, K.; Ishikawa, A.; Kawashima, K.; et al. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 2000, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Nakamura, Y.; Sato, S.; Minamisawa, K.; Uchiumi, T.; Sasamoto, S.; Watanabe, A.; Idesawa, K.; Iriguchi, M.; Kawashima, K.; et al. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wielbo, J.; Mazur, A.; Król, J.; Marczak, M.; Kutkowska, J.; Skorupska, A. Complexity of phenotypes and symbiotic behaviour of Rhizobium leguminosarum biovar trifolii exopolysaccharide mutants. Arch. Microbiol. 2004, 182, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.A.; Jiménez, J.C.; Zaldívar, M.; Álvarez, S.A.; Marolda, C.L.; Valvano, M.A.; Contreras, I. The cellular level of O-antigen polymerase Wzy determines chain length regulation by WzzB and WzzpHS-2 in Shigella flexneri 2a. Microbiology 2009, 155, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Jofré, E.; Becker, A. Production of succinoglycan polymer in Sinorhizobium meliloti is affected by SMb21506 and requires the N-terminal domain of ExoP. Mol. Plant Microbe Interact. 2009, 22, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Garcia-de los Santos, A.; Brom, S. Characterization of two plasmid-borne lpsβ loci of Rhizobium etli required for lipopolysaccharide synthesis and for optimal interaction with plants. Mol. Plant Microbe Interact. 1997, 10, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, G.; Mazur, A.; Wielbo, J.; Marczak, M.; Zebracki, K.; Koper, P.; Skorupska, A. Functional relationships between plasmids and their significance for metabolism and symbiotic performance of Rhizobium leguminosarum bv. trifolii. J. Appl. Genet. 2014, 55, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Stasiak, G.; Wielbo, J.; Koper, P.; Kubik-Komar, A.; Skorupska, A. Phenotype profiling of Rhizobium leguminosarum bv. trifolii clover nodule isolates reveal their both versatile and specialized metabolic capabilities. Arch. Microbiol. 2013, 195, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Rinaudi, L.V.; González, J.E. The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J. Bacteriol. 2009, 191, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Sorroche, F.G.; Rinaudi, L.V.; Zorreguieta, A.; Giordano, W. EPS II-dependent autoaggregation of Sinorhizobium meliloti planktonic cells. Curr. Microbiol. 2010, 61, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sorroche, F.G.; Spesia, M.B.; Zorreguieta, A.; Giordano, W. A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl. Environ. Microbiol. 2012, 78, 4092–4101. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; Oliva, M.D.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marczak, M.; Mazur, A.; Koper, P.; Żebracki, K.; Skorupska, A. Synthesis of Rhizobial Exopolysaccharides and Their Importance for Symbiosis with Legume Plants. Genes 2017, 8, 360. https://doi.org/10.3390/genes8120360
Marczak M, Mazur A, Koper P, Żebracki K, Skorupska A. Synthesis of Rhizobial Exopolysaccharides and Their Importance for Symbiosis with Legume Plants. Genes. 2017; 8(12):360. https://doi.org/10.3390/genes8120360
Chicago/Turabian StyleMarczak, Małgorzata, Andrzej Mazur, Piotr Koper, Kamil Żebracki, and Anna Skorupska. 2017. "Synthesis of Rhizobial Exopolysaccharides and Their Importance for Symbiosis with Legume Plants" Genes 8, no. 12: 360. https://doi.org/10.3390/genes8120360




