Genome-Wide Transcriptional Changes and Lipid Profile Modifications Induced by Medicago truncatula N5 Overexpression at an Early Stage of the Symbiotic Interaction with Sinorhizobium meliloti
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Medicago truncatula Stable Transformation with MtN5-Silencing and MtN5-Overexpression Constructs
2.3. Plant Growth and Symbiont Inoculation
2.4. LacZ Histochemical Staining
2.5. Cutin Treatment
2.6. Trypan Blue Staining
2.7. RNA Extraction and Quantitative RT-PCR
2.8. Microarray and Data Analyses
2.9. Probe Mapping to M. truncatula Genes and Annotation
2.10. Lipid Extraction
2.11. LC-MS, Metabolite Annotation and Data Analysis
3. Results
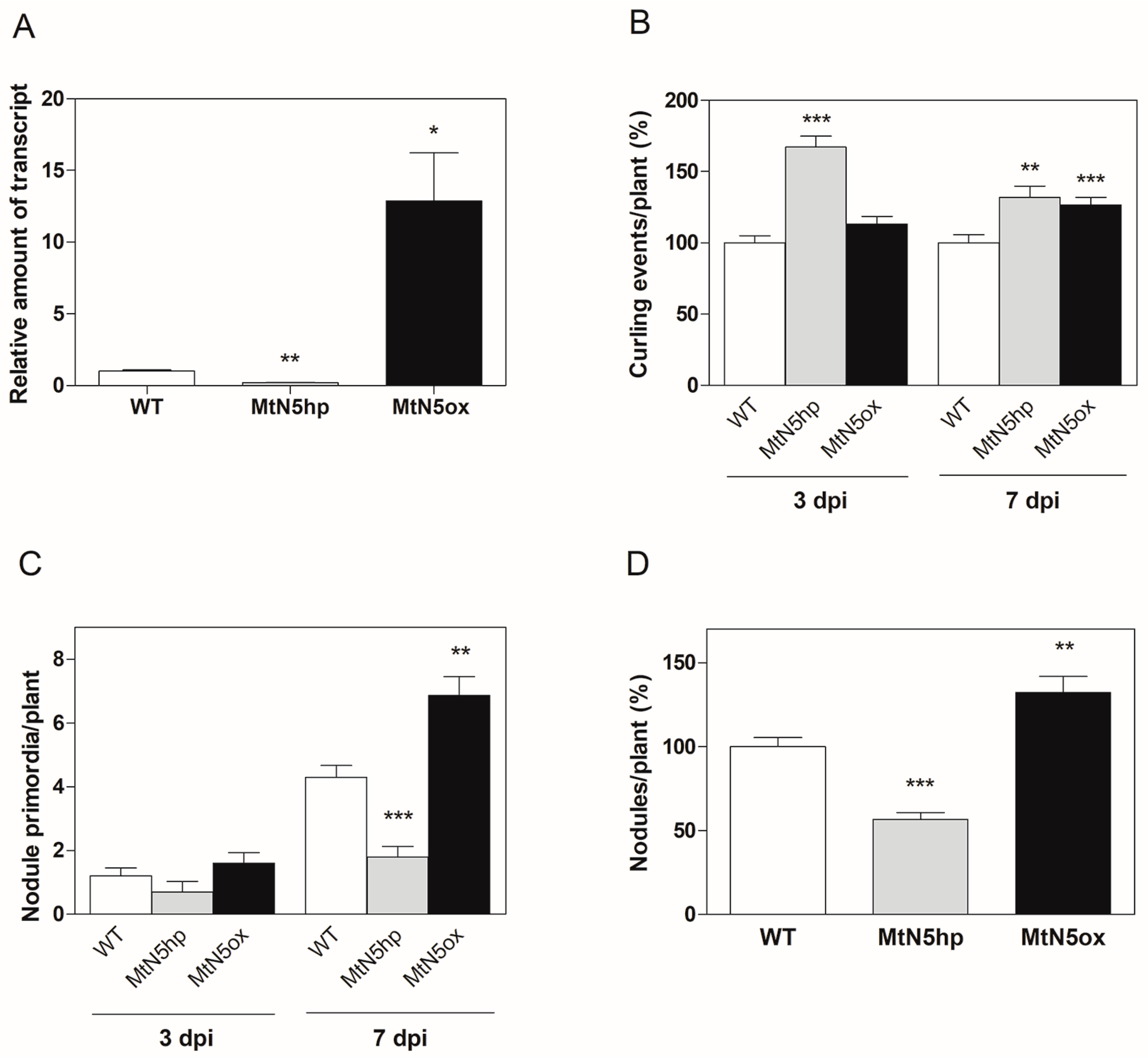
3.1. Transcriptional Analysis of MtN5-Silenced and MtN5–Overexpressing Plants at an Early Stage of S. meliloti Infection
3.2. Changes in Gene Expression Observed in MtN5hp Inoculated Roots
3.3. Changes in Gene Expression Observed in MtN5ox Inoculated Roots
3.3.1. Transcription Factors
3.3.2. Transport
3.3.3. Genes Related to Metabolic Changes Relevant for the Early Response to Rhizobia
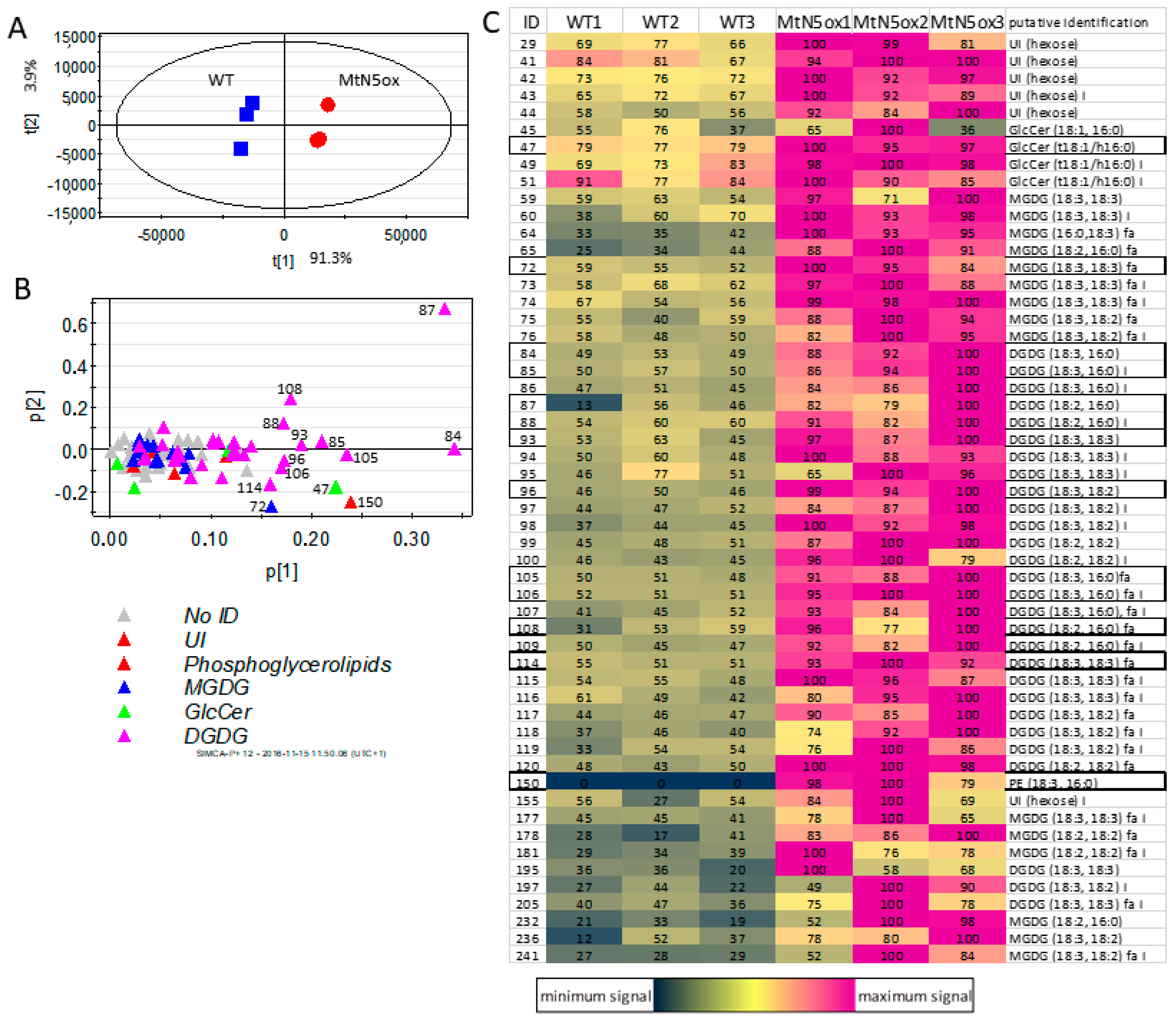
3.3.4. DEGs Related to Lipid Metabolism
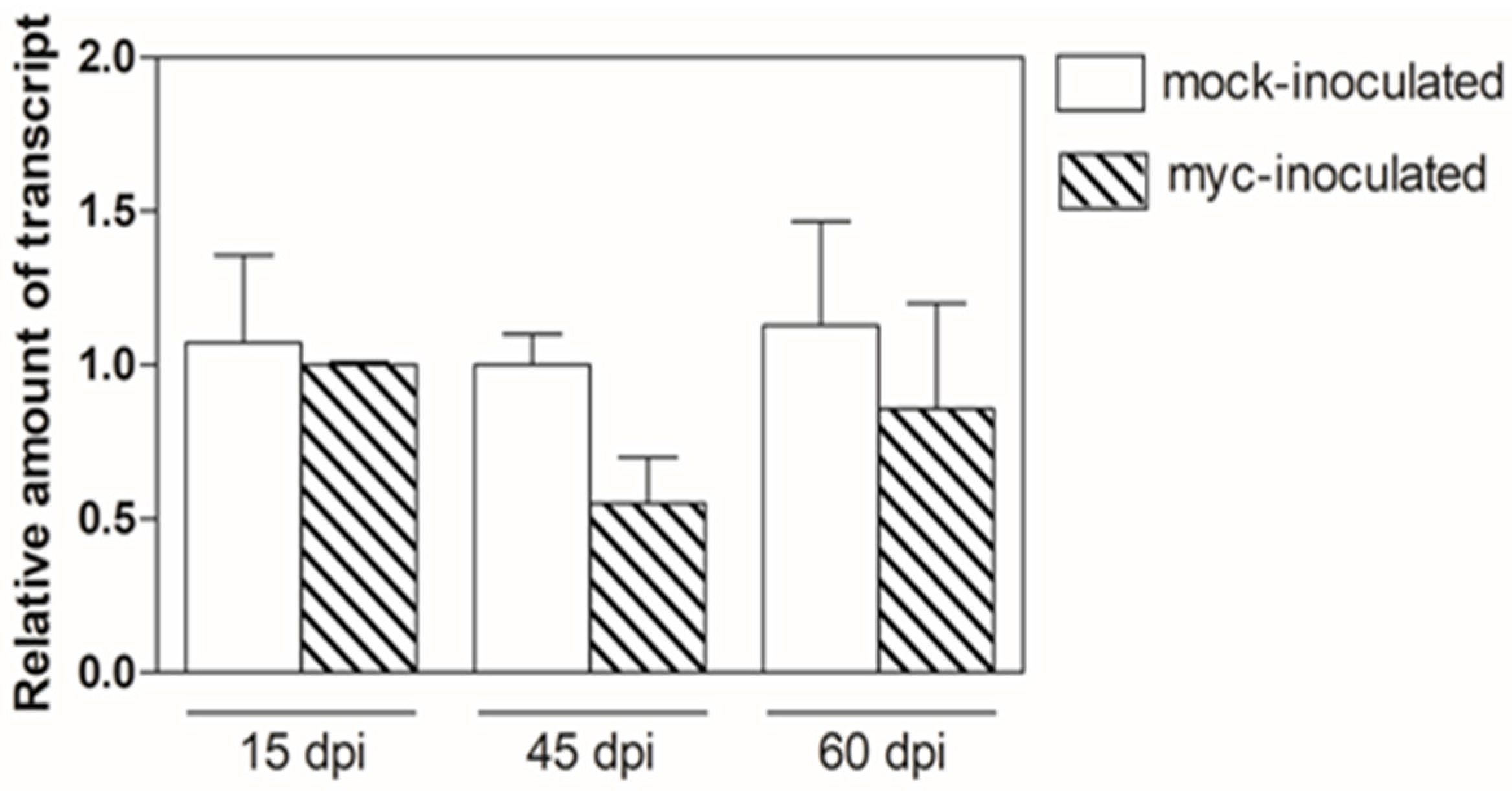
3.4. Lipid Profiles of Rhizobia-inoculated MtN5ox and WT Roots
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kader, J.C. Lipid-transfer proteins in plants. Annu. Rev. Plant Biol. 1996, 47, 627–654. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Carvalho, A.; Gomes, V.M. Role of plant lipid transfer proteins in plant cell physiology—A concise review. Peptides 2007, 28, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.J.; Lee, C.C.; Cheng, C.S.; Lo, W.C.; Yang, Y.F.; Chen, M.N.; Lyu, P.C. Construction and analysis of a plant non-specific lipid transfer protein database (nsLTPDB). BMC Genom. 2012, 13, S9. [Google Scholar] [CrossRef] [PubMed]
- Sy, D.; Gravier, Y.L.; Goodfellow, J.; Vovelle, F. Protein stability and plasticity of the hydrophobic cavity in wheat ns-LTP. J. Biomol. Struct. Dyn. 2003, 21, 15–29. [Google Scholar] [CrossRef] [PubMed]
- José-Estanyol, M.; Gomis-Rüth, F.X.; Puigdomenech, P. The eight-cysteine motif, a versatile structure in plant proteins. Plant Physiol. Biochem. 2004, 42, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [PubMed]
- Gonorazky, A.G.; Regente, M.C.; de la Canal, L. Stress induction and antimicrobial properties of a lipid transfer protein in germinating sunflower seeds. J. Plant Physiol. 2005, 162, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Go, Y.S.; Bae, H.J.; Park, J.H.; Cho, S.H.; Cho, H.J.; Lee, D.S.; Park, O.K.; Hwang, I.; Suh, M.C. Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plastoglobules, and enhanced susceptibility to infection by the fungal pathogen Alternaria brassicicola. Plant Physiol. 2009, 150, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.G.; Barros, L.M.; de Almeida, J.D.; de Lamotte, F.; Barbosa, E.A.; Vieira, N.G.; Alves, G.S.; Vinecky, F.; Andrade, A.C.; Marraccini, P. Lipid transfer proteins in coffee: Isolation of Coffea orthologs, Coffea arabica homeologs, expression during coffee fruit development and promoter analysis in transgenic tobacco plants. Plant Mol. Biol. 2014, 85, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Segura, A.; García-Olmedo, F. Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993, 316, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Segura, A.; Moreno, M.; García-Olmedo, F.X. Purification and antipathogenic activity of lipid transfer proteins (LTPs) from the leaves of Arabidopsis and spinach. FEBS Lett. 1993, 332, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Park, C.J.; Shin, R.; Park, J.M.; Lee, G.J.; You, J.S.; Paek, K.H. Induction of pepper cDNA encoding a lipid transfer protein during the resistance response to tobacco mosaic virus. Plant Mol. Biol. 2002, 48, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.S.; Johnson, J.W.; Seo, Y.W. Differential expression of TaLTP3 and TaCOMT1 induced by Hessian fly larval infestation in a wheat line possessing H21 resistance gene. Plant Sci. 2005, 168, 1319–1326. [Google Scholar] [CrossRef]
- Salminen, T.A.; Blomqvist, K.; Edqvist, J. Lipid transfer proteins: Classification, nomenclature, structure, and function. Planta 2015, 244, 971–997. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Astegno, A.; Peroni, E.; Zaccardelli, M.; Pandolfini, T.; Crimi, M. The Medicago truncatula N5 gene encoding a root-specific lipid transfer protein is required for the symbiotic interaction with Sinorhizobium meliloti. Mol. Plant Microbe Interact. 2009, 22, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Molesini, B.; Masiero, S.; Pandolfini, T. The non-specific lipid transfer protein N5 of Medicago truncatula is implicated in epidermal stages of rhizobium-host interaction. BMC Plant Biol. 2012, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, L.; Shi, X.; Li, Y.; Wang, J.; Chen, D.; Xie, F.; Li, Y. A nodule-specific lipid transfer protein AsE246 participates in transport of plant-synthesized lipids to symbiosome membrane and is essential for nodule organogenesis in Chinese milk vetch. Plant Physiol. 2014, 164, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.P.S.; Hong, Z.L. Biogenesis of the peribacteroid membrane in root nodules. Trends Microbiol. 1996, 4, 364–368. [Google Scholar] [CrossRef]
- Catalano, C.M.; Lane, W.S.; Sherrier, D.J. Biochemical characterization of symbiosome membrane proteins from Medicago truncatula root nodules. Electrophoresis 2004, 25, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.D. Invasion by invitation: Rhizobial infection in legumes. Mol. Plant Microbe Interact. 2011, 24, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.H.; Gris, C.; Cruveiller, S.; Pouzet, C.; Tasse, L.; Leru, A.; Maillard, A.; Médigue, C.; Batut, J.; Masson-Boivin, C.; et al. Experimental evolution of nodule intracellular infection in legume symbionts. ISME J. 2013, 7, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Maróti, G.; Kondorosi, E. Nitrogen-fixing Rhizobium-legume symbiosis: Are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front. Microbiol. 2014, 5, 326. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Uchiumi, T.; Alunni, B.; Evanno, G.; Cheron, A.; Catrice, O.; Mausset, A.E.; Barloy-Hubler, F.; Galibert, F.; Kondorosi, A.; et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5230–5235. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Rivas, J.C.; Guefrachi, I.; Mok, K.C.; Villaécija-Aguilar, J.A.; Acosta-Jurado, S.; Pierre, O.; Ruiz-Sainz, J.E.; Taga, M.E.; Mergaert, P.; Vinardell, J.M. Sinorhizobium fredii HH103 bacteroids are not terminally differentiated and show altered O-antigen in nodules of the Inverted Repeat-Lacking Clade legume Glycyrrhiza uralensis. Environ. Microbiol. 2016, 18, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Nikovics, K.; Kelemen, Z.; Maunoury, N.; Vaubert, D.; Kondorosi, A.; Kondorosi, E. A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 2003, 132, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Haag, A.F.; Arnold, M.F.; Myka, K.K.; Kerscher, B.; Dall’Angelo, S.; Zanda, M.; Mergaert, P.; Ferguson, G.P. Molecular insights into bacteroid development during Rhizobium–legume symbiosis. FEMS Microbiol. Rev. 2013, 37, 364–383. [Google Scholar] [CrossRef] [PubMed]
- Nallu, S.; Silverstein, K.A.; Zhou, P.; Young, N.D.; Vandenbosch, K.A. Patterns of divergence of a large family of nodule cysteine-rich peptides in accessions of Medicago truncatula. Plant J. 2014, 78, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Alunni, B.; Gourion, B. Terminal bacteroid differentiation in the legume-rhizobium symbiosis: Nodule-specific cysteine-rich peptides and beyond. New Phytol. 2016, 211, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Clarke, V.C.; Loughlin, P.C.; Day, D.A.; Smith, P.M.C. Transport processes of the legume symbiosome membrane. Front. Plant Sci. 2014, 5, 699. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M.; Lloret, J.; Daniele, J.R.; Walker, G.C. The type IV secretion system of Sinorhizobium meliloti strain 1021 is required for conjugation but not for intracellular symbiosis. J. Bacteriol. 2007, 189, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Gamas, P.; Niebel Fde, C.; Lescure, N.; Cullimore, J. Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol. Plant Microbe Interact. 1996, 9, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Haney, C.H.; Long, S.R. Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Ané, J.M.; Kiss, G.B.; Riely, B.K.; Penmetsa, R.V.; Oldroyd, G.E.; Ayax, C.; Lévy, J.; Debellé, F.; Baek, J.M.; Kalo, P.; et al. Medicago truncatula DMI1 is required for bacterial and fungal symbioses in legumes. Science 2004, 303, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Molesini, B.; Pandolfini, T. The involvement of Medicago truncatula non-specific lipid transfer protein N5 in the control of rhizobial infection. Plant Signal. Behav. 2013, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Meade, H.M.; Long, S.R.; Ruvkun, G.B.; Brown, S.E.; Ausubel, F.M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 1982, 149, 114–122. [Google Scholar]
- Veereshlingam, H.; Haynes, J.G.; Penmetsa, R.V.; Cook, D.R.; Sherrier, D.J.; Dickstein, R. Nip, a symbiotic Medicago truncatula mutant that forms root nodules with aberrant infection threads and plant defense-like response. Plant Physiol. 2004, 136, 3692–3702. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984, 12, 8711–8721. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, M.; Ratet, P.; De Sousa Araújo, S.; Roldão Lopes Amaral Duque, A.; Harrison, M.; Barker, D. Agrobacterium tumefaciens-mediated transformation and in vitro plant regeneration of M. truncatula. In Medicago truncatula Handbook; The Samuel Roberts Noble Foundation, Inc.: Ardmore, OK, USA, 2006; pp. 1–34. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Molesini, B.; Cecconi, D.; Pii, Y.; Pandolfini, T. Local and systemic proteomic changes in Medicago truncatula at an early phase of Sinorhizobium meliloti infection. J. Proteome Res. 2014, 13, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast-spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Manara, A.; Dal Corso, G.; Guzzo, F.; Furini, A. Loss of the atypical kinases ABC1K7 and ABC1K8 changes the lipid composition of the chloroplast membrane. Plant Cell Physiol. 2015, 56, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Kamide, Y.; Hirai, M.Y.; Saito, K. Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 2013, 9, S121–S131. [Google Scholar] [CrossRef] [PubMed]
- Penmetsa, R.V.; Cook, D.R. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 1997, 275, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Arbuscular mycorrhizal dialogues: Do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 2015, 20, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Ellingson, S.R.; Roberts, D.M. Ammonia permeability of the soybean nodulin 26 channel. FEBS Lett. 2010, 584, 4339–4343. [Google Scholar] [CrossRef] [PubMed]
- Felle, H.H.; Kondorosi, E.; Kondorosi, A.; Schultze, M. Elevation of cytosolic free [Ca2+] is indispensable for the transduction of the Nod factor signal from alfalfa. Plant Phys. 1999, 121, 273–279. [Google Scholar] [CrossRef]
- Esseling, J.J.; Lhuissier, F.G.P.; Emons, A.M.C. Nod factor-induced root hair curling: Continuous polar growth towards the point of Nod factor application. Plant Phys. 2003, 132, 1982–1988. [Google Scholar] [CrossRef]
- DeHoff, P.L.; Brill, M.A.; Hirsch, A.M. Plant lectins: The ties that bind in root symbiosis and plant defence. Mol. Genet. Genom. 2009, 282, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, K.; Govers, F. Arabidopsis l-type lectin receptor kinases: Phylogeny, classification, and expression profiles. J. Exp. Bot. 2009, 60, 4383–4396. [Google Scholar] [CrossRef] [PubMed]
- Ranf, S.; Gisch, N.; Schäffer, M.; Illig, T.; Westphal, L.; Knirel, Y.A.; Sánchez-Carballo, P.M.; Zähringer, U.; Hückelhoven, R.; Lee, J.; et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015, 16, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Zehirov, G.; Szatmari, A.; Debreczeny, M.; Ishihara, H.; Kevei, Z.; Farkas, A.; Mikulass, K.; Nagy, A.; Tiricz, H.; et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 2010, 327, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Salavati, A.; Bushehri, A.; Taleei, A.; Hiraga, S.; Komatsu, S. A comparative proteomic analysis of the early response to compatible symbiotic bacteria in the roots of a supernodulating soybean variety. J. Proteom. 2012, 75, 819–832. [Google Scholar] [CrossRef] [PubMed]
- D’haeseleer, K.; Den Herder, G.; Laffont, C.; Plet, J.; Mortier, V.; Lelandais-Brière, C.; De Bodt, S.; de Keyser, A.; Crespi, M.; Holsters, M.; et al. Transcriptional and posttranscriptional regulation of a NAC1 transcription factor in Medicago truncatula roots. New Phytol. 2011, 191, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zélicourt, A.; Diet, A.; Marion, J.; Laffont, C.; Ariel, F.; Moison, M.; Zahaf, O.; Crespi, M.; Gruber, V.; Frugier, F. Dual involvement of a Medicago truncatula NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J. 2012, 70, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, S.S.; Liang, Z.; Feng, B.R.; Liu, L.; Huang, Y.B. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 2012, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Joshi, T.; Benedito, V.A.; Xu, D.; Udvardi, M.K.; Stacey, G. Legume transcription factor genes: What makes legumes so special? Plant Physiol. 2009, 151, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D.; Andriankaja, A.; Allen, S.; Kakar, K.; Wandrey, M.; Verdier, J.; Zuber, H.; Ott, T.; et al. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008, 55, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Godiard, L.; Lepage, A.; Moreau, S.; Laporte, D.; Verdenaud, M.; Timmers, T.; Gamas, P. MtbHLH1, a bHLH transcription factor involved in Medicago truncatula nodule vascular patterning and nodule to plant metabolic exchanges. New Phytol. 2011, 191, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Karas, B.; Amyot, L.; Johansen, C.; Sato, S.; Tabata, S.; Kawaguchi, M.; Szczyglowski, K. Conservation of Lotus and Arabidopsis basic helix-loop-helix proteins reveals new players in root hair development. Plant Physiol. 2009, 151, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, D.M.; Loughlina, P.C.; Mazurkiewicz, D.; Mohammadidehcheshmeh, M.; Fedorova, E.E.; Okamoto, M.; McLean, E.; Glass, A.D.; Smith, S.E.; Bisseling, T.; et al. Soybean SAT1 (Symbiotic Ammonium Transporter 1) encodes a bHLH transcription factor involved in nodule growth and NH4+ transport. Proc. Natl. Acad. Sci. USA 2014, 111, 4814–4819. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.K.; Kakar, K.; Wandrey, M.; Montanari, O.; Murray, J.; Andriankaja, A.; Zhang, J.Y.; Benedito, V.; Hofer, J.M.; Chueng, F.; et al. Legume transcription factors: Global regulators of plant development and response to the environment. Plant Physiol. 2007, 144, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Gordon, A.J.; Minchin, F.R.; James, C.L.; Komina, O. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol. 1999, 120, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Barsch, A.; Küster, H.; Hohnjec, N. Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiol. 2007, 145, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.S.; Rempe, C.S.; Davitt, J.; Staton, M.E.; Peng, Y.; Soltis, D.E.; Melkonian, M.; Deyholos, M.; Leebens-Mack, J.H.; Chase, M.; et al. Diversity of ABC transporter genes across the plant kingdom and their potential utility in biotechnology. BMC Biotechnol. 2016, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Haney, C.H.; Riely, B.K.; Tricoli, D.M.; Cook, D.R.; Ehrhardt, D.W.; Long, S.R. Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell 2011, 23, 2774–2787. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Timmers, T.; Mbengue, M.; Moreau, S.; Hervé, C.; Tóth, K.; Bittencourt-Silvestre, J.; Klaus, D.; Deslandes, L.; Godiard, L.; et al. A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc. Natl. Acad. Sci. USA 2010, 107, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; Stratil, T.F.; Madsen, E.B.; Ye, J.Y.; Popp, C.; Antolin-Llovera, M.; Grossmann, C.; Jensen, O.N.; Schussler, A.; Parniske, M.; et al. Functional domain analysis of the remorin protein LjSYMREM1 in Lotus japonicus. PLoS ONE 2012, 7, e30817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernié, T.; Camut, S.; Camps, C.; Rembliere, C.; de Carvalho-Niebel, F.; Mbengue, M.; Timmers, T.; Gasciolli, V.; Thompson, R.; le Signor, C.; et al. PUB1 Interacts with the receptor kinase DMI2 and negatively regulates rhizobial and arbuscular mycorrhizal symbioses through its ubiquitination activity in Medicago truncatula. Plant Physiol. 2016, 170, 2312–2324. [Google Scholar] [CrossRef] [PubMed]
- Kevei, Z.; Lougnon, G.; Mergaert, P.; Horváth, G.V.; Kereszt, A.; Jayaraman, D.; Zaman, N.; Marcel, F.; Regulski, K.; Kiss, G.B.; et al. 3-Hydroxy-3-Methylglutaryl coenzyme A reductase1 interacts with NORK and is crucial for nodulation in Medicago truncatula. Plant Cell 2007, 19, 3974–3989. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.K.; Schorderet, M.; Reinhardt, D. The role of the cell wall compartment in mutualistic symbioses of plants. Front. Plant Sci. 2014, 5, 238. [Google Scholar] [CrossRef] [PubMed]
- Giordano, W.; Hirsch, A.M. The expression of MaEXP1, a Melilotus alba expansin gene, is upregulated during the sweetclover-Sinorhizobium meliloti interaction. Mol. Plant Microbe Interact. 2004, 17, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U.; Schlaman, H.R.; Spaink, H.P.; Of Sautter, C.; Rolfe, B.G.; Djordjevic, M.A. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Alunni, B.; Kevei, Z.; Redondo-Nieto, M.; Kondorosi, A.; Mergaert, P.; Kondorosi, E. Genomic organization and evolutionary insights on GRP and NCR genes, two large nodule-specific gene families in Medicago truncatula. Mol. Plant Microbe Interact. 2007, 20, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Kevei, Z.; Vinardell, J.M.; Kiss, G.B.; Kondorosi, A.; Kondorosi, E. Glycine-rich proteins encoded by a nodule-specific gene family are implicated in different stages of symbiotic nodule development in Medicago spp. Mol. Plant Microbe Interact. 2002, 15, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Bapaume, L.; Reinhardt, D. How membranes shape symbioses: Signalling and transport in nodulation and arbuscular mycorrhiza. Front. Plant Sci. 2012, 3, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Gaude, N.; Tippmann, H.; Flemetakis, E.; Katinakis, P.; Udvardi, M.; Dörmann, P. The galactolipid digalactosyldiacylglycerol accumulates in the peribacteroid membrane of nitrogen-fixing nodules of soybean and Lotus. J. Biol. Chem. 2004, 279, 34624–34630. [Google Scholar] [CrossRef] [PubMed]
- Bankaitis, V.A.; Mousley, C.J.; Schaaf, G. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem. Sci. 2010, 35, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P.; Chua, M.; Nogue, F.; Fairbrother, A.; Mekeel, H.; Xu, Y.; Allen, N.; Bibikova, T.N.; Gilroy, S.; Bankaitis, V.A. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J. Cell Biol. 2005, 168, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.B.; McGee, T.P.; McMaster, C.R.; Fry, M.R.; Bell, R.M.; Bankaitis, V.A. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc. Natl. Acad. Sci. USA 1995, 92, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Venkateshwaran, M.; Boersma, M.; Harms, A.; Howes-Podoll, M.; den Os, D.; Ané, J.M.; Sussman, M.R. Metabolomic profiling reveals suppression of oxylipin biosynthesis during the early stages of legume-rhizobia symbiosis. FEBS Lett. 2012, 586, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Oldroyd, G.E. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Marx, H.; Minogue, C.E.; Jayaraman, D.; Richards, A.L.; Kwiecien, N.W.; Siahpirani, A.F.; Rajasekar, S.; Maeda, J.; Garcia, K.; del Valle-Echevarria, A.R. A proteomic atlas of the legume Medicago truncatula and its nitrogen-fixing endosymbiont Sinorhizobium meliloti. Nat. Biotechnol. 2016, 34, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Benning, C. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu. Rev. Cell Dev. Biol. 2009, 25, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and glycolipids in plants and algae. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer: Cham, Switzerland, 2016; Volume 86. [Google Scholar]
- Dörmann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Pant, B.D.; Burgos, A.; Pant, P.; Cuadros-Inostroza, A.; Willmitzer, L.; Scheible, W.R. The transcription factor PHR1 regulates lipid remodeling and triacylglycerol accumulation in Arabidopsis thaliana during phosphorus starvation. J. Exp. Bot. 2015, 66, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Leidi, E.O.; Rodríguez-Navarro, D.N. Nitrogen and phosphorus availability limit N2 fixation in bean. New Phytol. 2000, 147, 337–346. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Haliem, A.M.; Joosten, M.H. Plant phosphatidylinositol-specific phospholipase C at the center of plant innate immunity. J. Integr. Plant Biol. 2017, 59, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Den Hartog, M.; Verhoef, N.; Munnik, T. Nod factor and elicitors activate different phospholipid signaling pathways in suspension-cultured alfalfa cells. Plant Physiol. 2003, 132, 311–317. [Google Scholar] [CrossRef] [PubMed]

| Genome ID | Description | Fold Change (MtN5ox vs. WT) |
|---|---|---|
| Medtr4g098800 | peptide transporter | −10.74 |
| Medtr3g100980 | peptide/nitrate transporter | −7.32 |
| Medtr2g094270 | major intrinsic protein (MIP) family transporter | −6.91 |
| Medtr8g089340 | cationic amino acid transporter | −5.82 |
| Medtr8g085630 | neutral amino acid transporter | −5.47 |
| Medtr8g087710 | MIP family transporter | −5.41 |
| Medtr4g114340 | peptide/nitrate transporter | −5.1 |
| Medtr3g070210 | MIP family transporter | −5.04 |
| Medtr4g099260 | high-affinity potassium transporter | −4.99 |
| Medtr4g104510 | transmembrane amino acid transporter family protein | −4.88 |
| Medtr4g134730 | chloride channel (ClC1) protein | −4.76 |
| Medtr3g087740 | sulphate/bicarbonate/oxalate exchanger and transporter sat-1 | −4.59 |
| Medtr3g082700 | high affinity inorganic phosphate transporter | −4.49 |
| Medtr1g041695 | phosphate transporter PHO1-like protein | −4.4 |
| Medtr5g038060 | peptide/nitrate transporter | −4.37 |
| Medtr1g071530 | sulphate/bicarbonate/oxalate exchanger and transporter sat-1 | −4.11 |
| Medtr6g016275 | transmembrane amino acid transporter family protein | −4.03 |
| Medtr1g006490 | MIP family transporter | −3.47 |
| Medtr8g061090 | oligopeptide transporter (OPT) family protein | −3.47 |
| Medtr2g012470 | transmembrane amino acid transporter family protein | −3.44 |
| Medtr1g109380 | amino-acid permease BAT1-like protein | −3.43 |
| Medtr2g101090 | drug resistance transporter-like ABC domain protein | −3.1 |
| Medtr4g069500 | polyol/monosaccharide transporter 1 | −3.02 |
| Medtr0294s0020 | proton-dependent oligopeptide transport family protein | −2.9 |
| Medtr3g108320 | potassium channel KAT3 protein | −2.81 |
| Medtr4g083570 | ZIP zinc/iron transport family protein | −2.7 |
| Medtr1g098240 | nucleotide/sugar transporter family protein | −2.67 |
| Medtr5g075960 | transporter family ABC domain protein | −2.55 |
| Medtr4g011600 | sulphate transporter-like protein | −2.39 |
| Medtr1g075640 | phosphate transporter PHO1-like protein | −2.35 |
| Medtr7g017630 | transmembrane amino acid transporter family protein | −2.18 |
| Medtr4g123990 | ABC transporter B family protein | −2.14 |
| Medtr6g008690 | peptide/nitrate transporter plant-like protein | −1.94 |
| Medtr6g065650 | ClC1 protein | −1.88 |
| Medtr7g088820 | nitrate transporter 1 | −1.78 |
| Medtr2g020710 | sugar porter (SP) family MFS transporter | 2.33 |
| Medtr2g028770 | magnesium transporter CorA family protein | 2.33 |
| Medtr8g088200 | high-affinity potassium transporter | 2.76 |
| Medtr5g068580 | vacuolar iron transporter-like protein | 3.42 |
| Medtr4g069430 | nucleotide-diphospho-sugar transferase family protein | 3.52 |
| Medtr1g104780 | SP family MFS transporter | 3.75 |
| Genome ID | Description | Fold Change (MtN5ox vs. WT) |
|---|---|---|
| Medtr7g091990 | Carboxy-terminal region remorin | −4.57 |
| Medtr8g080180 | Carboxy-terminal region remorin | −4.54 |
| Medtr8g031370 | Carboxy-terminal region remorin | −1.94 |
| Medtr5g083030 | Ubiquitin-protein ligase, PUB17 | −1.93 |
| Medtr5g084080 | Nodule-specific Glycine Rich Peptide | 5.45 |
| Medtr3g106430 | Flotillin-like 4 | 3.45 |
| Medtr5g024880 | 3-hydroxy-3-methylglutaryl-coenzyme A reductase-like protein | 5.05 |
| Genome ID | Description | Fold Change (MtN5ox vs. WT) |
|---|---|---|
| Medtr4g087830 | phospholipase A1 | −6.41 |
| Medtr3g087590 | myo-inositol 1-phosphate synthase | −6.32 |
| Medtr8g018420 | seed linoleate 9S-lipoxygenase | −5.48 |
| Medtr4g053785 | gland-specific fatty acyl-CoA reductase | −4.97 |
| Medtr7g076900 | calcium-dependent lipid-binding (CaLB domain) family protein | −4.01 |
| Medtr8g018730 | seed linoleate 9S-lipoxygenase | −3.91 |
| Medtr5g071010 | phosphatidylinositol-specific phospholipase C | −3.49 |
| Medtr4g107850 | (CaLB domain) family protein | −3.49 |
| Medtr6g086060 | serinc-domain serine and sphingolipid biosynthesis protein | −3.16 |
| Medtr7g104220 | GDSL-like lipase/acylhydrolase | −3.15 |
| Medtr2g031610 | Sec14p-like phosphatidylinositol transfer family protein | −3.06 |
| Medtr5g094230 | glycerophosphoryl diester phosphodiesterase family protein | −2.95 |
| Medtr5g009120 | phosphatidylinositol 3-and 4-kinase family protein | −2.86 |
| Medtr8g070040 | lipid transfer protein | −2.86 |
| Medtr1g106875 | lipase | −2.45 |
| Medtr3g079340 | enhanced disease susceptibility protein | −2.39 |
| Medtr3g098530 | polyphosphoinositide-binding protein | −2.22 |
| Medtr7g017360 | phosphatidylinositol 3-and 4-kinase | −2.22 |
| Medtr4g115920 | monogalactosyldiacylglycerol synthase | −2.19 |
| Medtr1g062740 | phosphatidylinositol-4-phosphate 5-kinase family protein | −2.16 |
| Medtr7g113660 | mevalonate kinase | −1.81 |
| Medtr6g006910 | phospholipid-transporting ATPase-like protein | −1.81 |
| Medtr7g117475 | phospholipid methyltransferase | −1.63 |
| Medtr1g097850 | choline/ethanolamine kinase | 1.49 |
| Medtr1g041495 | glycolipid transfer protein (GLTP) family protein | 1.62 |
| Medtr2g020020 | alpha/beta-hydrolase superfamily protein | 1.70 |
| Medtr5g012880 | phosphatidylserine decarboxylase | 1.86 |
| Medtr8g031400 | GDSL-like lipase/acylhydrolase | 2.23 |
| Medtr0009s0120 | phosphatidylinositol 3-and 4-kinase | 2.26 |
| Medtr1g070195 | alpha/beta-hydrolase superfamily protein | 2.87 |
| Medtr1g082300 | breast carcinoma amplified sequence 3 protein | 3.02 |
| Medtr2g049790 | CBL-interacting kinase | 3.21 |
| Medtr7g109830 | long-chain fatty acyl CoA ligase | 4.12 |
| Medtr7g083130 | esterase/lipase/thioesterase family protein | 4.37 |
| Medtr7g090470 | triacylglycerol lipase SDP1 | 5.41 |
| Medtr1g068945 | protein transporter Sec24-plant-like protein | 23.83 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, C.; Molesini, B.; Guzzo, F.; Pii, Y.; Vitulo, N.; Pandolfini, T. Genome-Wide Transcriptional Changes and Lipid Profile Modifications Induced by Medicago truncatula N5 Overexpression at an Early Stage of the Symbiotic Interaction with Sinorhizobium meliloti. Genes 2017, 8, 396. https://doi.org/10.3390/genes8120396
Santi C, Molesini B, Guzzo F, Pii Y, Vitulo N, Pandolfini T. Genome-Wide Transcriptional Changes and Lipid Profile Modifications Induced by Medicago truncatula N5 Overexpression at an Early Stage of the Symbiotic Interaction with Sinorhizobium meliloti. Genes. 2017; 8(12):396. https://doi.org/10.3390/genes8120396
Chicago/Turabian StyleSanti, Chiara, Barbara Molesini, Flavia Guzzo, Youry Pii, Nicola Vitulo, and Tiziana Pandolfini. 2017. "Genome-Wide Transcriptional Changes and Lipid Profile Modifications Induced by Medicago truncatula N5 Overexpression at an Early Stage of the Symbiotic Interaction with Sinorhizobium meliloti" Genes 8, no. 12: 396. https://doi.org/10.3390/genes8120396






