In Vivo Imaging of Local Gene Expression Induced by Magnetic Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Animals Handling
2.2. MTN Synthesis and Characterization
2.3. In Vitro MTN Experiments (Phantoms)
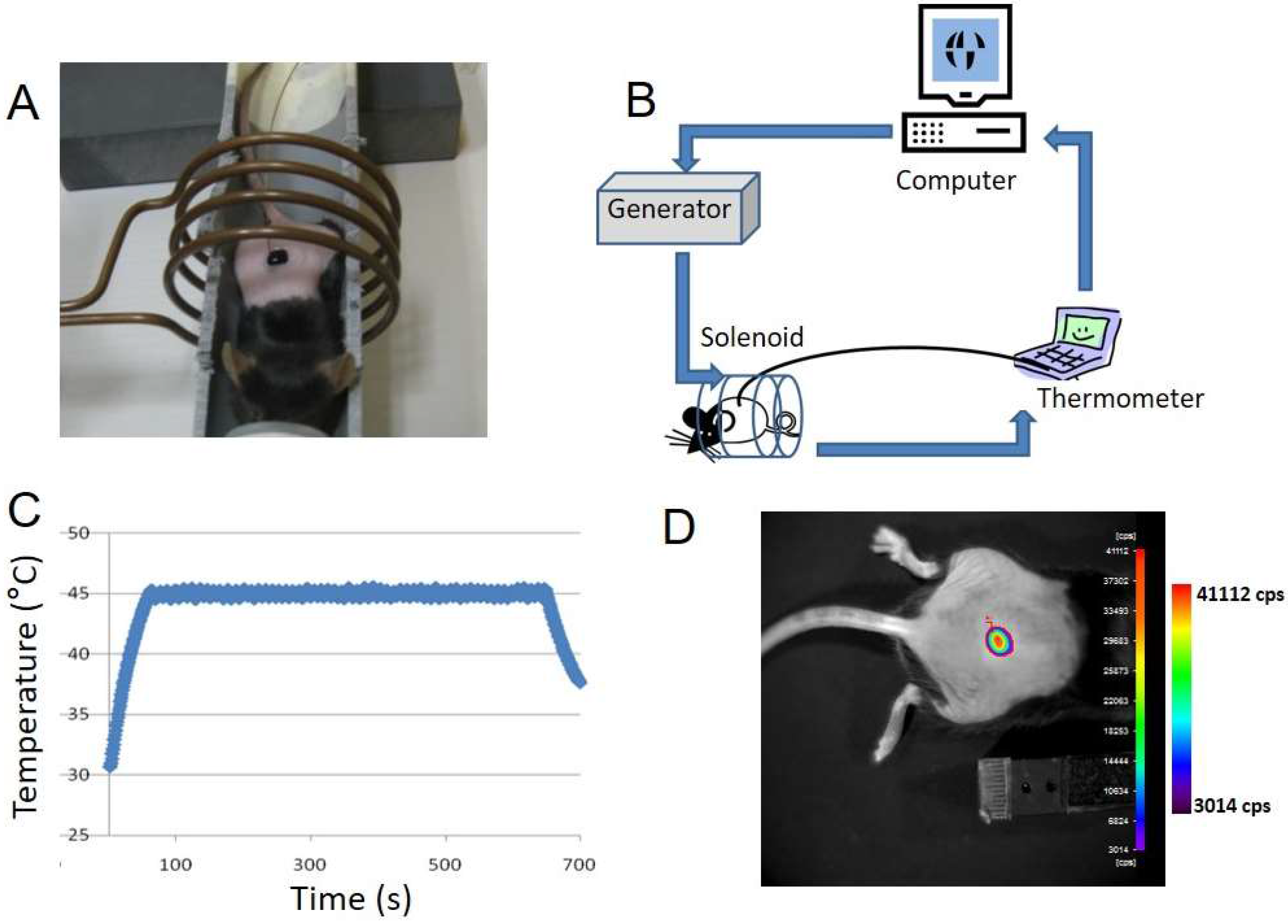
2.4. In Vivo MTN Experiments (Animals)
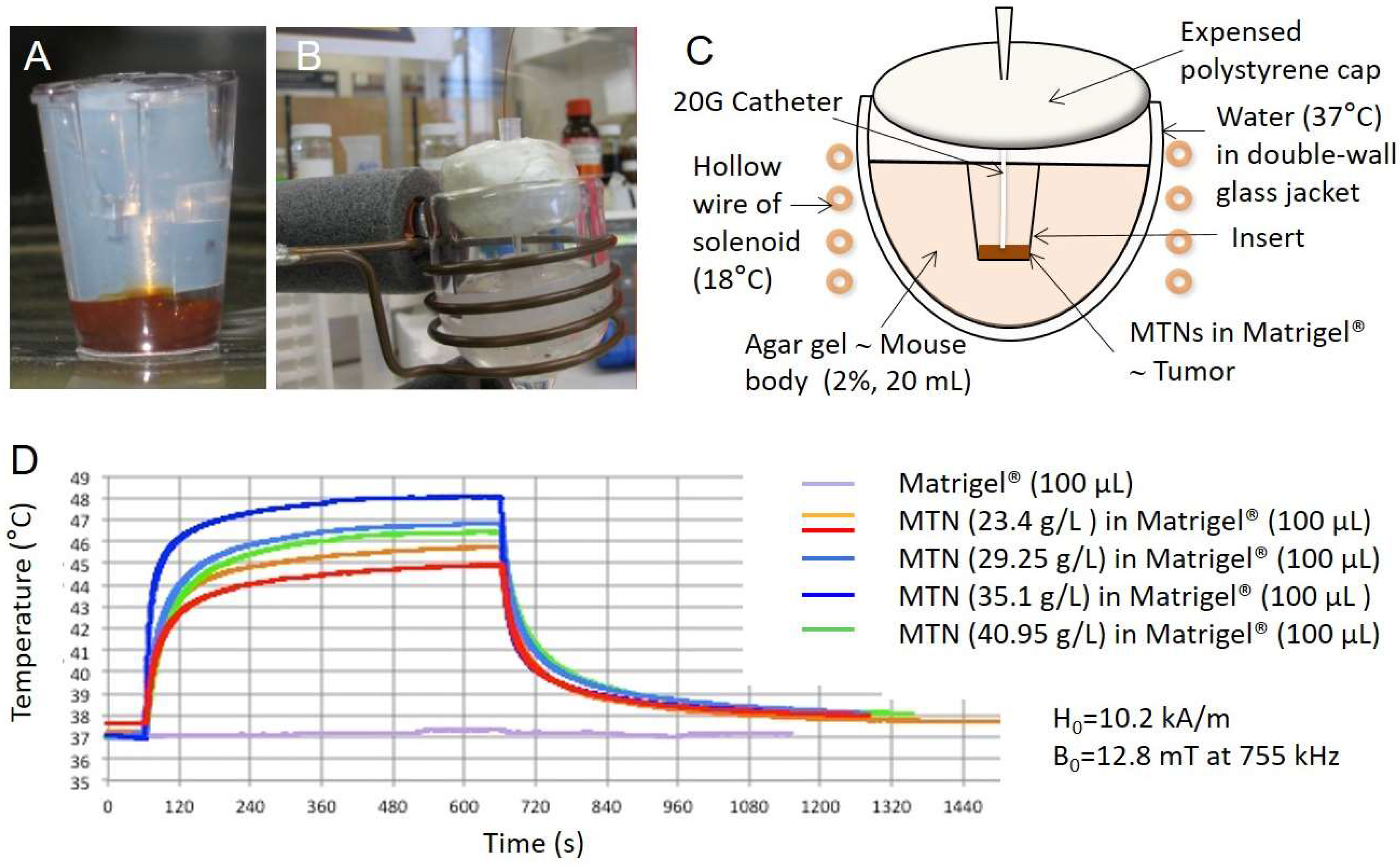
2.5. Hyperthermia Setup and Protocol
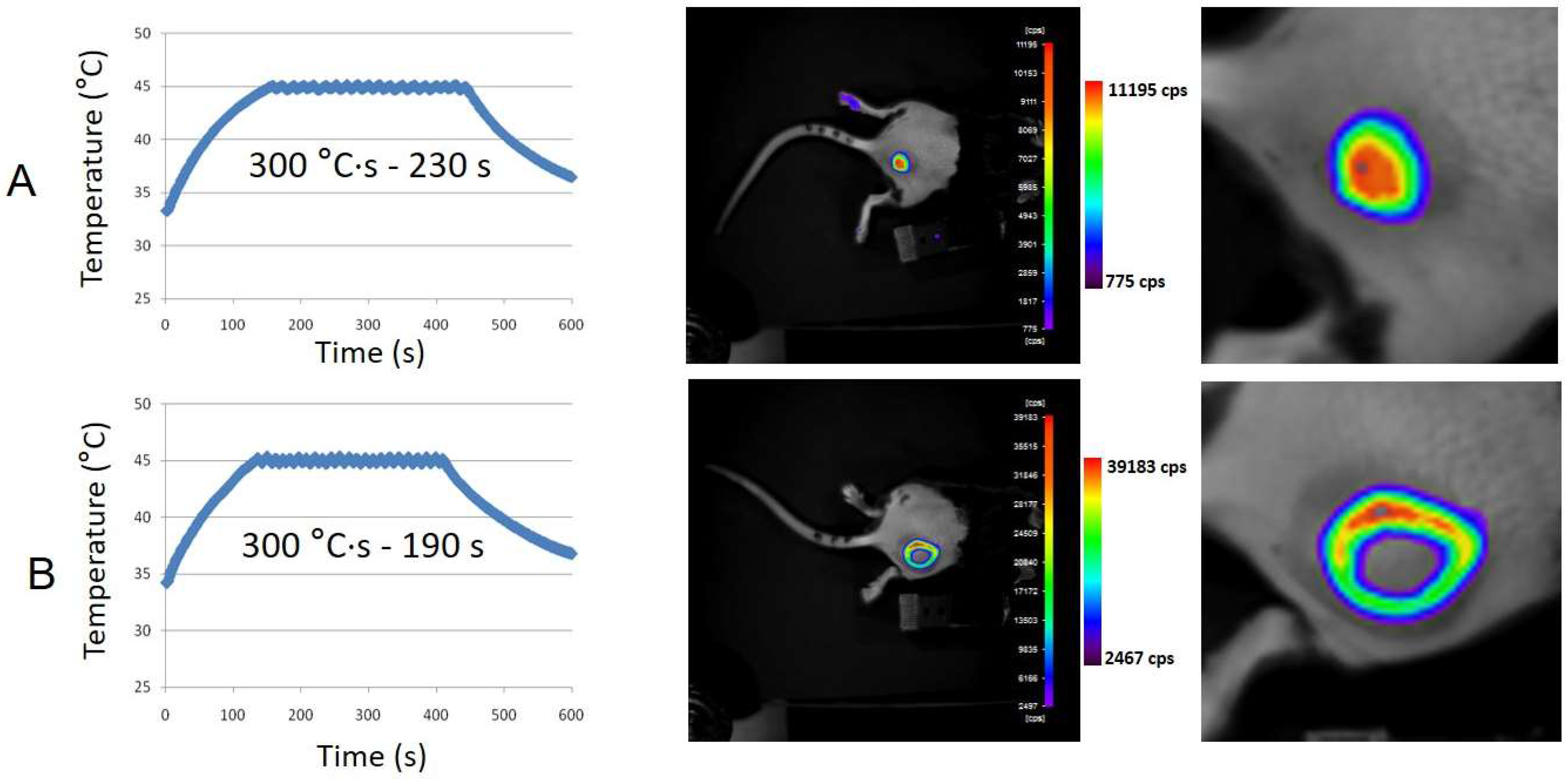
2.6. In Vivo Bioluminescence Imaging (BLI)
3. Results
3.1. Heating Performances of MTN In Vitro on Bio-Inspired Phantom
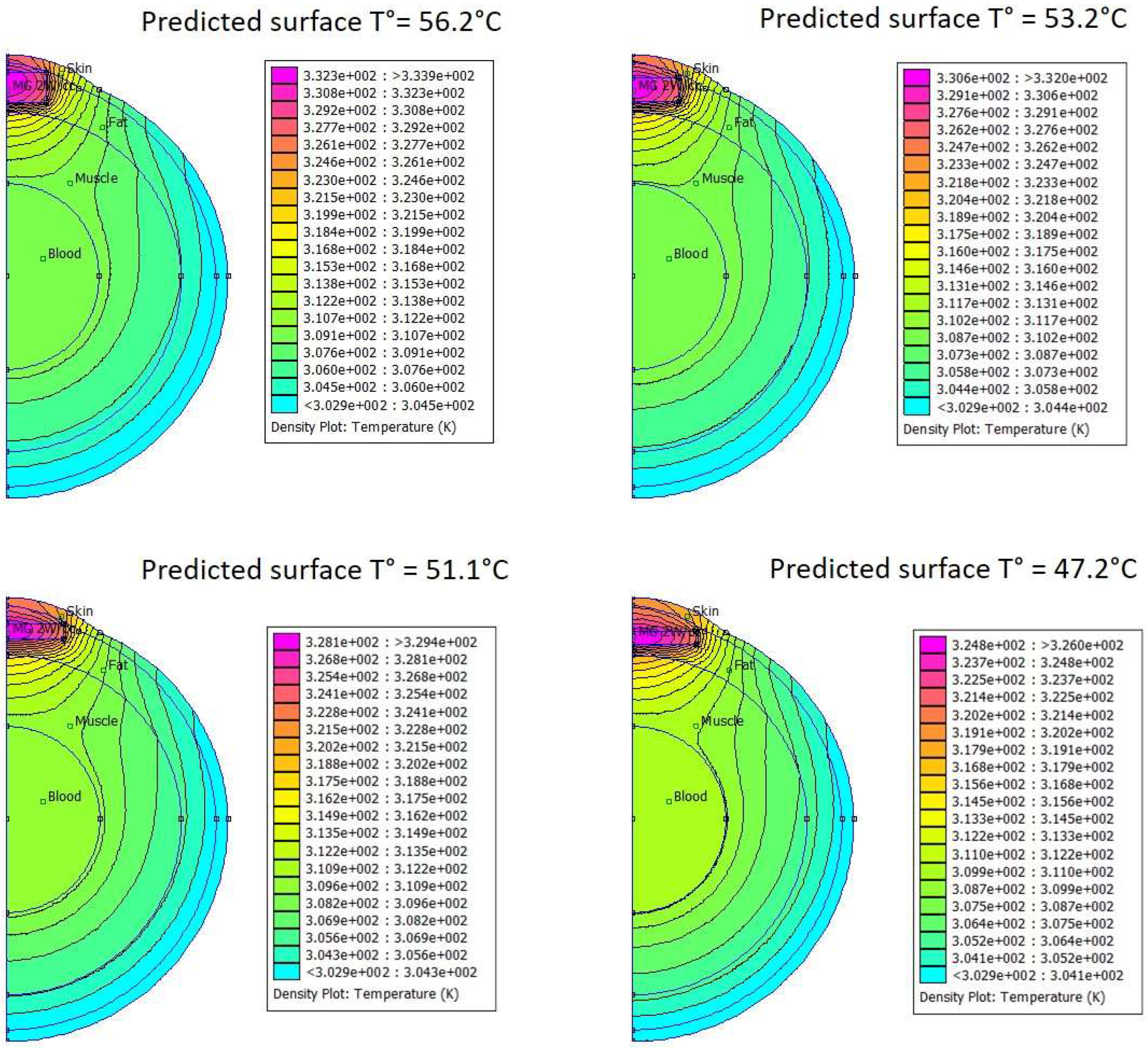
3.2. In Silico Modeling of Temperature Distribution in the Bio-Inspired Phantom
3.3. Topical Application of MTN Solution on Transgenic Mouse Skin
3.4. Subcutaneous MTN-Containing Matrigel™ Pseudotumors in Mice
3.5. In Silico Modeling of Temperature Distribution in MTN-Containing Pseudotumors
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cavazzana-Calvo, M.; Hacein-Bey, S.; de Saint Basile, G.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.L.; et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2007—An update. J. Gene Med. 2007, 9, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Chen, S.T.; Friedmann, T.; Yee, J.K. Inducible gene expression by retrovirus-mediated transfer of a modified tetracycline-regulated system. J. Virol. 1996, 70, 6054–6059. [Google Scholar] [PubMed]
- Clackson, T. Controlling mammalian gene expression with small molecules. Curr. Opin. Chem. Biol. 1997, 1, 210–218. [Google Scholar] [CrossRef]
- Deckers, R.; Quesson, B.; Arsaut, J.; Eimer, S.; Couillaud, F.; Moonen, C.T. Image-guided, noninvasive, spatiotemporal control of gene expression. Proc. Natl. Acad Sci. USA 2009, 106, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Eker, O.F.; Quesson, B.; Rome, C.; Arsaut, J.; Deminière, C.; Moonen, C.T.; Grenier, N.; Couillaud, F. Combination of cell delivery and thermoinducible transcription for in vivo spatiotemporal control of gene expression: A feasibility study. Radiology 2011, 258, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Fortin, P.-Y.; Lepetit-Coiffé, M.; Genevois, C.; Debeissat, C.; Quesson, B.; Moonen, C.T.W.; Konsman, J.P.; Couillaud, F. Spatiotemporal control of gene expression in bone-marrow derived cells of the tumor microenvironment induced by MRI guided focused ultrasound. Oncotarget 2015, 6, 23417–23426. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Creixell, M.; Bohórquez, A.C.; Torres-Lugo, M.; Rinaldi, C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011, 5, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Kubista, B.; Trieb, K.; Blahovec, H.; Kotz, R.; Micksche, M. Hyperthermia increases the susceptibility of chondro- and osteosarcoma cells to natural killer cell-mediated lysis. Anticancer Res. 2002, 22, 789–792. [Google Scholar] [PubMed]
- Ito, A.; Matsuoka, F.; Honda, H.; Kobayashi, T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol. Immunother. 2004, 53, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Moros, M.; Ambrosone, A.; Stepien, G.; Fabozzi, F.; Marchesano, V.; Castaldi, A.; Tino, A.; de la Fuente, J.M.; Tortiglione, C. Deciphering intracellular events triggered by mild magnetic hyperthermia in vitro and in vivo. Nanomed. 2015, 10, 2167–2183. [Google Scholar] [CrossRef] [PubMed]
- Deckers, R.; Debeissat, C.; Fortin, P.-Y.; Moonen, C.T.W.; Couillaud, F. Arrhenius analysis of the relationship between hyperthermia and Hsp70 promoter activation: A comparison between ex vivo and in vivo data. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2012, 28, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Fortin, P.-Y.; Genevois, C.; Chapolard, M.; Santalucía, T.; Planas, A.M.; Couillaud, F. Dual-reporter in vivo imaging of transient and inducible heat-shock promoter activation. Biomed. Opt. Express 2014, 5, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015. [Google Scholar] [CrossRef]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.-M. Magnetic nanoparticles and their applications in medicine. Nanomed. 2006, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Garaio, E.; Sandre, O.; Collantes, J.-M.; Garcia, J.A.; Mornet, S.; Plazaola, F. Specific absorption rate dependence on temperature in magnetic field hyperthermia measured by dynamic hysteresis losses (ac magnetometry). Nanotechnology 2015. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, F. A.; Franck, R.; Massart, R. Aqueous ferrofluids based on manganese and cobalt ferrites. J. Mater. Sci. 1990, 25, 3249–3254. [Google Scholar] [CrossRef]
- Massart, R.; Dubois, E.; Cabuil, V.; Hasmonay, E. Preparation and properties of monodisperse magnetic fluids. J. Magn. Magn. Mater. 1995, 149, 1–5. [Google Scholar] [CrossRef]
- Arosio, P.; Thévenot, J.; Orlando, T.; Orsini, F.; Corti, M.; Mariani, M.; Bordonali, L.; Innocenti, C.; Sangregorio, C.; Oliveira, H.; et al. Hybrid iron oxide-copolymer micelles and vesicles as contrast agents for MRI: Impact of the nanostructure on the relaxometric properties. J. Mater. Chem. B 2013, 1, 5317–5328. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Rabin, Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int. J. Hyperthermia 2002, 18, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Redondo, Y.; Bañobre-López, M.; Pardiñas-Blanco, I.; Goya, G.; López-Quintela, M.A.; Rivas, J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res. Lett. 2011. [Google Scholar] [CrossRef] [PubMed]
- Sandre, O.; Genvois, C.; Garaio, E.; Adumeau, L.; Mornet, S.; Couillaud, F. University Bordeaux: Bordeaux, France, Data not shown. 2017.
- Levy, A.; Dayan, A.; Ben-David, M.; Gannot, I. A new thermography-based approach to early detection of cancer utilizing magnetic nanoparticles theory simulation and in vitro validation. Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Poincloux, R.; Lizárraga, F.; Chavrier, P. Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 2009, 122, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Van Rhoon, G.C.; Samaras, T.; Yarmolenko, P.S.; Dewhirst, M.W.; Neufeld, E.; Kuster, N. CEM43 °C thermal dose thresholds: A potential guide for magnetic resonance radiofrequency exposure levels? Eur. Radiol. 2013, 23, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Cells in stress: transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Borrelli, M.J.; Syed, K.; Senisterra, G.; Stafford, D.M.; Lepock, J.R. Characterization of a signal generated by oxidation of protein thiols that activates the heat shock transcription factor. J. Cell. Physiol. 1995, 164, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. Bethesda Md 1985 2002, 92, 2177–2186. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandre, O.; Genevois, C.; Garaio, E.; Adumeau, L.; Mornet, S.; Couillaud, F. In Vivo Imaging of Local Gene Expression Induced by Magnetic Hyperthermia. Genes 2017, 8, 61. https://doi.org/10.3390/genes8020061
Sandre O, Genevois C, Garaio E, Adumeau L, Mornet S, Couillaud F. In Vivo Imaging of Local Gene Expression Induced by Magnetic Hyperthermia. Genes. 2017; 8(2):61. https://doi.org/10.3390/genes8020061
Chicago/Turabian StyleSandre, Olivier, Coralie Genevois, Eneko Garaio, Laurent Adumeau, Stéphane Mornet, and Franck Couillaud. 2017. "In Vivo Imaging of Local Gene Expression Induced by Magnetic Hyperthermia" Genes 8, no. 2: 61. https://doi.org/10.3390/genes8020061






