Identification, Characterization and Expression Profiling of Stress-Related Genes in Easter Lily (Lilium formolongi)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Defense-Related EST Sequence Retrieval
2.2. Plant Materials
2.3. Molecular Cloning of EST Sequences
2.4. Sequence Analysis of mRNA and Deduced Proteins
2.5. Phylogenetic Relationship and Motif Analysis
2.6. Biotic Stress Treatments with Botrytis Spp.
2.7. Abiotic Stress Treatments
2.8. RNA Extraction from Various Plant Organs and Stress-Treated (Biotic and Abiotic) Samples
2.9. Real-Time Quantitative PCR Expression Analysis of Different Organs and Stress-Treated Samples
2.10. Statistical Analyses
3. Results
3.1. Sequence Analysis of Defense-Related ESTs from L. formolongi
3.2. Molecular Cloning and Sequence Analysis of Stress-Related Genes
3.3. Phylogenetic Relatedness and Motif Distribution of Defense-Related Proteins
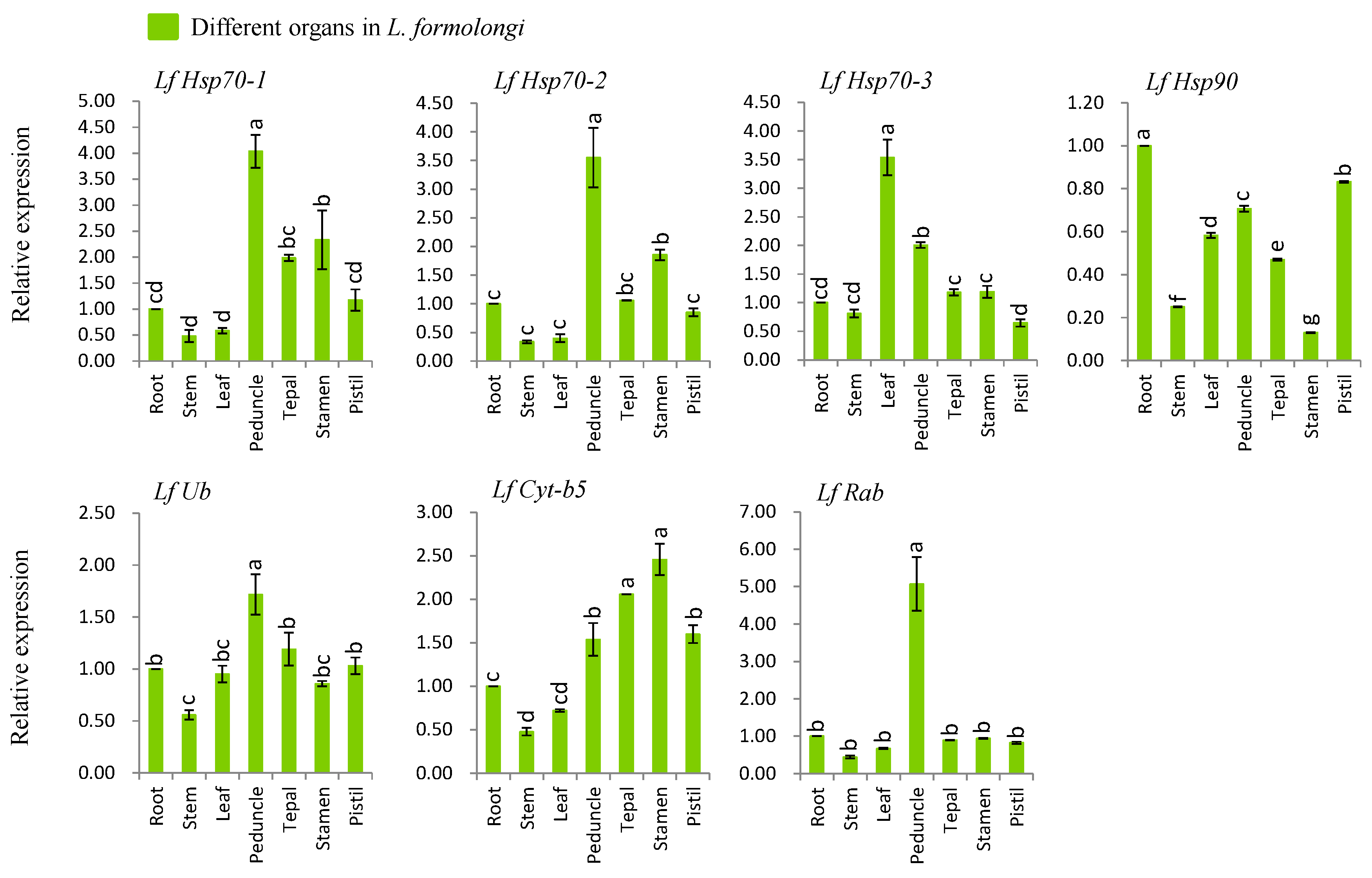
3.4. Organ-Specific Expression Analysis of Defense-Related Genes
3.5. B. elliptica Is More Virulent than B. cinerea
3.6. qPCR Expression Analysis after Biotic Stress
3.6.1. Gene Expression in B. cinerea-Inoculated Susceptible L. formolongi
3.6.2. Gene Expression in B. elliptica-Inoculated Susceptible L. formolongi
3.7. Gene Expression under Abiotic Stress Conditions
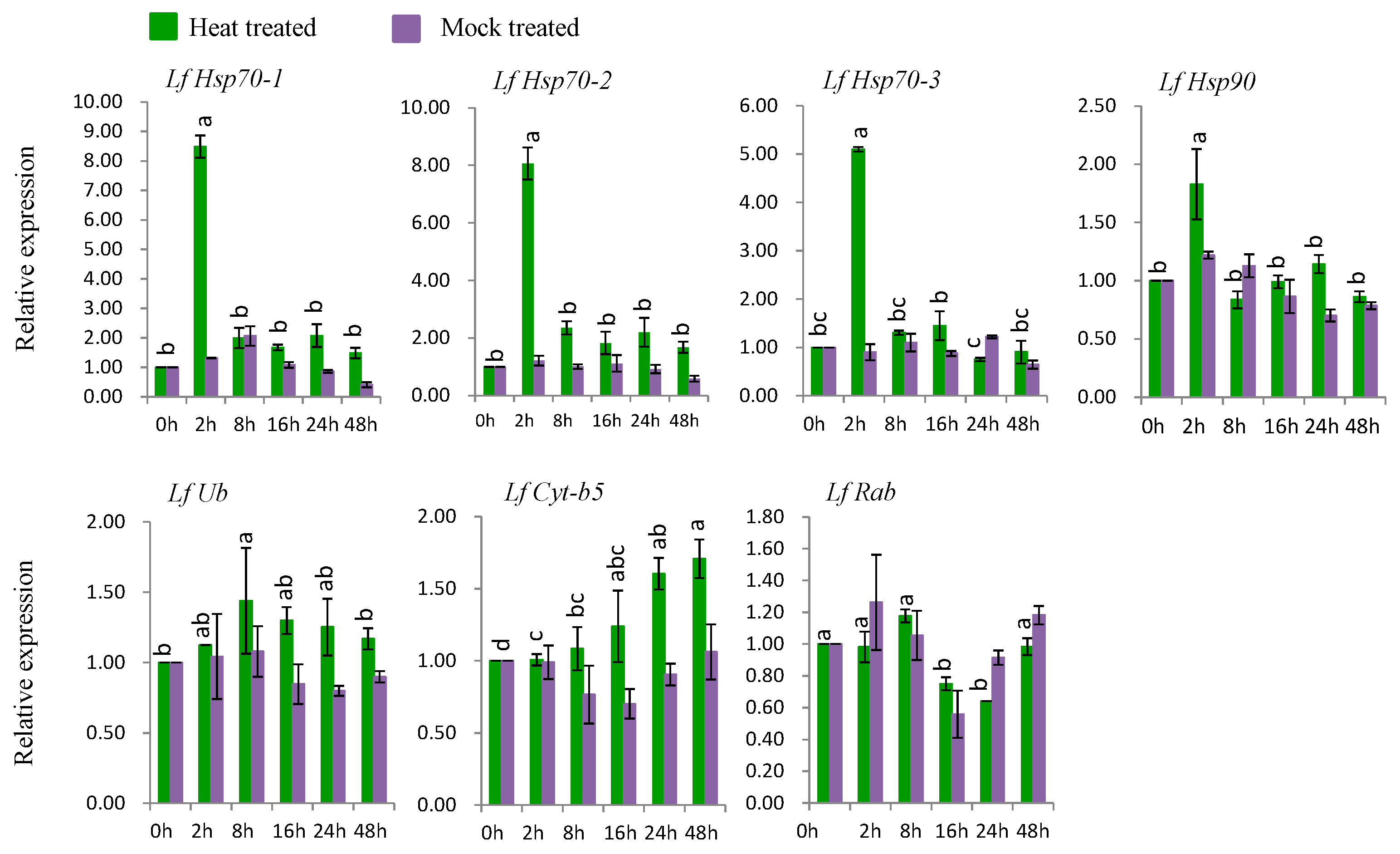
3.7.1. Gene Expression under Heat Stress
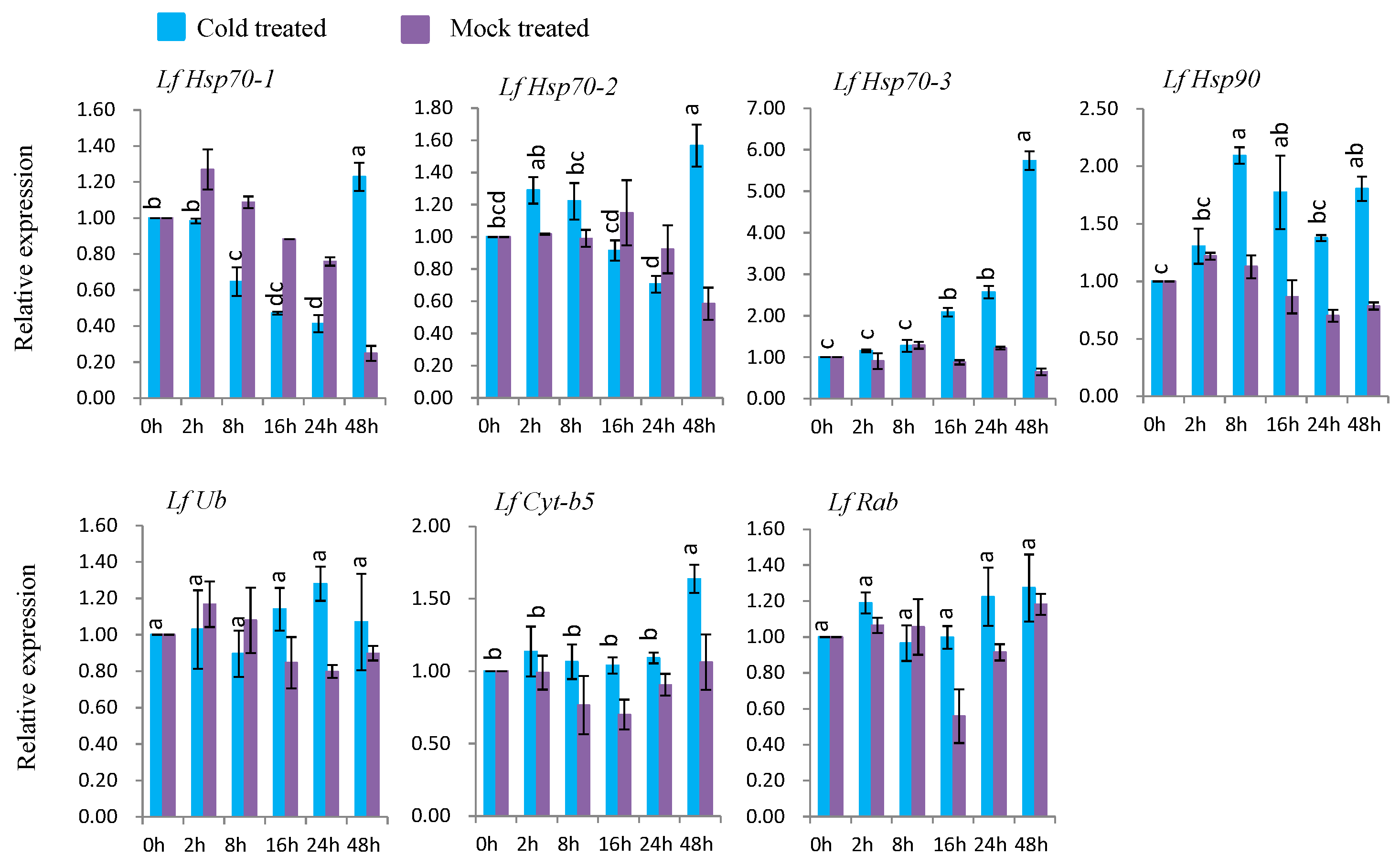
3.7.2. Gene Expression under Cold Stress
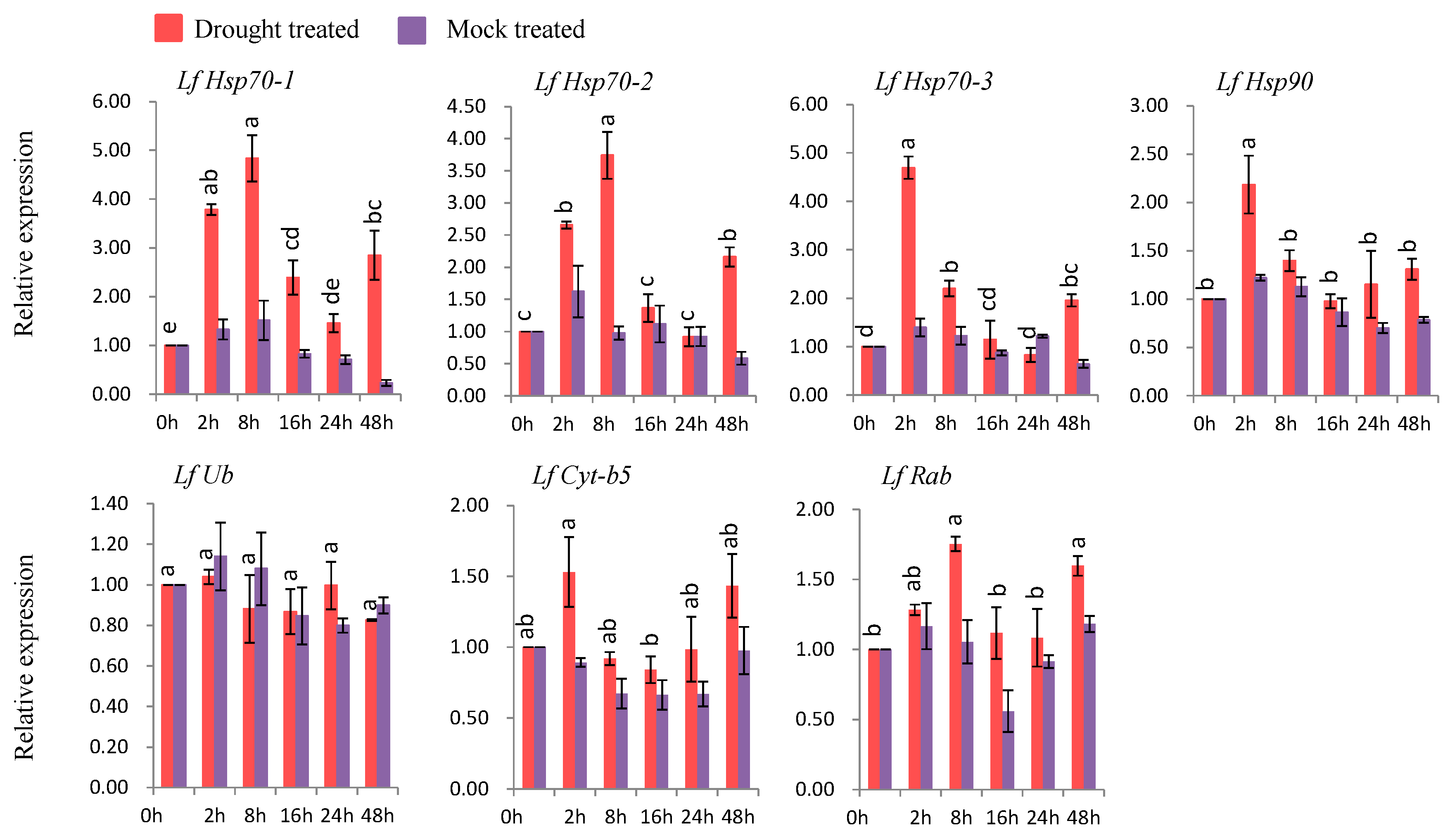
3.7.3. Gene Expression under Drought Stress
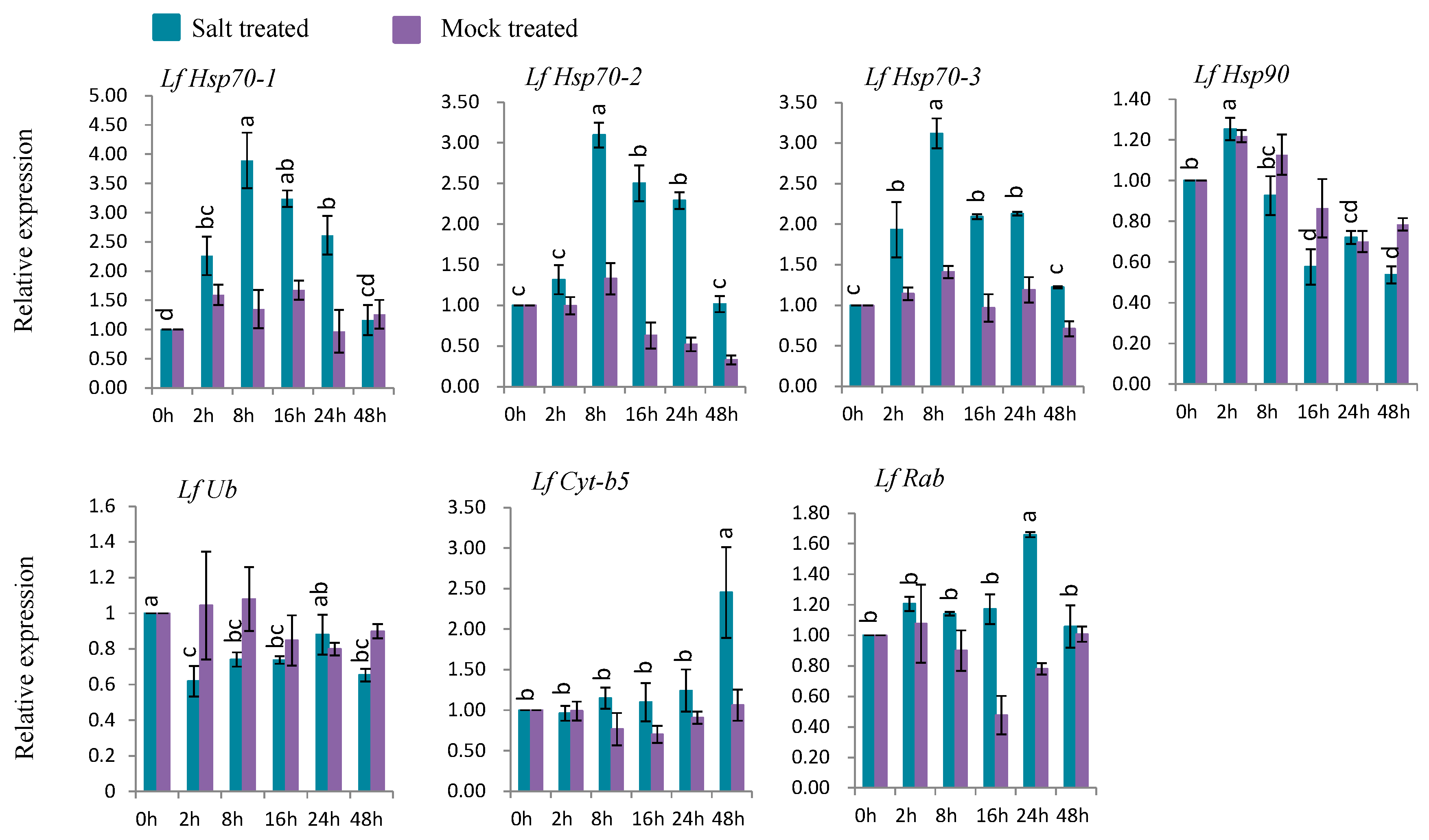
3.7.4. Gene Expression under Salt Stress
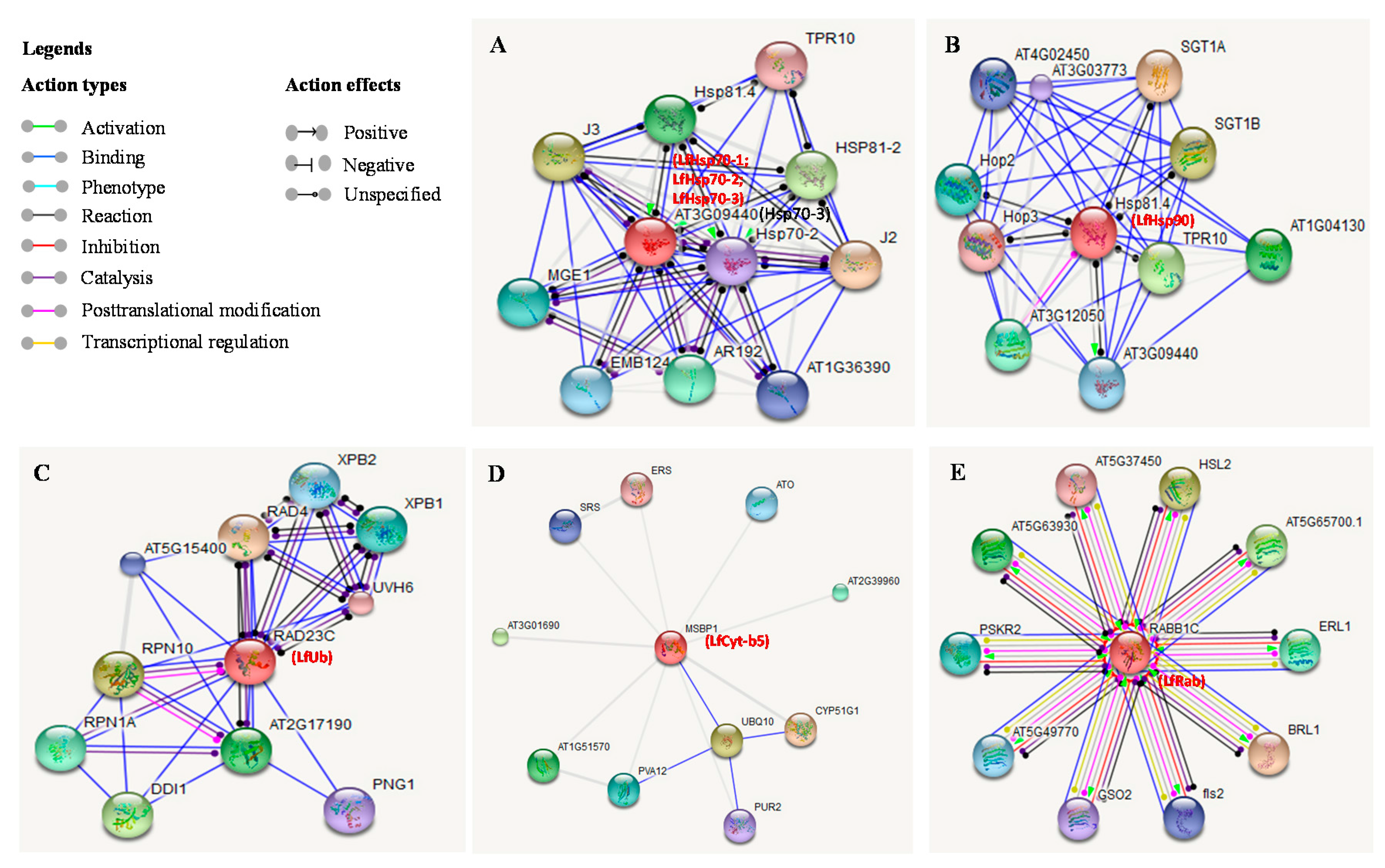
3.8. Analysis of Stress-Related Protein Interactions
4. Discussion
4.1. Importance of Sequence Variation in Defense-Related Proteins
4.2. L. formolongi Defense-Related Genes Are Active in All Organs
4.3. Expression Analysis of L. formolongi Defense-Related Genes under Botrytis Infection
4.4. Expression Analysis of L. formolongi Defense-Related Genes under Heat Stress
4.5. Expression Analysis of L. formolongi Defense-Related Genes under Cold Stress
4.6. Expression Analysis of L. formolongi Defense-Related Genes under Drought Stress
4.7. Expression Analysis Defense-Related of L. formolongi Genes Under Salt Stress
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shahin, A.; Kaauwen, M.V.; Esselink, D.; Bargsten, J.W.; van Tuyl, J.M.; Visser, R.G.; Arens, P. Generation and analysis of expressed sequence tags in the extreme large genomes. Lilium Tulipa. BMC Genom. 2012, 13, 640. [Google Scholar] [CrossRef]
- McRae, E.A. Lily species. In Lilies: A Guide for Growers and Collectors; Timber Press: Portland, OR, USA, 1998; pp. 105–204. [Google Scholar]
- Liang, S.Y.; Tamura, M. Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press/Missouri Botanical Garden Press: Beijing, China, 2000; Volume 24, pp. 135–159. [Google Scholar]
- Lim, K.B.; Barba-Gonzalez, R.; Zhou, S.; Ramanna, M.S.; Tuyl, J.M. Interspecific Hybridization in Lily (Lilium): Taxonomic and Commercial Aspects of Using Species Hybrids in Breeding. In Floriculture, Ornamental and Plant Biotechnology; Teixeira da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2008; Volume V. [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Statistics for Floricultural Industry in 2011. 2012. Available online: http://www.mafra.go.kr/list.jsp?id=28564&pageNo=1&NOW_YEAR=2012&group_id=2&menu_id=52&link_menu_id=&division=B&board_kind=C&board_skin_id=C1&parent_code=34&link_url=&depth=2 (accessed on 31 July 2012).
- Korea International Trade Association (KITA). Available online: http://www.kita.net (accessed on 1 April 2016).
- Doss, R.P.; Chastagner, G.A.; Riley, K.L. Techniques for inoculum production and inoculation of lily leaves with Botrytis elliptica. Plant Dis. 1984, 68, 854–856. [Google Scholar] [CrossRef]
- Hahm, S.S.; Lee, K.H.; Lee, J.W.; Lee, H.D.; Yu, S.H. Control and incidence of leaf blight on lily with different cultural systems. Res. Plant Dis. 2007, 13, 152–156. [Google Scholar] [CrossRef]
- Cho, W.D.; Shin, H.D. List of Plant Disease in Korea, 5th ed.; Korean Society of Plant Pathology (KSPP): Suwon, Korea, 2009; p. 853. [Google Scholar]
- Bray, E.A.; Bailey-Serres, J. Plant Responses to abiotic stresses. In Biochemistry and Molecular Biology of Plants; Gruissem, W., Buchannan, B., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1249. [Google Scholar]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kaoru, U.; Yukio, K.; Motoaki, S.; Kazuo, S. Omics: Analyses of regulatory networks in plant abiotic stress responses. Curr. Opin. Plant Biol. 2010, 13, 132–138. [Google Scholar] [CrossRef]
- Park, J.T.; Hwang, Y.J.; Lee, H.I.; Younis, A.; Lim, K.B. Ecological analysis of Lilium tsingtauense native in Korea. Hort. Environ. Biotechnol. 2014, 55, 230–236. [Google Scholar] [CrossRef]
- Mao, M.; Fu, G.; Wu, J.S.; Zhang, Q.H.; Zhou, J.; Kan, L.X. Identification of genes expressed in human CD34+ hematopoietic stem/progenitor cells by expressed sequence tags and efficient full-length cDNA cloning. Proc. Natl. Acad. Sci. USA 1998, 95, 8175–8180. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Bhalla, P.L.; Singh, M.B. Expressed sequence tag analysis of Lilium longiflorum generative cells. Plant Cell Physiol. 2006, 47, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.H.; Gasser, R.B.; Ranganathan, S. A hitchhiker’s guide to expressed sequence tag (EST) analysis. Brief Bioinform. 2007, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Takizawa, M.; Goto, A.; Watanabe, Y. The tobacco ubiquitin activating enzymes NtE1A and NtE1B are induced by tobacco mosaic virus, wounding and stress hormones. Mol. Cells 2005, 19, 228–231. [Google Scholar] [PubMed]
- Dreher, K.; Callis, J. Ubiquitin, hormones and biotic stress in plants. Ann. Bot. 2007, 99, 787–822. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, J.B.; Jansson, I. The many roles of cytochrome b5. Pharmacol. Ther. 2003, 97, 139–152. [Google Scholar] [CrossRef]
- Gostinčar, C.; Turk, M.; Gunde-Cimerman, N. The Evolution of Fatty Acid Desaturases and Cytochrome b5 in Eukaryotes. J. Membr. Biol. 2010, 233, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H.; Olkkonen, V.M. The RabGTPase family. Genome Biol 2001, 2, reviews 3007.1–reviews 3007.7. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ding, X.; Chang, T.; Wang, Z.; Liu, R.; Zeng, X.; Cai, Y.; Zhu, Y. Overexpression of a vesicle trafficking gene, OsRab7, enhances salt tolerance in rice. Sci. World J. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Benning, C. Unraveling plant metabolism by EST analysis. Curr. Opin. Plant Biol. 2000, 3, 224–228. [Google Scholar] [CrossRef]
- White, J.A.; Todd, J.; Newman, T.; Focks, N.; Girke, T.; de Ilarduya, O.M.; Jaworski, J.G.; Ohlrogge, J.B.; Benning, C. A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol. 2000, 124, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Brandle, J.E.; Richman, A.; Swanson, A.K.; Chapman, B.P. Leaf ESTs from Stevia rebaudiana: A resource for gene discovery in diterpene synthesis. Plant Mol. Biol. 2002, 50, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Arasan, S.K.T.; Park, J.I.; Ahmed, N.U.; Jung, H.J.; Lee, I.H.; Cho, Y.G.; Lim, Y.P.; Kang, K.K.; Nou, I.S. Gene ontology based characterization of Expressed Sequence Tags (ESTs) of Brassica rapa cv. Osome. Indian J. Exp. Biol. 2013, 51, 522–530. [Google Scholar] [PubMed]
- National Center for Biotechnology Information (NCBI) genetic database. Available online: http://www.ncbi.nlm.nih.gov/gene (accessed on 25 January 2017).
- Basic Local Alignment Search Tool (BLAST) from NCBI. Available online: http://www.ncbi.nlm.nih.gov/BLAST/ (accessed on 25 January 2017).
- Letunic, I.; Doerks, T.; Bork, P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Walker, J.M., Ed.; The Proteomics Protocols Handbook, Humana Press: New York, NY, USA, 2005; pp. 571–607. [Google Scholar]
- MolQuest–Bioinformatics Toolbox for analysis of biomedical data. Available online: http://linux1.softberry.com/berry.phtml (accessed on 25 January 2017).
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Alva, V.; Nam, S.Z.; Söding, J.; Lupas, A.N. The MPI bioinformatics Toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res. 2016, 44, W410–W415. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Wang, J.S.; Liu, H.C.; Chen, R.W.; Meyer, Y.; Barakat, A.; Delseny, M. Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperon. 2001, 6, 201–208. [Google Scholar] [CrossRef]
- Krishna, P.; Gloor, G. The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones 2001, 6, 238–246. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Chen, C.Y. Molecular analysis of lily leaves in response to salicylic acid effective towards protection against Botrytis elliptica. Plant Sci. 2005, 169, 1–9. [Google Scholar] [CrossRef]
- Mori, S.; Adachi, Y.; Horimoto, S.; Suzuki, S.; Nakano, M. Callus formation and plant regeneration in various Lilium species and cultivars. In vitro Cell Dev. Biol. Plant. 2005, 41, 783–788. [Google Scholar] [CrossRef]
- Kedra, M.; Bach, A. Morphogenesis of Lilium martagon L. explants in callus culture. Acta Biol. Cracov. Ser. Bot. 2005, 47, 65–73. [Google Scholar]
- Wang, J.; Wang, Q.; Yang, Y.; Liu, X.; Gu, J.; Li, W.; Ma, S.; Lu, Y. De novo assembly and characterization of stress transcriptome and regulatory networks under temperature, salt and hormone stresses in Lilium lancifolium. Mol. Biol. Rep. 2014, 41, 8231–8245. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Park, J.I.; Junga, H.J.; Kang, K.K.; Limc, Y.P.; Hurd, Y.; Nou, I.-S. Molecular characterization of thaumatin family genes related to stresses in Brassica rapa. Sci. Hort. 2013, 152, 26–34. [Google Scholar] [CrossRef]
- Khatun, K.; Robin, A.H.K.; Park, J.-I.; Nath, U.K.; Kim, C.K.; Lim, K.-B.; Nou, I.S.; Chung, M.-Y. Molecular Characterization and Expression Profiling of Tomato GRF Transcription Factor Family Genes in Response to Abiotic Stresses and Phytohormones. Int. J. Mol. Sci. 2017, 18, 1056. [Google Scholar] [CrossRef] [PubMed]
- Khatun, K.; Robin, A.H.K.; Park, J.-I.; Kim, C.K.; Lim, K.-B.; Kim, M.-B.; Lee, D.-J.; Nou, I.S.; Chung, M.-Y. Genome-Wide Identification, Characterization and Expression Profiling of ADF Family Genes in Solanum lycopersicum L. Genes 2016, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Zhang, H.; Chen, L.; Li, X.; Lian, Q.; Yuan, X.; Hu, X.; Cao, L.; He, X.; Yi, M. Cloning and characterization of HsfA2 from Lily (Lilium longiflorum). Plant Cell Rep. 2010, 29, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −∆∆ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- SAS. SAS/STAT. Statistical Analysis Systems for Windows, Release 9.1; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Mochida, K.; Uehara-Yamaguchi, Y.; Takahashi, F.; Yoshida, T.; Sakurai, T.; Shinozaki, K. Large-Scale Collection and Analysis of Full-Length cDNAs from Brachypodium distachyon and Integration with Pooideae Sequence Resources. PLoS ONE 2013, 8, e75265. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; Clarke, J.D.; Budworth, P.; Kreps, J.; Hutchison, D.; Park, S.; Guimil, S.; Dunn, M.; Luginbühl, P.; Ellero, C.; Goff, S.A.; Glazebrook, J. A network of rice genes associated with stress response and seed development. Proc. Natl. Acad. Sci. USA 2003, 100, 4945–4950. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Su’udi, M.; Kim, W.Y.; Kim, D.R.; Kwak, Y.S.; Son, D. Functional characterization of orchardgrass cytosolic Hsp70 (DgHsp70) and the negative regulation by Ca2+/AtCaM2 binding. Plant Physiol. Biochem. 2012, 58, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Xu, Q.; Harter, K.; Gruissem, W.; Luan, S. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 1999, 96, 4718–4723. [Google Scholar] [CrossRef] [PubMed]
- Salanoubat, M.; Lemcke, K.; Rieger, M.; Ansorge, W.; Unseld, M.; Fartmann, B.; Valle, G.; Blöcker, H.; Perez-Alonso, M.; Obermaier, B.; et al. Sequence and analysis of chromosome 3 of the plant Arabidopsis thaliana. Nature 2000, 408, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.B.; Anderson, J.V.; Guy, C.L. A cDNA clone encoding a spinach 70-kiloDalton heat-shock cognate. Plant Physiol. 1994, 105, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Yabe, N.; Takahashi, T.; Komeda, Y. Analysis of Tissue-Specific Expression of Arabidopsis thaliana HSP90-Family Gene. Plant Cell Physiol. 1994, 35, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.H.; Sharrock, R.A.; Quail, P.H. Maize polyubiquitin genes: Structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 1992, 18, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Motamayor, J.C.; Mockaitis, K.; Schmutz, J.; Haiminen, N.; Livingstone, D., 3rd; Cornejo, O.; Findley, S.D.; Zheng, P.; Utro, F.; Royaert, S.; et al. The genome sequence of the most widely cultivated cacao type and its use to identify candidate genes regulating pod color. Genome Biol. 2013, 14, r53. [Google Scholar] [CrossRef] [PubMed]
- Rice Chromosomes 11 and 12 Sequencing Consortia. The Sequence of Rice Chromosomes 11 and 12, Rich in Disease Resistance Genes and Recent Gene Duplications. BMC Biol. 2005, 3. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, R. Structure and expression of a rice hsp70 gene. Sci. China C Life Sci. 1996, 39, 291–299. [Google Scholar] [PubMed]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Krishnakumar, V.; Bidwell, S.; Rosen, B.; Chan, A.; Zhou, S.; Gentzbittel, L.; Childs, K.L.; Yandell, M.; Gundlach, H.; et al. An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genom. 2014, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Wei, X.; Fan, R.; Zhou, H.; Wang, X.; Yu, C.; Dong, L.; Dong, Z.; Wang, X.; Kang, Z.; et al. Molecular analysis of common wheat genes encoding three types of cytosolic heat shock protein 90 (Hsp90): Functional involvement of cytosolic Hsp90s in the control of wheat seedling growth and disease resistance. New Phytol. 2011, 191, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Yano, K.; Suzuki, A.; Kawamura, S.; Sakurai, N.; Suda, K.; Kurabayashi, A.; Suzuki, T.; Tsugane, T.; Watanabe, M.; et al. Large-scale analysis of full-length cDNAs from the tomato (Solanum lycopersicum) cultivar Micro-Tom, a reference system for the Solanaceae genomics. BMC Genom. 2010, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, Y.A.; Ramos, C.H. Cloning, purification and characterization of a 90kDa heat shock protein from Citrus sinensis (sweet orange). Plant Physiol. Biochem. 2012, 50, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Hasebe, M.; Fang, X. Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nat. Ecol. Evol. 2017, 1. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Zapata, L.; Ding, J.; Willing, E.M.; Hartwig, B.; Bezdan, D.; Jiao, W.B. Chromosome-level assembly of Arabidopsis thaliana Ler reveals the extent of translocation and inversion polymorphisms. Proc. Natl. Acad. Sci. USA 2016, 113, E4052–E4060. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Schüller, C.; Wambutt, R.; Murphy, G.; Volckaert, G.; Pohl, T.; Düsterhöft, A.; Stiekema, W.; Entian, K.D.; Terryn, N.; et al. Sequence and analysis of chromosome 4 of the plant Arabidopsis thaliana. Nature 1999, 402, 769–7777. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.L.; Rouzé, P.; Verhelst, B.; Lin, Y.C.; Bayer, T.; Collen, J. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 2016, 530, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.Y.; Vierling, E.; Guy, C.L. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001, 126, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Li, Z.Y.; Chen, Y.; Chen, M.; Li, L.C.; Ma, Y.Z. Heat shock protein 90 in plants: Molecular mechanisms and roles in stress responses. Int. J. Mol. Sci. 2012, 13, 15706–15723. [Google Scholar] [CrossRef] [PubMed]
- Brukhin, V.; Gheyselinck, J.; Gagliardini, V.; Genschik, P.; Grossniklaus, U. The RPN1 subunit of the 26S proteasome in Arabidopsis is essential for embryogenesis. Plant Cell. 2005, 17, 2723–2737. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kurepa, J.; Smalle, J.A. The Arabidopsis 26S Proteasome Subunit RPN1a Is Required for Optimal Plant Growth and Stress Responses. Plant Cell Physiol. 2009, 50, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Shi, Q.M.; Yang, X.H.; Xu, Z.H.; Xue, H.W. Membrane steroid-binding protein 1 (MSBP1) negatively regulates brassinosteroid signaling by enhancing the endocytosis of BAK1. Cell Res. 2009, 19, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.M.; DeLuca-Flaherty, C.; McKay, D.B. Three-dimensional structure of the ATPase fragment of the 70 K heat-shock cognate protein. Nature 1990, 346, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, X.; Burkholder, W.F.; Gragerov, A.; Ogata, C.M.; Gottesman, M.E.; Hendrickson, W.A. Structural analysis of substrate binding by the molecular chaperone Dnak. Science 1996, 272, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Bukau, B.; Horwich, A.L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998, 92, 351–366. [Google Scholar] [CrossRef]
- Guy, C.L.; Li, Q.B. The organization and evolution of the spinach stress 70 molecular chaperone gene family. Plant Cell. 1998, 10, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Milioni, D.; Hatzopoulos, P. Genomic organization of Hsp90 gene family in Arabidopsis. Plant Mol. Biol. 1997, 35, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.H.; Prodromou, C. Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 2000, 10, 46–51. [Google Scholar] [CrossRef]
- Panaretou, B.; Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998, 17, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T.; Ziff, E. Leucine zippers of fos, jun and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature 1989, 340, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, W.T. Regulation of abiotic stress signal transduction by E3 ubiquitin ligases in Arabidopsis. Mol. Cells. 2011, 31, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.M.; Seth, A.K. The ubiquitin-mediated protein degradation pathway in cancer: Therapeutic implications. Eur. J. Cancer 2004, 40, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997, 18, 306–360. [Google Scholar] [CrossRef] [PubMed]
- Lässle, M.; Blatch, G.L.; Kundra, V.; Takatoriand, T.; Zetter, B.R. Stress-inducible, murine protein mSTI1 characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J. Biol. Chem. 1997, 272, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burch-Smith, T.; Schiff, M.; Feng, S.; Dinesh-Kumar, S.P. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 2004, 279, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Sangster, T.A.; Bahrami, A.; Wilczek, A.; Watanabe, E.; Schellenberg, K.; McLellan, C.; Kelley, A.; Kong, S.W.; Queitsch, C.; Lindquist, S. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE 2007, 2, e648. [Google Scholar] [CrossRef] [PubMed]
- Marcote, M.J.; Gu, F.; Gruenberg, J.; Aniento, F. Membrane transport in the endocytic pathway: Animal versus plant cells. Protoplasma 2000, 210, 123–132. [Google Scholar] [CrossRef]
- Lee, J.H.; Yun, H.S.; Kwon, C. Molecular communications between plant heat shock responses and disease resistance. Mol. Cells 2012, 34, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Staats, M.; van Kan, J.A.L. Induction of programmed cell death in lily by the fungal pathogen Botrytis elliptica. Mol. Plant Pathol. 2004, 5, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Govrin, E.M.; Rachmilevitch, S.; Tiwari, B.S.; Solomon, M.; Levine, A. An elicitor from Botrytis cinerea induces the hypersensitive response in Arabidopsis thaliana and other plants and promotes the gray mold disease. Phytopathol 2006, 96, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Seo, Y.S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Whitham, S.A. Overexpression of a soybean nuclear localized type-III DnaJ domain-containing HSP40 reveals its roles in cell death and disease resistance. Plant J. 2013, 74, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Van Ooijen, G.; Lukasik, E.; Van Den Burg, H.A.; Vossen, J.H.; Cornelissen, B.J.; Takken, F.L. The small heat shock protein 20 RSI2 interacts with and is required for stability and function of tomato resistance protein I-2. Plant J. 2010, 63, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009, 60, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Casais, C.; Ichimura, K.; Shirasu, K. HSP90 interacts with RAR1 and SGT1, and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 11777–11782. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, M.; Hardy, K.D.; Milne, G.L.; Morrow, J.D.; Chen, C.; Gonzalez, F.J.; Gu, J.; Ding, X.; Johnson, D.A.; Johnson, J.A. Development of Oxidative Stress by Cytochrome P450 Induction in Rodents Is Selective for Barbiturates and Related to Loss of Pyridine Nucleotide-dependent Protective Systems. J. Biol. Chem. 2008, 283, 17147–17157. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Zhu, X.; Chen, F.; Gao, H.; Jiang, J.; Chen, S. A chrysanthemum heat shock protein confers tolerance to abiotic stress. Int. J. Mol. Sci. 2014, 15, 5063–5078. [Google Scholar] [CrossRef] [PubMed]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Mazel, A.; Leshem, Y.; Tiwari, B.S.; Levine, A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 2004, 134, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.B.; Haskell, D.W.; Guy, C.L. Coordinate and non-coordinate expression of the stress 70 family and other molecular chaperones at high and low temperature in spinach and tomato. Plant Mol. Biol. 1999, 39, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.Y.; Guy, C.L. Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis. Evidence for pleiotropic consequences. Plant Physiol. 2003, 132, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Li, B.; Shang, Z.L.; Li, X.Z.; Mu, R.L.; Sun, D.; Zhou, R.G. Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 2003, 132, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.; Sacco, M.; Cherutti, J.F.; Hill, S. Cold-induced accumulation of hsp90 transcripts in Brassica napus. Plant Physiol. 1995, 107, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Singla, S.L.; Grover, A. Immunological evidence for accumulation of two high-molecular-weight (104 and 90 kDa) HSPs in response to different stresses in rice and in response to high temperature stress in diverse plant genera. Plant Mol. Biol. 1995, 29, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Alvim, F.C.; Carolino, S.M.; Cascardo, J.C.; Nunes, C.C.; Martinez, C.A.; Otoni, W.C.; Fontes, E.P. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol. 2001, 126, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Sugino, M.; Hibino, T.; Tanaka, Y.; Nii, N.; Takabe, T. Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytica acquires resistance to salt stress in transgenic tobacco plants. Plant Sci. 1999, 146, 81–88. [Google Scholar] [CrossRef]
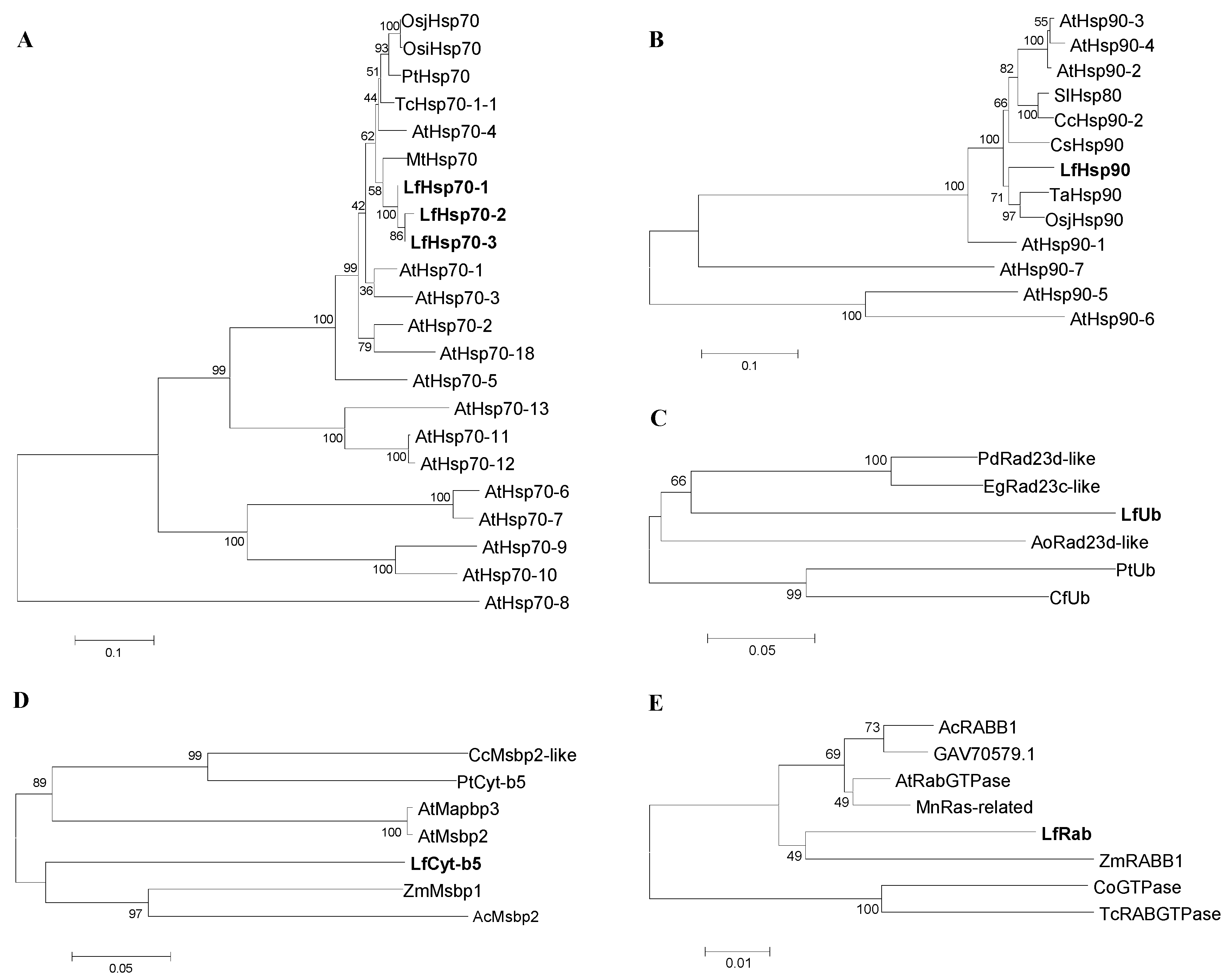



| EST Gene Name | Gene Name (mRNA) | GenBank Accession | Location | Domain Name | E-Value | Domain Position | CDS (bp) | Protein | Retrieved Sequence | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Length (aa) | MW (kDa) | PI | ||||||||
| Lf6 | LfHsp70-1 | KX683998 | Cytoplasm and Nucleus | HSP70 | 1.2 × 10−217 | 1 | 513 | 1623 | 540 | 59.51 | 5.04 | 1821 |
| Lf7 | LfHsp70-2 | KX683999 | Cytoplasm and Nucleus | HSP70 | 3.2 × 10−113 | 1 | 306 | 1002 | 333 | 36.58 | 4.78 | 1238 |
| Lf8 | LfHsp70-3 | KX684000 | Cytoplasm and Nucleus | HSP70 | 2.6 × 10−209 | 1 | 493 | 1563 | 520 | 57.27 | 5.17 | 1752 |
| Lf9 | LfHsp90 | KX684001 | Cytoplasm and Membrane | HATPase_c HSP90 | 2.81 × 10−8 6.3 × 10−236 | 28 185 | 183 700 | 2103 | 700 | 80.23 | 4.90 | 2422 |
| Lf10 | LfUb | KX683995 | Cytoplasm and Nucleus | UBQ UBA STI1 UBA | 2.3 × 10−18 8.0 × 10−5 4.81 × 10−6 1.38 × 10−8 | 1 155 253 336 | 75 195 296 373 | 1143 | 380 | 40.36 | 4.55 | 1505 |
| Lf11 | LfCyt-b5 | KX683996 | Plasma membrane | TM Cyt-b5 | - 2.68 × 10−20 | 13 71 | 35 167 | 795 | 264 | 28.65 | 4.56 | 1144 |
| Lf12 | LfRab | KX683997 | Cytoplasm and Golgi and Endoplasmic Reticulum | RAB | 2.63 × 10−105 | 7 | 170 | 627 | 208 | 22.86 | 6.51 | 950 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howlader, J.; Park, J.-I.; Robin, A.H.K.; Sumi, K.R.; Nou, I.-S. Identification, Characterization and Expression Profiling of Stress-Related Genes in Easter Lily (Lilium formolongi). Genes 2017, 8, 172. https://doi.org/10.3390/genes8070172
Howlader J, Park J-I, Robin AHK, Sumi KR, Nou I-S. Identification, Characterization and Expression Profiling of Stress-Related Genes in Easter Lily (Lilium formolongi). Genes. 2017; 8(7):172. https://doi.org/10.3390/genes8070172
Chicago/Turabian StyleHowlader, Jewel, Jong-In Park, Arif Hasan Khan Robin, Kanij Rukshana Sumi, and Ill-Sup Nou. 2017. "Identification, Characterization and Expression Profiling of Stress-Related Genes in Easter Lily (Lilium formolongi)" Genes 8, no. 7: 172. https://doi.org/10.3390/genes8070172







