Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets
Abstract
:1. Introduction
2. Acetylation-Related Protein Families
2.1. Histone Acetyltransferases (HATs)
2.2. Bromodomain-Containing Proteins
2.3. Histone Deacetylases (HDACs)
3. Methylation-Related Protein Families
3.1. Methyltransferases
3.2. Demethylases
3.3. Methyl Binding Proteins
4. Epigenetics and Human Diseases
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suñer, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Humpherys, D.; Eggan, K.; Akutsu, H.; Hochedlinger, K.; Rideout, W.M.; Biniszkiewicz, D.; Yanagimachi, R.; Jaenisch, R. Epigenetic instability in ES cells and cloned mice. Science 2001, 293, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C. Preliminary notes on the development of the wings in normal and mutant strains of Drosophila. Proc. Natl. Acad. Sci. USA 1939, 25, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.E.; Martienssen, R.A.; Riggs, A.D. Epigenetic Mechanisms of Gene Regulation; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1996. [Google Scholar]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997, 389, 251–260. [Google Scholar] [PubMed]
- Meneghini, M.D.; Wu, M.; Madhani, H.D. Conserved histone variant H2A. Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 2003, 112, 725–736. [Google Scholar] [CrossRef]
- Ng, H.H.; Ciccone, D.N.; Morshead, K.B.; Oettinger, M.A.; Struhl, K. Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: A potential mechanism for position-effect variegation. Proc. Natl. Acad. Sci. USA 2003, 100, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosa, H.; Bannister, A.J.; Dehe, P.M.; Géli, V.; Kouzarides, T. Methylation of H3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J. Biol. Chem. 2004, 279, 47506–47512. [Google Scholar] [CrossRef] [PubMed]
- Suka, N.; Luo, K.; Grunstein, M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat. Genet. 2002, 32, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Hebbes, T.R.; Thorne, A.W.; Clayton, A.L.; Crane-Robinson, C. Histone acetylation and globin gene switching. Nucleic Acids Res. 1992, 20, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Hebbes, T.R.; Thorne, A.W.; Crane-Robinson, C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988, 7, 1395. [Google Scholar] [PubMed]
- Logie, C.; Tse, C.; Hansen, J.C.; Peterson, C.L. The core histone N-terminal domains are required for multiple rounds of catalytic chromatin remodeling by the SWI/SNF and RSC complexes. Biochemistry 1999, 38, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.G.; Smith, M.M.; Roth, S.Y. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 1996, 10, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Hecht, A.; Laroche, T.; Strahl-Bolsinger, S.; Gasser, S.M.; Grunstein, M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: A molecular model for the formation of heterochromatin in yeast. Cell 1995, 80, 583–592. [Google Scholar] [CrossRef]
- Allfrey, V.G. Structural modifications of histones and their possible role in the regulation of ribonucleic acid synthesis. In Proceedings of the Canadian Cancer Conference, Honey Harbour, ON, Canada, 1 January 1965; pp. 313–335. [Google Scholar]
- Wolffe, A.P.; Hayes, J.J. Chromatin disruption and modification. Nucleic Acids Res. 1999, 27, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Schmitges, F.W.; Prusty, A.B.; Faty, M.; Stützer, A.; Lingaraju, G.M.; Aiwazian, J.; Sack, R.; Hess, D.; Li, L.; Zhou, S. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol. Cell 2011, 42, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Xu, M.; Huang, C.; Liu, N.; Chen, S.; Zhu, B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J. Biol. Chem. 2011, 286, 7983–7989. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sweet, S.M.; Popovic, R.; Martinez-Garcia, E.; Tipton, J.D.; Thomas, P.M.; Licht, J.D.; Kelleher, N.L. Total kinetic analysis reveals how combinatorial methylation patterns are established on lysines 27 and 36 of histone H3. Proc. Natl. Acad. Sci. USA 2012, 109, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Ma, B.; Yuan, W.; Zhang, Z.; Chen, P.; Ding, X.; Feng, L.; Shen, X.; Chen, S.; Li, G. Histone H2A ubiquitination inhibits the enzymatic activity of H3 lysine 36 methyltransferases. J. Biol. Chem. 2013, 288, 30832–30842. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.; Salmon-Divon, M.; Dvinge, H.; Hynes-Allen, A.; Balasooriya, G.; Leaford, D.; Behrens, A.; Bertone, P.; Hendrich, B. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J. 2012, 31, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kang, B.H.; Jang, H.; Kwak, S.; Shin, J.; Kim, H.; Lee, S.E.; Lee, S.M.; Lee, J.H.; Kim, J.H. Ctbp2 modulates NuRD-mediated deacetylation of H3K27 and facilitates PRC2-mediated H3K27me3 in active embryonic stem cell genes during exit from pluripotency. Stem Cells 2015, 33, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Zahradka, P.; Ebisuzaki, K. A Shuttle Mechanism for DNA-Protein Interactions. FEBS J. 1982, 127, 579–585. [Google Scholar] [CrossRef]
- Oei, S.L.; Griesenbeck, J.; Schweiger, M.; Ziegler, M. Regulation of RNA polymerase II-dependent transcription by poly (ADP-ribosyl) ation of transcription factors. J. Biol. Chem. 1998, 273, 31644–31647. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.L.; Griesenbeck, J.; Ziegler, M.; Schweiger, M. A novel function of poly (ADP-ribosyl) ation: Silencing of RNA polymerase II-dependent transcription. Biochemistry 1998, 37, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Węsierska-Gądek, J.; Schmid, G.; Cerni, C. ADP-Ribosylation of Wild-Type p53in Vitro: Binding of p53 protein to specific p53 consensus sequence prevents its modification. Biochem. Biophys. Res. Commun. 1996, 224, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.L.; Griesenbeck, J.; Schweiger, M.; Babich, V.; Kropotov, A.; Tomilin, N. Interaction of the transcription factor YY1 with human poly (ADP-ribosyl) transferase. Biochem. Biophys. Res. Commun. 1997, 240, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-J.; Alvarez-Gonzalez, R. The sequence-specific DNA binding of NF-κB is reversibly regulated by the automodification reaction of poly (ADP-ribose) polymerase 1. J. Biol. Chem. 2001, 276, 47664–47670. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Alvarez, H.; Alvarez-Gonzalez, R. Regulation of p53 sequence-specific DNA-binding by covalent poly (ADP-ribosyl) ation. J. Biol. Chem. 2001, 276, 36425–36430. [Google Scholar] [CrossRef] [PubMed]
- Simbulan-Rosenthal, C.M.; Rosenthal, D.S.; Luo, R.; Smulson, M.E. Poly (ADP-ribose) polymerase upregulates E2F-1 promoter activity and DNA pol α expression during early S phase. Oncogene 1999, 18, 5015–5023. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Covic, M.; Hasan, S.; Imhof, R.; Hottiger, M.O. The enzymatic and DNA binding activity of PARP-1 are not required for NF-κB coactivator function. J. Biol. Chem. 2001, 276, 45588–45597. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Haenni, S.S.; Buerki, C.; Meier, N.I.; Lane, W.S.; Owen, H.; Gersbach, M.; Imhof, R.; Hottiger, M.O. Acetylation of poly (ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-κB-dependent transcription. J. Biol. Chem. 2005, 280, 40450–40464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrozza, M.J.; Li, B.; Florens, L.; Suganuma, T.; Swanson, S.K.; Lee, K.K.; Shia, W.-J.; Anderson, S.; Yates, J.; Washburn, M.P. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 2005, 123, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Botuyan, M.V.; Lee, J.; Ward, I.M.; Kim, J.-E.; Thompson, J.R.; Chen, J.; Mer, G. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 2006, 127, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Mowen, K.A.; Tang, J.; Zhu, W.; Schurter, B.T.; Shuai, K.; Herschman, H.R.; David, M. Arginine methylation of STAT1 modulates IFNα/β-induced transcription. Cell 2001, 104, 731–741. [Google Scholar] [CrossRef]
- Murr, R.; Loizou, J.I.; Yun-Gui, Y.; Cuenin, C.; Li, H.; Zhao-Qi, W.; Herceg, Z. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat. Cell Biol. 2006, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Hayes, J.J.; Pruss, D.; Wolffe, A.P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 1993, 72, 73–84. [Google Scholar] [CrossRef]
- Vogelauer, M.; Rubbi, L.; Lucas, I.; Brewer, B.J.; Grunstein, M. Histone acetylation regulates the time of replication origin firing. Mol. Cell 2002, 10, 1223–1233. [Google Scholar] [CrossRef]
- Basnet, H.; Su, X.B.; Tan, Y.; Meisenhelder, J.; Merkurjev, D.; Ohgi, K.A.; Hunter, T.; Pillus, L.; Rosenfeld, M.G. Tyrosine phosphorylation of histone H2A by CK2 regulates transcriptional elongation. Nature 2014, 516, 267. [Google Scholar] [CrossRef] [PubMed]
- Van Attikum, H.; Fritsch, O.; Hohn, B.; Gasser, S.M. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 2004, 119, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Psakhye, I.; Jentsch, S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012, 151, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Best, J.L.; Zon, L.I.; Gill, G. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell 2002, 10, 831–842. [Google Scholar] [CrossRef]
- Xu, K.; Shimelis, H.; Linn, D.E.; Jiang, R.; Yang, X.; Sun, F.; Guo, Z.; Chen, H.; Li, W.; Chen, H. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell 2009, 15, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Hoege, C.; Pfander, B.; Moldovan, G.-L.; Pyrowolakis, G.; Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002, 419, 135. [Google Scholar] [CrossRef] [PubMed]
- Olabisi, O.A.; Soto-Nieves, N.; Nieves, E.; Yang, T.T.; Yang, X.; Raymond, Y.; Suk, H.Y.; Macian, F.; Chow, C.-W. Regulation of transcription factor NFAT by ADP-ribosylation. Mol. Cell. Biol. 2008, 28, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Kreimeyer, A.; Wielckens, K.; Adamietz, P.; Hilz, H. DNA repair-associated ADP-ribosylation in vivo. Modification of histone H1 differs from that of the principal acceptor proteins. J. Biol. Chem. 1984, 259, 890–896. [Google Scholar] [PubMed]
- Boulikas, T. Poly (ADP-ribosylated) histones in chromatin replication. J. Biol. Chem. 1990, 265, 14638–14647. [Google Scholar] [PubMed]
- Nelson, C.J.; Santos-Rosa, H.; Kouzarides, T. Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell 2006, 126, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, G.L.; Daujat, S.; Snowden, A.W.; Erdjument-Bromage, H.; Hagiwara, T.; Yamada, M.; Schneider, R.; Gregory, P.D.; Tempst, P.; Bannister, A.J. Histone deimination antagonizes arginine methylation. Cell 2004, 118, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Brownell, J.E.; Zhou, J.; Ranalli, T.; Kobayashi, R.; Edmondson, D.G.; Roth, S.Y.; Allis, C.D. Tetrahymena histone acetyltransferase A: A homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 1996, 84, 843–851. [Google Scholar] [CrossRef]
- Sterner, D.E.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Wolffe, A. Chromatin: Structure and Function; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Ait-Si-Ali, S.; Ramirez, S.; Robin, P.; Trouche, D.; Harel-Bellan, A. A rapid and sensitive assay for histone acetyl-transferase activity. Nucleic Acids Res. 1998, 26, 3869–3870. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carrillo, A.; Wangh, L.J.; Allfrey, V.G. Processing of newly synthesized histone molecules. Science 1975, 190, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Brownell, J.E.; Allis, C.D. Special HATs for special occasions: Linking histone acetylation to chromatin assembly and gene activation. Curr. Opin. Genet. Dev. 1996, 6, 176–184. [Google Scholar] [CrossRef]
- Kuo, M.-H.; Brownell, J.E.; Sobel, R.E.; Ranalli, T.A. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 1996, 383, 269. [Google Scholar] [CrossRef] [PubMed]
- Dhalluin, C.; Carlson, J.E.; Zeng, L.; He, C.; Aggarwal, A.K.; Zhou, M.-M. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999, 399, 491–496. [Google Scholar] [PubMed]
- Akhtar, A.; Becker, P.B. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 2000, 5, 367–375. [Google Scholar] [CrossRef]
- Borrow, J.; Stanton, V.P.; Andresen, J.M.; Becher, R.; Behm, F.G.; Chaganti, R.S.; Civin, C.I.; Disteche, C.; Dubé, I.; Frischauf, A.M. The translocation t (8; 16)(p11; p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB–binding protein. Nat. Genet. 1996, 14, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.S.; Lowell, J.E.; Jacobson, S.J.; Pillus, L. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol. Cell. Biol. 1999, 19, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Reifsnyder, C.; Lowell, J.; Clarke, A.; Pillus, L. Yeast SAS silencing genes and human genes associated with AML and HIV–1 Tat interactions are homologous with acetyltransferases. Nat. Genet. 1996, 14, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Zink, D.; Becker, P.B. Chromodomains are protein–RNA interaction modules. Nature 2000, 407, 405–409. [Google Scholar] [PubMed]
- Neuwald, A.F.; Landsman, D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 1997, 22, 154–155. [Google Scholar] [CrossRef]
- Dutnall, R.N.; Tafrov, S.T.; Sternglanz, R.; Ramakrishnan, V. Structure of the histone acetyltransferase Hat1: A paradigm for the GCN5-related N-acetyltransferase superfamily. Cell 1998, 94, 427–438. [Google Scholar] [CrossRef]
- Yan, Y.; Barlev, N.A.; Haley, R.H.; Berger, S.L.; Marmorstein, R. Crystal structure of yeast Esa1 suggests a unified mechanism for catalysis and substrate binding by histone acetyltransferases. Mol. Cell 2000, 6, 1195–1205. [Google Scholar] [CrossRef]
- Rojas, J.R.; Trievel, R.C.; Zhou, J.; Mo, Y.; Li, X.; Berger, S.L.; Allis, C.D.; Marmorstein, R. Structure of Tetrahymena GCN5 bound to coenzyme A and a histone H3 peptide. Nature 1999, 401, 93–98. [Google Scholar] [PubMed]
- Trievel, R.C.; Rojas, J.R.; Sterner, D.E.; Venkataramani, R.N.; Wang, L.; Zhou, J.; Allis, C.D.; Berger, S.L.; Marmorstein, R. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc. Natl. Acad. Sci. USA 1999, 96, 8931–8936. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.C.; Minucci, S.; Lu, J.; Yang, X.-J.; Walker, K.K.; Chen, H.; Evans, R.M.; Nakatani, Y.; Ozato, K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998, 12, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.G.; Trievel, R.C.; Kuo, M.-H.; Howard, R.M.; Berger, S.L.; Allis, C.D.; Marmorstein, R.; Denu, J.M. Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J. Biol. Chem. 1999, 274, 18157–18160. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Muñoz, F.; Schilcher, P.; Imhof, A.; Almouzni, G.; Loyola, A. Sequential establishment of marks on soluble histones H3 and H4. J. Biol. Chem. 2011, 286, 17714–17721. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Che, Y.-Z.; Zou, H.-S.; Cui, Y.-P.; Guo, W.; Zou, L.-F.; Biddle, E.M.; Yang, C.-H.; Chen, G.-Y. Hpa2 required by HrpF to translocate Xanthomonas oryzae transcriptional activator-like effectors into rice for pathogenicity. Appl. Environ. Microbiol. 2011, 77, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Lu, J.; Duan, J.; Su, D.; Hou, X.; Li, F.; Wang, X.; Huang, B. Gcn5-and Elp3-induced histone H3 acetylation regulates hsp70 gene transcription in yeast. Biochem. J. 2008, 409, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, I.; Aikawa, Y.; Yokoyama, A.; Hosoda, F.; Nagai, M.; Kakazu, N.; Abe, T.; Ohki, M. Fusion of MOZ and p300 histone acetyltransferases in acute monocytic leukemia with at (8; 22)(p11; q13) chromosome translocation. Leukemia 2001, 15, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Miotto, B.; Struhl, K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol. Cell 2010, 37, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, A.; Hilfiker-Kleiner, D.; Pannuti, A.; Lucchesi, J.C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997, 16, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Horikoshi, M. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J. Biol. Chem. 1997, 272, 30595–30598. [Google Scholar] [CrossRef] [PubMed]
- Katan-Khaykovich, Y.; Struhl, K. Dynamics of global histone acetylation and deacetylation in vivo: Rapid restoration of normal histone acetylation status upon removal of activators and repressors. Genes Dev. 2002, 16, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Mizzen, C.A.; Yang, X.-J.; Kokubo, T.; Brownell, J.E.; Bannister, A.J.; Owen-Hughes, T.; Workman, J.; Wang, L.; Berger, S.L.; Kouzarides, T. The TAF II 250 subunit of TFIID has histone acetyltransferase activity. Cell 1996, 87, 1261–1270. [Google Scholar] [CrossRef]
- Srinivasan, L.; Gopinathan, K.P. Characterization of RNA polymerase III transcription factor TFIIIC from the mulberry silkworm, Bombyx mori. FEBS J. 2002, 269, 1780–1789. [Google Scholar] [CrossRef]
- Liu, Z.; Myers, L.C. Med5 (Nut1) and Med17 (Srb4) are direct targets of mediator histone H4 tail interactions. PLoS ONE 2012, 7, e38416. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Xu, J. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol. Endocrinol. 2010, 24, 1917–1934. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.-A.; Rose, D.W.; Haque, Z.K.; Kurokawa, R.; McInerney, E.; Westin, S.; Thanos, D.; Rosenfeld, M.G.; Glass, C.K.; Collins, T. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 1999, 19, 6367–6378. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Kohli, K.; Trivedi, A.; Johnson, D.L.; Stallcup, M.R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 4948–4952. [Google Scholar] [CrossRef] [PubMed]
- Bhoumik, A.; Singha, N.; O’Connell, M.J.; Ze’ev, A.R. Regulation of TIP60 by ATF2 modulates ATM activation. J. Biol. Chem. 2008, 283, 17605–17614. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.R.; Dollard, C.; Winston, F.; Beck, S.; Trowsdale, J.; Dawid, I.B. The bromodomain: A conserved sequence found in human, Drosophila and yeast proteins. Nucleic Acids Res. 1992, 20, 2603. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Alsarraj, J.; Faraji, F.; Geiger, T.R.; Mattaini, K.R.; Williams, M.; Wu, J.; Ha, N.-H.; Merlino, T.; Walker, R.C.; Bosley, A.D. BRD4 short isoform interacts with RRP1B, SIPA1 and components of the LINC complex at the inner face of the nuclear membrane. PLoS ONE 2013, 8, e80746. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Knapp, S. Targeting bromodomains: Epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 2014, 13, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Schlegl, J.; Hahne, H.; Gholami, A.M.; Lieberenz, M.; Savitski, M.M.; Ziegler, E.; Butzmann, L.; Gessulat, S.; Marx, H. Mass-spectrometry-based draft of the human proteome. Nature 2014, 509, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sabari, B.R.; Panchenko, T.; Wen, H.; Zhao, D.; Guan, H.; Wan, L.; Huang, H.; Tang, Z.; Zhao, Y. Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. Mol. Cell 2016, 62, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Gamsjaeger, R.; Webb, S.R.; Lamonica, J.M.; Billin, A.; Blobel, G.A.; Mackay, J.P. Structural basis and specificity of acetylated transcription factor GATA1 recognition by BET family bromodomain protein Brd3. Mol. Cell. Biol. 2011, 31, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.; He, Y.; Zeng, L.; Yan, S.; Plotnikova, O.; Sanchez, R.; Zeleznik-Le, N.J.; Ronai, Z.E.; Zhou, M.-M. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol. Cell 2004, 13, 251–263. [Google Scholar] [CrossRef]
- Jacobson, R.H.; Ladurner, A.G.; King, D.S.; Tjian, R. Structure and function of a human TAFII250 double bromodomain module. Science 2000, 288, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.J.; Ornaghi, P.; Yang, J.C.; Lowe, N.; Evans, P.R.; Ballario, P.; Neuhaus, D.; Filetici, P.; Travers, A.A. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 2000, 19, 6141–6149. [Google Scholar] [CrossRef] [PubMed]
- Ruthenburg, A.J.; Li, H.; Milne, T.A.; Dewell, S.; McGinty, R.K.; Yuen, M.; Ueberheide, B.; Dou, Y.; Muir, T.W.; Patel, D.J. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell 2011, 145, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Morinière, J.; Rousseaux, S.; Steuerwald, U.; Soler-López, M.; Curtet, S.; Vitte, A.-L.; Govin, J.; Gaucher, J.; Sadoul, K.; Hart, D.J. Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature 2009, 461, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-W.; Wang, Z.; Yiu, T.T.; Akdemir, K.C.; Xia, W.; Winter, S.; Tsai, C.-Y.; Shi, X.; Schwarzer, D.; Plunkett, W. TRIM24 links a non-canonical histone signature to breast cancer. Nature 2010, 468, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Cho, S.; Zeng, L.; Zhang, Q.; Kaehlcke, K.; Mak, L.; Lau, J.; Bisgrove, D.; Schnölzer, M.; Verdin, E. Two-pronged binding with bromodomain-containing protein 4 liberates positive transcription elongation factor b from inactive ribonucleoprotein complexes. J. Biol. Chem. 2012, 287, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Zeng, L.; Wu, Y.; Deng, J.; Zhang, Q.; Lin, Y.; Li, J.; Kang, T.; Tao, M. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 2014, 25, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, X.; Diaz-Trelles, R.; Ruiz-Lozano, P.; Wang, Z. Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Dev. Biol. 2008, 319, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.; Gebuhr, T.; Yee, D.; La Mantia, C.; Nicholson, J.; Gilliam, A.; Randazzo, F.; Metzger, D.; Chambon, P.; Crabtree, G. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell 2000, 6, 1287–1295. [Google Scholar] [CrossRef]
- Reyes, J.; Barra, J.; Muchardt, C.; Camus, A.; Babinet, C.; Yaniv, M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2α). EMBO J. 1998, 17, 6979–6991. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Popowicz, G.M.; Krajewski, M.; Holak, T.A. Structural Ramification for Acetyl-Lysine Recognition by the Bromodomain of Human BRG1 Protein, a Central ATPase of the SWI/SNF Remodeling Complex. ChemBioChem 2007, 8, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Graumann, J.; Wu, G.; Araúzo-Bravo, M.J.; Han, D.W.; Greber, B.; Gentile, L.; Mann, M.; Schöler, H.R. Chromatin-remodeling components of the BAF complex facilitate reprogramming. Cell 2010, 141, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.; Khochbin, S. Histone acylation beyond acetylation: Terra incognita in chromatin biology. Cell J. 2015, 17, 1. [Google Scholar] [PubMed]
- Chen, Y.; Sprung, R.; Tang, Y.; Ball, H.; Sangras, B.; Kim, S.C.; Falck, J.R.; Peng, J.; Gu, W.; Zhao, Y. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteom. 2007, 6, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Luo, H.; Lee, S.; Jin, F.; Yang, J.S.; Montellier, E.; Buchou, T.; Cheng, Z.; Rousseaux, S.; Rajagopal, N. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 2011, 146, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Tang, Z.; Huang, H.; Yong-Gonzalez, V.; Molina, H.; Kong, H.E.; Dai, L.; Shimada, M.; Cross, J.R.; Zhao, Y. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 2015, 58, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, A.; Zhang, D.; Huang, H.; Barral, S.; Kwon, O.K.; Qi, S.; Tang, Z.; Buchou, T.; Vitte, A.-L.; He, T. Dynamic competing histone H4 K5K8 acetylation and butyrylation are hallmarks of highly active gene promoters. Mol. Cell 2016, 62, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Bowser, R.; Giambrone, A.; Davies, P. FAC1, a novel gene identified with the monoclonal antibody Alz50, is developmentally regulated in human brain (Part 1 of 2). Dev. Neurosci. 1995, 17, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Banting, G.S.; Barak, O.; Ames, T.M.; Burnham, A.C.; Kardel, M.D.; Cooch, N.S.; Davidson, C.E.; Godbout, R.; McDermid, H.E.; Shiekhattar, R. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum. Mol. Genet. 2005, 14, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Fairbridge, N.A.; Dawe, C.E.; Niri, F.H.; Kooistra, M.K.; King-Jones, K.; McDermid, H.E. Cecr2 mutations causing exencephaly trigger misregulation of mesenchymal/ectodermal transcription factors. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Herrema, H.; Salazar, M.; Cakir, I.; Cabi, S.; Sahin, F.B.; Chiu, Y.-H.; Cantley, L.C.; Ozcan, U. BRD7 regulates XBP1s’ activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 2014, 20, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, M.D.; Aslanian, A.; Dong, M.-Q.; Yates, J.R.; Emerson, B.M. BRD7, a novel PBAF-specific SWI/SNF subunit, is required for target gene activation and repression in embryonic stem cells. J. Biol. Chem. 2008, 283, 32254–32263. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, P.M.; Chambers, A.L.; Cloney, R.; Bianchi, A.; Downs, J.A. BAF180 promotes cohesion and prevents genome instability and aneuploidy. Cell Rep. 2014, 6, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Saare, M.; Hämarik, U.; Venta, R.; Panarina, M.; Zucchelli, C.; Pihlap, M.; Remm, A.; Kisand, K.; Toots, U.; Möll, K. SP140L, an evolutionarily recent member of the SP100 family, is an autoantigen in primary biliary cirrhosis. J. Immunol. Res. 2015, 2015, 526518. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.E.; Smogorzewska, A.; Elledge, S.J. Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proc. Natl. Acad. Sci. USA 2010, 107, 14280–14285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Schmitz, K.-M.; Mayer, C.; Yuan, X.; Akhtar, A.; Grummt, I. Reversible acetylation of the chromatin remodelling complex NoRC is required for non-coding RNA-dependent silencing. Nat. Cell Biol. 2009, 11, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Poot, R.A.; Kukimoto, I.; García-Jiménez, C.; Dellaire, G.; Varga-Weisz, P.D. An ACF1–ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat. Genet. 2002, 32, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Li, H.; Shechter, D.; Ahn, S.H.; Fabrizio, L.A.; Erdjument-Bromage, H.; Ishibe-Murakami, S.; Wang, B.; Tempst, P.; Hofmann, K. WSTF regulates the H2A. X DNA damage response via a novel tyrosine kinase activity. Nature 2009, 457, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kasper, L.H.; Fukuyama, T.; Biesen, M.A.; Boussouar, F.; Tong, C.; De Pauw, A.; Murray, P.J.; van Deursen, J.M.; Brindle, P.K. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol. Cell. Biol. 2006, 26, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Kuttenkeuler, D.; Gesellchen, V.; Zeidler, M.P.; Boutros, M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 2005, 436, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Y.; Selleck, W.; Lane, W.S.; Tan, S.; Côté, J. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell. Biol. 2004, 24, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Krebs, A.R.; Karmodiya, K.; Lindahl-Allen, M.; Struhl, K.; Tora, L. SAGA and ATAC histone acetyl transferase complexes regulate distinct sets of genes and ATAC defines a class of p300-independent enhancers. Mol. Cell 2011, 44, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Milne, T.A.; Briggs, S.D.; Brock, H.W.; Martin, M.E.; Gibbs, D.; Allis, C.D.; Hess, J.L. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 2002, 10, 1107–1117. [Google Scholar] [CrossRef]
- Feng, Y.; Vlassis, A.; Roques, C.; Lalonde, M.E.; González-Aguilera, C.; Lambert, J.P.; Lee, S.B.; Zhao, X.; Alabert, C.; Johansen, J.V. BRPF3-HBO1 regulates replication origin activation and histone H3K14 acetylation. EMBO J. 2016, 35, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.J.; Muthurajan, U.M.; Lalonde, M.-E.; Gibson, M.D.; Andrews, F.H.; Hepler, M.; Machida, S.; Yan, K.; Kurumizaka, H.; Poirier, M.G. Bivalent interaction of the PZP domain of BRPF1 with the nucleosome impacts chromatin dynamics and acetylation. Nucleic Acids Res. 2016, 44, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Obri, A.; Ouararhni, K.; Papin, C.; Diebold, M.-L.; Padmanabhan, K.; Marek, M.; Stoll, I.; Roy, L.; Reilly, P.T.; Mak, T.W. ANP32E is a histone chaperone that removes H2A. Z from chromatin. Nature 2014, 505, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, H.; Xi, Y.; Tanaka, K.; Wang, H.; Peng, D.; Ren, Y.; Jin, Q.; Dent, S.Y.; Li, W. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell 2014, 159, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Andrews, F.H.; Shinsky, S.A.; Shanle, E.K.; Bridgers, J.B.; Gest, A.; Tsun, I.K.; Krajewski, K.; Shi, X.; Strahl, B.D.; Kutateladze, T.G. The Taf14 YEATS domain is a reader of histone crotonylation. Nat. Chem. Biol. 2016, 12, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.-P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Müller, S.; Pawson, T. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 2012, 149, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ilin, S.; Wang, W.; Duncan, E.M.; Wysocka, J.; Allis, C.D.; Patel, D.J. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 2006, 442, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Wang, Z.; Zaromytidou, A.-I.; Zhang, X.H.-F.; Chow-Tsang, L.-F.; Liu, J.X.; Kim, H.; Barlas, A.; Manova-Todorova, K.; Kaartinen, V. A poised chromatin platform for TGF-β access to master regulators. Cell 2011, 147, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Cress, W.D.; Seto, E. Histone deacetylases, transcriptional control, and cancer. J. Cell Physiol. 2000, 184, 1–16. [Google Scholar] [CrossRef]
- Zhou, D.-C.; Kim, S.H.; Ding, W.; Schultz, C.; Warrell, R.P.; Gallagher, R.E. Frequent mutations in the ligand-binding domain of PML-RARα after multiple relapses of acute promyelocytic leukemia: Analysis for functional relationship to response to all-transretinoic acid and histone deacetylase inhibitors in vitro and in vivo. Blood 2002, 99, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Minucci, S.; Nervi, C.; Coco, F.L.; Pelicci, P.G. Histone deacetylases: A common molecular target for differentiation treatment of acute myeloid leukemias? Oncogene 2001, 20, 3110. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.F.; Fazi, F.; Bianchini, A.; Padula, F.; Gelmetti, V.; Minucci, S.; Mancini, M.; Pelicci, P.G.; Coco, F.L.; Nervi, C. Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 2001, 61, 2–7. [Google Scholar] [PubMed]
- Kitamura, K.; Hoshi, S.; Koike, M.; Kiyoi, H.; Saito, H.; Naoe, T. Histone deacetylase inhibitor but not arsenic trioxide differentiates acute promyelocytic leukaemia cells with t (11; 17) in combination with all-trans retinoic acid. Br. J. Haematol. 2000, 108, 696–702. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Alland, L.; Hong, S.-H.; Wong, C.-W.; DePinho, R.A.; Dejean, A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene 1998, 16, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Bjerling, P.; Silverstein, R.A.; Thon, G.; Caudy, A.; Grewal, S.; Ekwall, K. Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol. Cell. Biol. 2002, 22, 2170–2181. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Sideris, M.L.; Polly, M.; Lorimer, D.D.; Mcintosh, B.; Clark, J.M. Cloning and characterization of a novel human histone deacetylase, HDAC8. Biochem. J. 2000, 350, 199–205. [Google Scholar]
- Galasinski, S.C.; Resing, K.A.; Goodrich, J.A.; Ahn, N.G. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J. Biol. Chem. 2002, 277, 19618–19626. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Lee, C.; Hou, T.; Boldogh, I.; Brasier, A.R. Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic Acids Res. 2008, 36, 4510–4520. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Seto, E. Unlocking the mechanisms of transcription factor YY1: Are chromatin modifying enzymes the key? Gene 1999, 236, 197–208. [Google Scholar] [CrossRef]
- Gobinet, J.; Carascossa, S.; Cavaillès, V.; Vignon, F.; Nicolas, J.-C.; Jalaguier, S. SHP represses transcriptional activity via recruitment of histone deacetylases. Biochemistry 2005, 44, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Watamoto, K.; Towatari, M.; Ozawa, Y.; Miyata, Y.; Okamoto, M.; Abe, A.; Naoe, T.; Saito, H. Altered interaction of HDAC5 with GATA-1 during MEL cell differentiation. Oncogene 2003, 22, 9176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Yang, Y.-C. Sumoylation and acetylation play opposite roles in the transactivation of PLAG1 and PLAGL2. J. Biol. Chem. 2005, 280, 40773–40781. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, M.; Heldin, C.-H.; Ericsson, J.; Grönroos, E. The balance between acetylation and deacetylation controls Smad7 stability. J. Biol. Chem. 2005, 280, 21797–21803. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Xie, Y.-B.; Choi, H.-S. Transcriptional corepressor SHP recruits SIRT1 histone deacetylase to inhibit LRH-1 transactivation. Nucleic Acids Res. 2010, 38, 4607–4619. [Google Scholar] [CrossRef] [PubMed]
- Wade, P.A. Transcriptional control at regulatory checkpoints by histone deacetylases: Molecular connections between cancer and chromatin. Hum. Mol. Genet. 2001, 10, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Finnin, M.S.; Donigian, J.R.; Cohen, A.; Richon, V.M.; Rifkind, R.A.; Marks, P.A.; Breslow, R.; Pavletich, N.P. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 1999, 401, 188–193. [Google Scholar] [PubMed]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar] [PubMed]
- Cameron, E.E.; Bachman, K.E.; Myöhänen, S.; Herman, J.G.; Baylin, S.B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 1999, 21, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.-F.; Yao, T.-P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Juan, L.-J.; Shia, W.-J.; Chen, M.-H.; Yang, W.-M.; Seto, E.; Lin, Y.-S.; Wu, C.-W. Histone deacetylases specifically down-regulate p53-dependent gene activation. J. Biol. Chem. 2000, 275, 20436–20443. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Sharif, J.; Muto, M.; Takebayashi, S.-I.; Suetake, I.; Iwamatsu, A.; Endo, T.A.; Shinga, J.; Mizutani-Koseki, Y.; Toyoda, T.; Okamura, K. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 2007, 450, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.; Hermann, A.; Pradhan, S.; Jeltsch, A. The activity of the murine DNA methyltransferase Dnmt1 is controlled by interaction of the catalytic domain with the N-terminal part of the enzyme leading to an allosteric activation of the enzyme after binding to methylated DNA. J. Mol. Biol. 2001, 309, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Rathert, P.; Laser, H.; Gowher, H.; Jeltsch, A. Phosphorylation of serine-515 activates the Mammalian maintenance methyltransferase Dnmt1. Epigenetics 2007, 2, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Bourc’his, D.; Bestor, T.H. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 2004, 431, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Okano, M.; Hata, K.; Sado, T.; Tsujimoto, N.; Li, E.; Sasaki, H. Essential role for de novo DNA methyltransferase DNMT3A in paternal and maternal imprinting. Nature 2004, 429, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Kareta, M.S.; Botello, Z.M.; Ennis, J.J.; Chou, C.; Chédin, F. Reconstitution and mechanism of the stimulation of de novo methylation by human DNMT3L. J. Biol. Chem. 2006, 281, 25893–25902. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Mann, J.R.; Hsieh, C.L.; Riggs, A.D.; Chédin, F. Physical and functional interactions between the human DNMT3L protein and members of the de novo methyltransferase family. J. Cell Biochem. 2005, 95, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Schubert, H.L.; Blumenthal, R.M.; Cheng, X. Many paths to methyltransfer: A chronicle of convergence. Trends Biochem. Sci. 2003, 28, 329–335. [Google Scholar] [CrossRef]
- Gowher, H.; Liebert, K.; Hermann, A.; Xu, G.; Jeltsch, A. Mechanism of stimulation of catalytic activity of DNMT3A and DNMT3B DNA-(cytosine-C5)-methyltransferases by DNMT3L. J. Biol. Chem. 2005, 280, 13341–13348. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Jurkowska, R.Z.; Zhang, X.; Jeltsch, A.; Cheng, X. Structure of DNMT3A bound to DNMT3L suggests a model for de novo DNA methylation. Nature 2007, 449, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Chédin, F.; Lieber, M.R.; Hsieh, C.-L. The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by DNMT3A. Proc. Natl. Acad. Sci. USA 2002, 99, 16916–16921. [Google Scholar] [CrossRef] [PubMed]
- Klimasauskas, S.; Kumar, S.; Roberts, R.J.; Cheng, X. Hhal methyltransferase flips its target base out of the DNA helix. Cell 1994, 76, 357–369. [Google Scholar] [CrossRef]
- Meissner, A.; Mikkelsen, T.S.; Gu, H.; Wernig, M.; Hanna, J.; Sivachenko, A.; Zhang, X.; Bernstein, B.E.; Nusbaum, C.; Jaffe, D.B. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 2008, 454, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.K.; Qiu, C.; Bernstein, E.; Li, K.; Jia, D.; Yang, Z.; Erdjument-Bromage, H.; Tempst, P.; Lin, S.-P.; Allis, C.D. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 2007, 448, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, D.N.; Su, H.; Hevi, S.; Gay, F.; Lei, H.; Bajko, J.; Xu, G.; Li, E.; Chen, T. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature 2009, 461, 415. [Google Scholar] [CrossRef] [PubMed]
- Otani, J.; Nankumo, T.; Arita, K.; Inamoto, S.; Ariyoshi, M.; Shirakawa, M. Structural basis for recognition of H3K4 methylation status by the DNA methyltransferase 3A ATRX–DNMT3–DNMT3L domain. EMBO Rep. 2009, 10, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Dhayalan, A.; Rajavelu, A.; Rathert, P.; Tamas, R.; Jurkowska, R.Z.; Ragozin, S.; Jeltsch, A. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J. Biol. Chem. 2010, 285, 26114–26120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jurkowska, R.; Soeroes, S.; Rajavelu, A.; Dhayalan, A.; Bock, I.; Rathert, P.; Brandt, O.; Reinhardt, R.; Fischle, W. Chromatin methylation activity of DNMT3A and DNMT3A/3L is guided by interaction of the ADD domain with the histone H3 tail. Nucleic Acids Res. 2010, 38, 4246–4253. [Google Scholar] [CrossRef] [PubMed]
- Hodges, E.; Smith, A.D.; Kendall, J.; Xuan, Z.; Ravi, K.; Rooks, M.; Zhang, M.Q.; Ye, K.; Bhattacharjee, A.; Brizuela, L. High definition profiling of mammalian DNA methylation by array capture and single molecule bisulfite sequencing. Genome Res. 2009, 19, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Liang, G.; Sharma, S.; Lin, J.C.; Choi, S.H.; Han, H.; Yoo, C.B.; Egger, G.; Yang, A.S.; Jones, P.A. Selective anchoring of DNA methyltransferases 3A and 3B to nucleosomes containing methylated DNA. Mol. Cell. Biol. 2009, 29, 5366–5376. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Milne, T.A.; Tackett, A.J.; Smith, E.R.; Fukuda, A.; Wysocka, J.; Allis, C.D.; Chait, B.T.; Hess, J.L.; Roeder, R.G. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell 2005, 121, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Lee, S.-K.; Lee, J.W. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol. Endocrinol. 2008, 22, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chun, J.; Wilson, J.R.; Walker, P.A. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature 2003, 421, 652. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, R.; Furukawa, Y.; Morita, M.; Iimura, Y.; Silva, F.P.; Li, M.; Yagyu, R.; Nakamura, Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat. Cell Biol. 2004, 6, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Feng, Q.; Ketel, C.S.; Wang, H.; Cao, R.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Simon, J.A.; Zhang, Y. Purification and functional characterization of SET8, a nucleosomal histone H4-lysine 20-specific methyltransferase. Curr. Biol. 2002, 12, 1086–1099. [Google Scholar] [CrossRef]
- Fuks, F.; Hurd, P.J.; Deplus, R.; Kouzarides, T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 2003, 31, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Jung, I.; Lee, H.; Kang, K.; Kim, M.; Jeong, K.; Kwon, C.S.; Han, Y.-M.; Kim, Y.S.; Kim, D. Human histone H3K79 methyltransferase DOT1L methyltransferase binds actively transcribing RNA polymerase II to regulate gene expression. J. Biol. Chem. 2012, 287, 39698–39709. [Google Scholar] [CrossRef] [PubMed]
- Viré, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.-M. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006, 439, 871. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sugimoto, K.; Nozaki, M.; Ueda, J.; Ohta, T.; Ohki, M.; Fukuda, M.; Takeda, N.; Niida, H.; Kato, H. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002, 16, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.C.; Ayyanathan, K.; Negorev, D.; Maul, G.G.; Rauscher, F.J. SETDB1: A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Branscombe, T.L.; Frankel, A.; Lee, J.-H.; Cook, J.R.; Yang, Z.-H.; Pestka, S.; Clarke, S. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J. Biol. Chem. 2001, 276, 32971–32976. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.M.; Dowhan, D.H.; Eriksson, N.A.; Muscat, G.E. CARM1/PRMT4 is necessary for the glycogen gene expression programme in skeletal muscle cells. Biochem. J. 2012, 444, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Frankel, A.; Cook, R.J.; Kim, S.; Paik, W.K.; Williams, K.R.; Clarke, S.; Herschman, H.R. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J. Biol. Chem. 2000, 275, 7723–7730. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.; Niveleau, A.; Walter, J.; Fundele, R.; Haaf, T. Embryogenesis: Demethylation of the zygotic paternal genome. Nature 2000, 403, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Taylor, S.M. Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980, 20, 85–93. [Google Scholar] [CrossRef]
- Morgan, H.D.; Santos, F.; Green, K.; Dean, W.; Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005, 14, R47–R58. [Google Scholar] [CrossRef] [PubMed]
- Bruniquel, D.; Schwartz, R.H. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat. Immunol. 2003, 4, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Jang, M.-H.; Guo, J.U.; Kitabatake, Y.; Chang, M.-L.; Pow-Anpongkul, N.; Flavell, R.A.; Lu, B.; Ming, G.-L.; Song, H. Neuronal activity–induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 2009, 323, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.K.; Bestor, T.H. The colorful history of active DNA demethylation. Cell 2008, 133, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zheng, Y.; Angliker, H.; Schwarz, S.; Thiry, S.; Siegmann, M.; Jost, J.-P. 5-Methylcytosine DNA glycosylase activity is also present in the human MBD4 (G/T mismatch glycosylase) and in a related avian sequence. Nucleic Acids Res. 2000, 28, 4157–4165. [Google Scholar] [CrossRef] [PubMed]
- Cortázar, D.; Kunz, C.; Saito, Y.; Steinacher, R.; Schär, P. The enigmatic thymine DNA glycosylase. DNA Repair 2007, 6, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.B.; Guy, J.; Sansom, O.J.; Selfridge, J.; MacDougall, E.; Hendrich, B.; Keightley, P.D.; Bishop, S.M.; Clarke, A.R.; Bird, A. Enhanced CpG mutability and tumorigenesis in MBD4-deficient mice. Science 2002, 297, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kondo, T.; Takada, I.; Youn, M.-Y.; Yamamoto, Y.; Takahashi, S.; Matsumoto, T.; Fujiyama, S.; Shirode, Y.; Yamaoka, I. DNA demethylation in hormone-induced transcriptional derepression. Nature 2009, 461, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.D.; Dean, W.; Coker, H.A.; Reik, W.; Petersen-Mahrt, S.K. Activation-induced cytidine deaminase deaminates 5-Methylcytosine in DNA and is expressed in pluripotent tissues implications for epigenetic reprogramming. J. Biol. Chem. 2004, 279, 52353–52360. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Huggins, I.J.; James, S.R.; Karpf, A.R.; Jones, D.A.; Cairns, B.R. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell 2008, 135, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.; Dean, W.; Feng, S.; Cokus, S.J.; Andrews, S.; Pellegrini, M.; Jacobsen, S.E.; Reik, W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 2010, 463, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, N.; Brady, J.J.; Damian, M.; Sacco, A.; Corbel, S.Y.; Blau, H.M. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 2010, 463, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef]
- Barreto, G.; Schäfer, A.; Marhold, J.; Stach, D.; Swaminathan, S.K.; Handa, V.; Döderlein, G.; Maltry, N.; Wu, W.; Lyko, F. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007, 445, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-G.; Guo, C.; Pfeifer, G.P. GADD45A does not promote DNA demethylation. PLoS Genet. 2008, 4, e1000013. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Tront, J.S.; Erinle, T.; Nguyen, N.; Latham, K.E.; Sapienza, C.; Hoffman, B.; Liebermann, D.A. Conserved DNA methylation in Gadd45a−/− mice. Epigenetics 2009, 4, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.-M.; Schmitt, N.; Hoffmann-Rohrer, U.; Schäfer, A.; Grummt, I.; Mayer, C. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol. Cell 2009, 33, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.V.; Cummings, A.; Teebor, G.W. 5-Hydroxymethylcytosine DNA glycosylase activity in mammalian tissue. Biochem. Biophys. Res. Commun. 1988, 151, 1173–1179. [Google Scholar] [CrossRef]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.-H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Chen, Y.; Zhao, Y.; Bruick, R.K. JMJD6 is a histone arginine demethylase. Science 2007, 318, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Webby, C.J.; Wolf, A.; Gromak, N.; Dreger, M.; Kramer, H.; Kessler, B.; Nielsen, M.L.; Schmitz, C.; Butler, D.S.; Yates, J.R. JMJD6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science 2009, 325, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Schmitz, C.; Böttger, A. Changing story of the receptor for phosphatidylserine-dependent clearance of apoptotic cells. EMBO Rep. 2007, 8, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.R.; Armstrong, M.I.; Flemington, E.K. CpG methylation as a mechanism for the regulation of E2F activity. Proc. Natl. Acad. Sci. USA 2000, 97, 6481–6486. [Google Scholar] [CrossRef] [PubMed]
- Iguchi-Ariga, S.; Schaffner, W. CpG methylation of the cAMP-responsive enhancer/promoter sequence TGACGTCA abolishes specific factor binding as well as transcriptional activation. Genes Dev. 1989, 3, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Veenstra, G.C.J.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [PubMed]
- Ballestar, E.; Paz, M.F.; Valle, L.; Wei, S.; Fraga, M.F.; Espada, J.; Cigudosa, J.C.; Huang, T.H.M.; Esteller, M. Methyl-CpG binding proteins identify novel sites of epigenetic inactivation in human cancer. EMBO J. 2003, 22, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Meehan, R.R.; Bird, A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993, 21, 4886–4892. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, B.; Bird, A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 1998, 18, 6538–6547. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.F.; Ben-Porath, I.; Bird, A.P. Mbd1 is recruited to both methylated and nonmethylated CpGs via distinct DNA binding domains. Mol. Cell. Biol. 2004, 24, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Esteve, P.-O.; Chin, H.G.; Samaranayke, M.; Kim, G.-D.; Pradhan, S. CXXC domain of human DNMT1 is essential for enzymatic activity. Biochemistry 2008, 47, 10000–10009. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ishikawa, F. The mCpG-binding domain of human MBD3 does not bind to mCpG but interacts with NuRD/Mi2 components HDAC1 and MTA2. J. Biol. Chem. 2002, 277, 35434–35439. [Google Scholar] [CrossRef] [PubMed]
- Wade, P.A.; Gegonne, A.; Jones, P.L.; Ballestar, E.; Aubry, F.; Wolffe, A.P. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat. Genet. 1999, 23, 62–66. [Google Scholar] [PubMed]
- Fraga, M.F.; Ballestar, E.; Montoya, G.; Taysavang, P.; Wade, P.A.; Esteller, M. The affinity of different MBD proteins for a specific methylated locus depends on their intrinsic binding properties. Nucleic Acids Res. 2003, 31, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Sarraf, S.A.; Schmiedeberg, L.; McDermott, S.M.; Stancheva, I.; Bird, A.P. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol. Cell 2005, 19, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Arita, K.; Ariyoshi, M.; Tochio, H.; Nakamura, Y.; Shirakawa, M. Recognition of hemi-methylated DNA by the SRA protein UHRF1 by a base-flipping mechanism. Nature 2008, 455, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Avvakumov, G.V.; Walker, J.R.; Xue, S.; Li, Y.; Duan, S.; Bronner, C.; Arrowsmith, C.H.; Dhe-Paganon, S. Structural basis for recognition of hemi-methylated DNA by the SRA domain of human UHRF1. Nature 2008, 455, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.M.; Spring, C.M.; Crawford, H.C.; Reynolds, A.B.; Baig, A. The p120ctn-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002, 30, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Filion, G.J.; Zhenilo, S.; Salozhin, S.; Yamada, D.; Prokhortchouk, E.; Defossez, P.-A. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell. Biol. 2006, 26, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Prokhortchouk, A.; Hendrich, B.; Jørgensen, H.; Ruzov, A.; Wilm, M.; Georgiev, G.; Bird, A.; Prokhortchouk, E. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 2001, 15, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.R.; Pontes, O.; Pikaard, C.S.; Richards, E.J. VIM1, a methylcytosine-binding protein required for centromeric heterochromatinization. Genes Dev. 2007, 21, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kokura, K.; Kaul, S.C.; Wadhwa, R.; Nomura, T.; Khan, M.M.; Shinagawa, T.; Yasukawa, T.; Colmenares, C.; Ishii, S. The Ski protein family is required for MeCP2-mediated transcriptional repression. J. Biol. Chem. 2001, 276, 34115–34121. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, L.E.; Caldenhoven, E.; Stunnenberg, H.G. In vivo repression of an erythroid-specific gene by distinct corepressor complexes. EMBO J. 2002, 21, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Fuks, F.; Hurd, P.J.; Wolf, D.; Nan, X.; Bird, A.P.; Kouzarides, T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 2003, 278, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, K.; Chow, M.Z.; Baker, E.K.; Pal, S.; Bassal, S.; Brasacchio, D.; Wang, L.; Craig, J.M.; Jones, P.L.; Sif, S. Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nat. Genet. 2005, 37, 254–264. [Google Scholar] [PubMed]
- Agarwal, N.; Hardt, T.; Brero, A.; Nowak, D.; Rothbauer, U.; Becker, A.; Leonhardt, H.; Cardoso, M.C. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res. 2007, 35, 5402–5408. [Google Scholar] [CrossRef] [PubMed]
- Ballas, N.; Grunseich, C.; Lu, D.D.; Speh, J.C.; Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 2005, 121, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Dintilhac, A.; Bernués, J. HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences. J. Biol. Chem. 2002, 277, 7021–7028. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Shiota, K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. J. Biol. Chem. 2003, 278, 4806–4812. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Hou, J.; Maclean, A.; Nasir, J.; Lafuente, M.J.; Shu, X.; Kriaucionis, S.; Bird, A. Interaction between chromatin proteins MECP2 and ATRX is disrupted by mutations that cause inherited mental retardation. Proc. Natl. Acad. Sci. USA 2007, 104, 2709–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.I.; Hong, E.P.; Castle, J.C.; Crespo-Barreto, J.; Bowman, A.B.; Rose, M.F.; Kang, D.; Richman, R.; Johnson, J.M.; Berget, S. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl. Acad. Sci. USA 2005, 102, 17551–17558. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Watanabe, S.; Ichimura, T.; Tsuruzoe, S.; Shinkai, Y.; Tachibana, M.; Chiba, T.; Nakao, M. Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression. J. Biol. Chem. 2003, 278, 24132–24138. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ichimura, T.; Fujita, N.; Tsuruzoe, S.; Ohki, I.; Shirakawa, M.; Kawasuji, M.; Nakao, M. Methylated DNA-binding domain 1 and methylpurine–DNA glycosylase link transcriptional repression and DNA repair in chromatin. Proc. Natl. Acad. Sci. USA 2003, 100, 12859–12864. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.; Morey, L.; Raker, V.A.; Buschbeck, M.; Gutierrez, A.; De Santis, F.; Corsaro, M.; Varas, F.; Bossi, D.; Minucci, S. The methyl-CpG binding protein MBD1 is required for PML-RARα function. Proc. Natl. Acad. Sci. USA 2006, 103, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Reese, B.E.; Bachman, K.E.; Baylin, S.B.; Rountree, M.R. The methyl-CpG binding protein MBD1 interacts with the p150 subunit of chromatin assembly factor 1. Mol. Cell. Biol. 2003, 23, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- Fukushige, S.; Kondo, E.; Gu, Z.; Suzuki, H.; Horii, A. RET finger protein enhances MBD2-and MBD4-dependent transcriptional repression. Biochem. Biophys. Res. Commun. 2006, 351, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Le Menuet, D.; Chung, A.C.-K.; Cooney, A.J. Differential recruitment of methylated CpG binding domains by the orphan receptor GCNF initiates the repression and silencing of Oct4 expression. Mol. Cell. Biol. 2006, 26, 9471–9483. [Google Scholar] [CrossRef] [PubMed]
- Tatematsu, K.I.; Yamazaki, T.; Ishikawa, F. MBD2-MBD3 complex binds to hemi-methylated DNA and forms a complex containing DNMT1 at the replication foci in late S phase. Genes Cells 2000, 5, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Angrisano, T.; Lembo, F.; Pero, R.; Natale, F.; Fusco, A.; Avvedimento, V.E.; Bruni, C.B.; Chiariotti, L. TACC3 mediates the association of MBD2 with histone acetyltransferases and relieves transcriptional repression of methylated promoters. Nucleic Acids Res. 2006, 34, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ego, T.; Tanaka, Y.; Shimotohno, K. Interaction of HTLV-1 Tax and methyl-CpG-binding domain 2 positively regulates the gene expression from the hypermethylated LTR. Oncogene 2005, 24, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Boeke, J.; Ammerpohl, O.; Kegel, S.; Moehren, U.; Renkawitz, R. The minimal repression domain of MBD2b overlaps with the methyl-CpG-binding domain and binds directly to Sin3A. J. Biol. Chem. 2000, 275, 34963–34967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ng, H.-H.; Erdjument-Bromage, H.; Tempst, P.; Bird, A.; Reinberg, D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999, 13, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.M.; Dai, Y.-S.; Kim, Y.; Kim, J.; Kimlin, L.; Gao, K.; Wong, D.T. Cdk2ap1 is required for epigenetic silencing of Oct4 during murine embryonic stem cell differentiation. J. Biol. Chem. 2009, 284, 6043–6047. [Google Scholar] [CrossRef] [PubMed]
- Le Guezennec, X.; Vermeulen, M.; Brinkman, A.B.; Hoeijmakers, W.A.; Cohen, A.; Lasonder, E.; Stunnenberg, H.G. MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties. Mol. Cell. Biol. 2006, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; Cicchillitti, L.; Schepis, F.; Riccio, A.; Yeung, A.T.; Matsumoto, Y.; Golemis, E.A.; Genuardi, M.; Neri, G. MED1, a novel human methyl-CpG-binding endonuclease, interacts with DNA mismatch repair protein MLH1. Proc. Natl. Acad. Sci. USA 1999, 96, 3969–3974. [Google Scholar] [CrossRef] [PubMed]
- Screaton, R.A.; Kiessling, S.; Sansom, O.J.; Millar, C.B.; Maddison, K.; Bird, A.; Clarke, A.R.; Frisch, S.M. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: A potential link between genome surveillance and apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E.; Gu, Z.; Horii, A.; Fukushige, S. The thymine DNA glycosylase MBD4 represses transcription and is associated with methylated p16INK4a and hMLH1 genes. Mol. Cell. Biol. 2005, 25, 4388–4396. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-G.; Chan, D.W.; Reynolds, A.B.; Qin, J.; Wong, J. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol. Cell 2003, 12, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.M.; Reynolds, A.B. The catenin p120 ctn interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol. Cell. Biol. 1999, 19, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Ruzov, A.; Hackett, J.A.; Prokhortchouk, A.; Reddington, J.P.; Madej, M.J.; Dunican, D.S.; Prokhortchouk, E.; Pennings, S.; Meehan, R.R. The interaction of xKaiso with xTcf3: A revised model for integration of epigenetic and Wnt signalling pathways. Development 2009, 136, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, J.E.; Costa, M. Epigenetics and the environment. Ann. N. Y. Acad. Sci. 2003, 983, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, C.L.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. The impact of metabolism on DNA methylation. Hum. Mol. Genet. 2005, 14, R139–R147. [Google Scholar] [CrossRef] [PubMed]
- Zaina, S.; Lindholm, M.W.; Lund, G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: More than just hyperhomocysteinemia? J. Nutr. 2005, 135, 5–8. [Google Scholar] [PubMed]
- Friso, S.; Choi, S.-W. Gene-nutrient interactions and DNA methylation. J. Nutr. 2002, 132, 2382S–2387S. [Google Scholar] [PubMed]
- Hiltunen, M.O.; Turunen, M.P.; Häkkinen, T.P.; Rutanen, J.; Hedman, M.; Mäkinen, K.; Turunen, A.-M.; Aalto-Setalä, K.; Ylä-Herttuala, S. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc. Med. 2002, 7, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Luch, A. Nature and nurture–lessons from chemical carcinogenesis. Nat. Rev. Cancer 2005, 5, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Ergul, E.; Sazci, A.; Utkan, Z.; Canturk, N.Z. Polymorphisms in the MTHFR gene are associated with breast cancer. Tumor Biol. 2004, 24, 286–290. [Google Scholar] [CrossRef]
- Le Marchand, L.; Haiman, C.A.; Wilkens, L.R.; Kolonel, L.N.; Henderson, B.E. MTHFR polymorphisms, diet, HRT, and breast cancer risk: The multiethnic cohort study. Cancer Epidemiol. Prev. Biomark. 2004, 13, 2071–2077. [Google Scholar]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases DNMT3A and DNMT3B are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Klose, R.; Bird, A. MeCP2 repression goes nonglobal. Science 2003, 302, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Hattori, D.; Wu, H.; Fouse, S.; He, F.; Hu, Y.; Fan, G.; Sun, Y.E. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 2003, 302, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, C.; Stanley, J.A.S.; Garrick, D.; Sharpe, J.A.; Ayyub, H.; Wood, W.G.; Higgs, D.R. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat. Genet. 2003, 34, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.F.; Loda, M.; Gaida, G.M.; Lipman, J.; Mishra, R.; Goldman, H.; Jessup, J.M.; Kolodner, R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997, 57, 808–811. [Google Scholar] [PubMed]
- Suter, C.M.; Martin, D.I.; Ward, R.L. Germline epimutation of MLH1 in individuals with multiple cancers. Nat. Genet. 2004, 36, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [PubMed]
- Jones, P.A.; Laird, P.W. Cancer-epigenetics comes of age. Nat. Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Hake, S.; Xiao, A.; Allis, C. Linking the epigenetic ‘language’of covalent histone modifications to cancer. Br. J. Cancer 2004, 90, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Grignani, F.; De Matteis, S.; Nervi, C.; Tomassoni, L.; Gelmetti, V.; Cioce, M.; Fanelli, M.; Ruthardt, M.; Ferrara, F.F.; Zamir, I. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature 1998, 391, 815–818. [Google Scholar] [PubMed]
- Jones, L.K.; Saha, V. Chromatin modification, leukaemia and implications for therapy. Br. J. Haematol. 2002, 118, 714–727. [Google Scholar] [PubMed]
- Roberts, C.W.; Orkin, S.H. The SWI/SNF complex—Chromatin and cancer. Nat. Rev. Cancer 2004, 4, 133–142. [Google Scholar] [CrossRef] [PubMed]
- He, L.-Z.; Tolentino, T.; Grayson, P.; Zhong, S.; Warrell, R.P.; Rifkind, R.A.; Marks, P.A.; Richon, V.M.; Pandolfi, P.P. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J. Clin. Investig. 2001, 108, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Sandor, V.; Bakke, S.; Robey, R.W.; Kang, M.H.; Blagosklonny, M.V.; Bender, J.; Brooks, R.; Piekarz, R.L.; Tucker, E.; Figg, W.D. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res. 2002, 8, 718–728. [Google Scholar] [PubMed]
- Butler, L.M.; Webb, Y.; Agus, D.B.; Higgins, B.; Tolentino, T.R.; Kutko, M.C.; LaQuaglia, M.P.; Drobnjak, M.; Cordon-Cardo, C.; Scher, H.I. Inhibition of transformed cell growth and induction of cellular differentiation by pyroxamide, an inhibitor of histone deacetylase. Clin. Cancer Res. 2001, 7, 962–970. [Google Scholar] [PubMed]
- Vigushin, D.M.; Ali, S.; Pace, P.E.; Mirsaidi, N.; Ito, K.; Adcock, I.; Coombes, R.C. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res. 2001, 7, 971–976. [Google Scholar] [PubMed]
- Nervi, C.; Borello, U.; Fazi, F.; Buffa, V.; Pelicci, P.G.; Cossu, G. Inhibition of histone deacetylase activity by trichostatin A modulates gene expression during mouse embryogenesis without apparent toxicity. Cancer Res. 2001, 61, 1247–1249. [Google Scholar] [PubMed]
- Coffey, D.C.; Kutko, M.C.; Glick, R.D.; Butler, L.M.; Heller, G.; Rifkind, R.A.; Marks, P.A.; Richon, V.M.; La Quaglia, M.P. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 2001, 61, 3591–3594. [Google Scholar] [PubMed]
- Constantinides, P.G.; Jones, P.A.; Gevers, W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature 1977, 267, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Matsen, C.B.; Gonzales, F.A.; Ye, W.; Greer, S.; Marquez, V.E.; Jones, P.A.; Selker, E.U. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J. Natl. Cancer Inst. 2003, 95, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Asgari, K.; Putzi, M.J.; Gage, W.R.; Yu, X.; Cornblatt, B.S.; Kumar, A.; Piantadosi, S.; DeWeese, T.L.; De Marzo, A.M. Reversal of GSTP1 CpG island hypermethylation and reactivation of π-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamide. Cancer Res. 2001, 61, 8611–8616. [Google Scholar] [PubMed]
- Duvic, M.; Dummer, R.; Becker, J.C.; Poulalhon, N.; Romero, P.O.; Bernengo, M.G.; Lebbé, C.; Assaf, C.; Squier, M.; Williams, D. Panobinostat activity in both bexarotene-exposed and-naive patients with refractory cutaneous T-cell lymphoma: Results of a phase II trial. Eur. J. Cancer 2013, 49, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Vose, J.M.; Harb, W.; Gordon, L.I.; Langdon, R.; Grant, B.; Sprague, J.; Plasencia, C.; Sirisawad, M.; Yue, J. A phase II multicenter study of the histone deacetylase inhibitor (HDACi) abexinostat (PCI-24781) in relapsed/refractory follicular lymphoma (FL) and mantle cell lymphoma (MCL). Am. Soc. Hematol. 2012, 120, 55. [Google Scholar]
- Giaccone, G.; Rajan, A.; Berman, A.; Kelly, R.J.; Szabo, E.; Lopez-Chavez, A.; Trepel, J.; Lee, M.-J.; Cao, L.; Espinoza-Delgado, I. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J. Clin. Oncol. 2011, 29, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Ning, Z.-Q.; Xing, P.-Y.; Xu, J.-L.; Cao, H.-X.; Dou, G.-F.; Meng, Z.-Y.; Shi, Y.-K.; Lu, X.-P.; Feng, F.-Y. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother. Pharmacol. 2012, 69, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Gore, L.; Rothenberg, M.L.; O’Bryant, C.L.; Schultz, M.K.; Sandler, A.B.; Coffin, D.; McCoy, C.; Schott, A.; Scholz, C.; Eckhardt, S.G. A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin. Cancer Res. 2008, 14, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Dellacasa, C.M.; Finazzi, G.; Carobbio, A.; Ferrari, M.L.; Guglielmelli, P.; Gattoni, E.; Salmoiraghi, S.; Finazzi, M.C.; Di Tollo, S. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br. J. Haematol. 2010, 150, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Oki, Y.; Bociek, R.G.; Kuruvilla, J.; Fanale, M.; Neelapu, S.; Copeland, A.; Buglio, D.; Galal, A.; Besterman, J. Mocetinostat for relapsed classical Hodgkin's lymphoma: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2011, 12, 1222–1228. [Google Scholar] [CrossRef]
- Razak, A.; Hotte, S.; Siu, L.; Chen, E.; Hirte, H.; Powers, J.; Walsh, W.; Stayner, L.; Laughlin, A.; Novotny-Diermayr, V. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2011, 104, 756. [Google Scholar] [CrossRef] [PubMed]
- Child, F.; Romero, P.O.; Alvarez, R.; Bagot, M.; Stadler, R.; Weichenthal, M.; Alves, R.; Bernengo, M.G.; Beylot-Barry, M.; Cowan, R. Phase 2 Multicenter trial of oral quisinostat, a histone deacetylase inhibitor, in patients with previously treated stage IB-IVA cutaneous T-cell lymphoma. Br. J. Dermatol. 2016, 175, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Santini, V.; Finelli, C.; Musto, P.; Pogliani, E.; Angelucci, E.; Fioritoni, G.; Alimena, G.; Maurillo, L.; Cortelezzi, A. Valproic acid at therapeutic plasma levels may increase 5-azacytidine efficacy in higher risk myelodysplastic syndromes. Clin. Cancer Res. 2009, 15, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.W.; Loaiza-Bonilla, A.; Juckett, M.; DiPersio, J.F.; Roy, V.; Slack, J.; Wu, W.; Laumann, K.; Espinoza-Delgado, I.; Gore, S.D. A phase 2 study of vorinostat in acute myeloid leukemia. Haematologica 2009, 94, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, C.C.; Liu, Z.; Bowers, M.A.; Porcu, P.; Flynn, J.M.; Christian, B.; Baiocchi, R.A.; Benson, D.M.; Andritsos, L.A.; Greenfield, C.N. Phase I Study of AR-42 in Relapsed Multiple Myeloma and Lymphoma. Blood 2012, 120, 2955. [Google Scholar]
- Banerji, U.; van Doorn, L.; Papadatos-Pastos, D.; Kristeleit, R.; Debnam, P.; Tall, M.; Stewart, A.; Raynaud, F.; Garrett, M.D.; Toal, M. A phase I pharmacokinetic and pharmacodynamic study of CHR-3996, an oral class I selective histone deacetylase inhibitor in refractory solid tumors. Clin. Cancer Res. 2012, 18, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Wijermans, P.; Lübbert, M.; Verhoef, G.; Klimek, V.; Bosly, A. An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2′-deoxycytidine (decitabine) in 177 patients. Ann. Hematol. 2005, 84, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Klisovic, R.B.; Stock, W.; Cataland, S.; Klisovic, M.I.; Liu, S.; Blum, W.; Green, M.; Odenike, O.; Godley, L.; Burgt, J.V. A phase I biological study of MG98, an oligodeoxynucleotide antisense to DNA methyltransferase 1, in patients with high-risk myelodysplasia and acute myeloid leukemia. Clin. Cancer Res. 2008, 14, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Burchenal, J.H.; Holmberg, E.A.; Fox, J.J.; Hemphill, S.C.; Reppert, J.A. The effects of 5-fluorodeoxycytidine, 5-fluorodeoxyuridine, and related compounds on transplanted mouse leukemias. Cancer Res. 1959, 19, 494–500. [Google Scholar] [PubMed]
- Yoo, C.; Cheng, J.; Jones, P. Zebularine: A New Drug for Epigenetic Therapy; Portland Press Limited: London, UK, 2004. [Google Scholar]
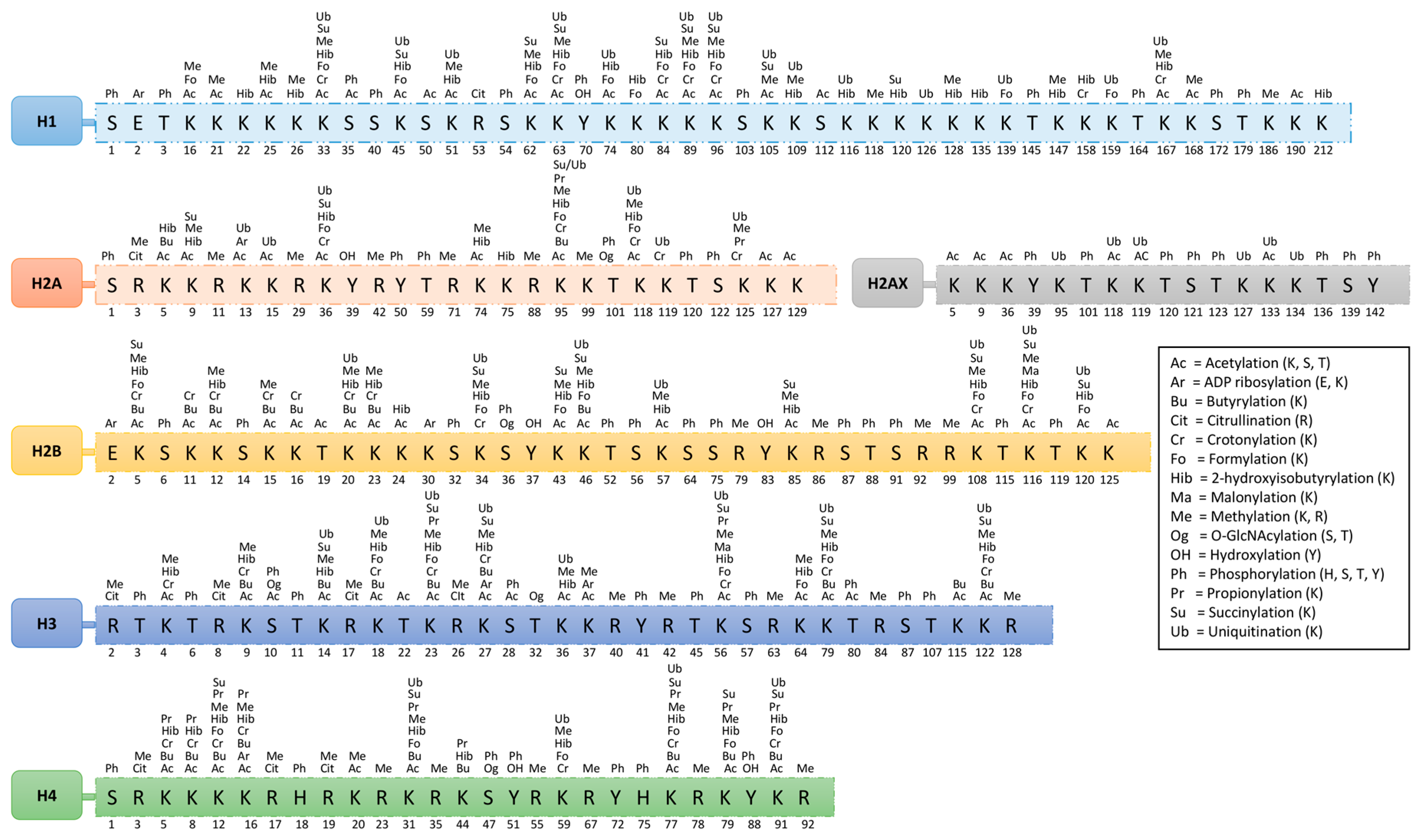

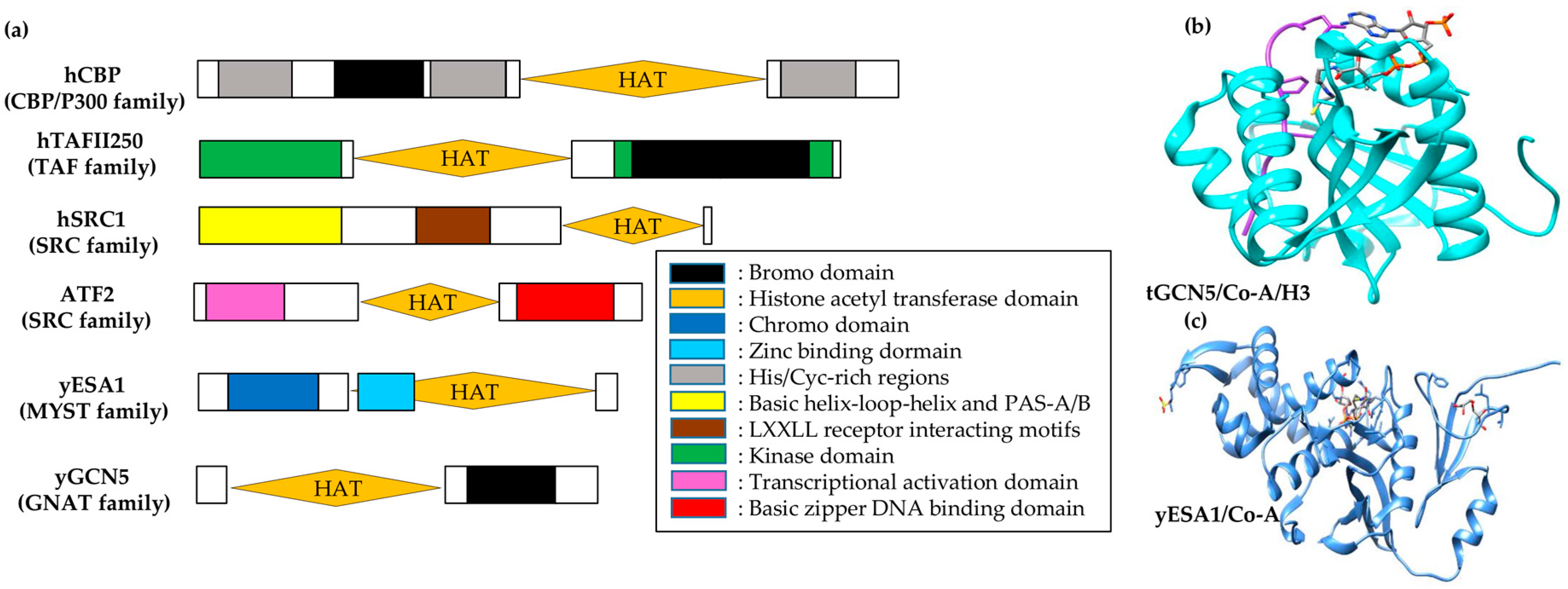

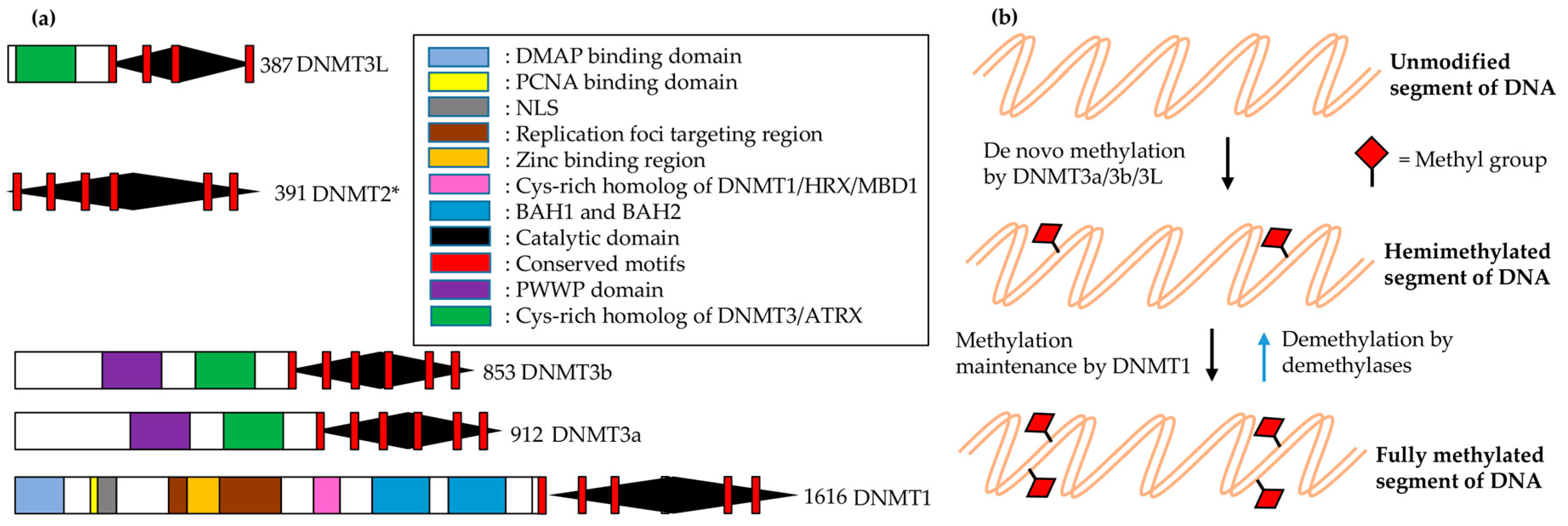
| Modification (Short Form) | Function | Target Residue/s | Reference |
|---|---|---|---|
| Methylation (me) | Repair, transcription | K-me1, K-me2, K-me3 | [34,35] |
| Transcription | R-me1, R-me2a, R-me2s | [36] | |
| Acetylation (ac) | Condensation, repair, transcription, replication | K-ac | [37,38,39] |
| Phosphorylation (ph) | Repair, transcription, condensation | T-ph, S-ph | [40,41] |
| Sumoylation (su) | Transcription, repair | K-su | [42,43] |
| Ubiquitylation (ub) | Repair, transcription | K-ub | [44,45] |
| ADP ribosylation (ar) | Transcription, repair, replication | E-ar | [46,47,48] |
| Proline isomerization | Transcription | P-cis > P-trans | [49] |
| Deimination | Transcription | R > Cit | [50] |
| HAT Family | Proteins | Enzyme | Organism | Orthologs | Substrate | Function | References |
|---|---|---|---|---|---|---|---|
| GNAT | PCAF | HAT A | Human | Mouse, chicken, lizard, zebrafish | H3 (nu) | Coactivator | [70] |
| GCN5 | HAT A | Human, yeast | Mouse, chicken, lizard, African clawed frog, zebrafish | H2A (nu), H2B (nu/free), H3 (K14), H4 (K8, K16) (nu), Sin1p, All core (nu) | Coactivator | [71] | |
| HAT1 | HAT B | Human | - | H4 (K12, K5) (free) | Acetylation of soluble histones | [72] | |
| HPA2 | Yeast | H3, H4 | Chromatin regulator, transferase | [73] | |||
| ELP3 | - | H4, H3, H2B, H2A | Transcription elongation | [74] | |||
| MYST | MOZ | Human | Mouse, chicken, lizard, zebrafish | Leukemogenesis | [75] | ||
| ESA1 | HAT | Yeast | H2A, H3, H4 (free) | Cell cycle progression | [62] | ||
| SAS3 (NuA3) | Yeast | H3 | Silencing | [63] | |||
| SAS2 | Yeast | Unknown | Silencing | [63] | |||
| HBO1 (KAT7) | Human | Mouse, chicken, lizard, African clawed frog, zebrafish | H3, H4 | Origin recognition interaction | [76] | ||
| MOF (KAT8/MSL) | Fruit fly | Mouse, lizard, African clawed frog, zebrafish | H2A, H3, H4 | Dosage compensation | [77] | ||
| TIP60 (KAT5) | HAT A | Human | Mouse, chicken, lizard, African clawed frog, zebrafish | H2A, H3, H4 | HIV TAT interaction | [78] | |
| P300/CBP | CBP (CREBBP) | HAT A | Humans, worms | - | H4 (K5, 9, 12, 16), all core (nu), p53 (K373, 382, peptide) TFIIF, TFIIEβ | Global coactivator | [79] |
| P300 | HAT A | - | Mouse, chicken, lizard, zebrafish | - | - | [79] | |
| Basal transcription | TAF1 (TAFII-250) | HAT A | Humans | Mouse, chicken, lizard, African clawed frog, zebrafish | TFIIEβ, H4 (free), H3 (K14) | Transcription initiation | [80] |
| TFC3 | HAT A | - | - | H2A, H3, H4 | - | [81] | |
| NUT1 | HAT A | Yeast | H3, H4 | Transcription mediator | [82] | ||
| SRC | NCOA3 (SRC-3) | HAT A | Humans | - | H4 (nu), H3 | Steroid receptor coactivators | [83] |
| NCOA1 (SRC-1) | HAT A | Human | Mouse, chicken, lizard, zebrafish | H4 (nu), H3 (K9, 14, peptide) | Steroid and nuclear hormone coactivator | [83] | |
| NCOA2 (SRC-2) | - | Mouse, chicken, lizard, African clawed frog, zebrafish | - | [84] | |||
| GRIP1 | - | Mouse, chicken, lizard, zebrafish | Trafficking and organization of transmembrane proteins | [85] | |||
| ATF2 (CREB2) | - | Mouse, chicken, lizard, African clawed frog, zebrafish | DNA sequence specific binding activator | [86] |
| Subfamily | Proteins | No. of BRDs | Other Domains | Function | Localization | Reference |
|---|---|---|---|---|---|---|
| I | PCAF | 1 | PCAF_nGNAT | HAT | Nu | [87] |
| GCN5L2 | 1 | PCAF_nGNAT | HAT | Nu | [87] | |
| FALZ/BPTF | 1 | WSD, PHD, WHIM1, DDT | Transcription factor | Nu | [88] | |
| CECR2 | 1 | - | Chromatin remodeler | Nu | [89] | |
| II | BAZ1A | 1 | PHD, WSD, WHIM1, DDT, WAC_Acf1_DNA_bd | Chromatin remodeler | Nu | [90] |
| BRDT | 2 | CTM, ET | Transcription regulator, chromatin remodeler, spermatogenesis | Nu | [91] | |
| BRD4 | 2 | CTM, ET | Transcription regulator, chromatin remodeler | Nu | [92] | |
| BRD3 | 2 | ET | Transcription regulator, erythropoiesis | Nu | [93] | |
| BRD2 | 2 | ET | Transcription regulator | Nu | [93] | |
| III | PHIP | 2 | WD40 | Insulin signaling | Nu | [94] |
| BRWD3 | 2 | WD40 | JAK-STAT signaling | Nu, Cyt | [95] | |
| BAZ1B | 1 | PHD, WSD, WHIM1, WAC_Acf1_DNA_bd | Spermatogenesis, tyrosine kinase, transcription regulator, chromatin remodeler | Nu | [59,96,97] | |
| BRD8 | 2 | - | Transcription regulator | Nu | [98,99] | |
| EP300 | 1 | CREB binding, ZZ, HAT, DUF902, KIX, zf-TAZ | HAT | Nu | [100] | |
| CREBBP | 1 | Same as above | HAT | Nu | [100] | |
| WDR9 | 2 | WD40 | Chromatin remodeler | Nu | [101,102] | |
| IV | BRD9 | 1 | DUF3512 | Component of SWI/SNF | Nu, Cyt | [103] |
| BRD7 | 1 | DUF3512 | Transcription regulator | Nu | [104] | |
| BRPF3 | 1 | PWWP, PHD-like, PHD, EPL1 | Same as above | Nu | [105] | |
| BRPF1 | 1 | Same as above | Same as above | Nu, Cyt | [106] | |
| BRD1 | 1 | Same as above | Same as above | Nu, Cyt | [107] | |
| ATAD2B | 1 | AAA | Same as above | Nu | [108] | |
| ATAD2 | 1 | AAA | Same as above | Nu | [109,110,111,112] | |
| V | BAZ2B | 1 | PHD, WSD, WHIM1, DDT, MBD | Unknown | Nu, Cyt | [113] |
| BAZ2A | 1 | Same as above | Transcription repressor | Nu, Cyt | [114] | |
| TRIM66 | 1 | PHD, zf-B_box | Same as above | Nu | [115] | |
| TRIM33 | 1 | PHD, zf-B_box, zf-RING | Transcription elongation, ligase (ubiquitin) | Nu | [116,117] | |
| TRIM24 | 1 | PHD, zf-B_box | Transcription regulator | Nu, Cyt | [118] | |
| SP110 | 1 | SAND, HSR | Same as above | Nu | [119] | |
| SP100 | 1 | SAND, HSR, HMG_box | Same as above | Nu, Cyt | [120] | |
| SP140L | 1 | PHD, SAND, HSR | Unknown | Nu | [121] | |
| SP140 | 1 | SAND, HSR | Transcription regulator | Nu, Cyt | [122] | |
| VI | TRIM28 | 1 | zf-B_box, zf-RING | Transcription regulator, ligase (E3 SUMO) | Nu | [123] |
| MLL | 1 | SET, FYRN, PHD-like, PHD, zf-CXXC | Methyltransferase (histone) | Nu | [124,125] | |
| VII | TAF1L | 2 | zf-CCHC_6, DUF3591, TBP- binding | Transcription initiation | Nu | [126] |
| TAF1 | 2 | Same as above | Transcription initiation, p53 transcription regulation | Nu | [127] | |
| ZMYND11 | 1 | PWWP | Transcription repressor | Nu | [128] | |
| ZMYND8 | 1 | PWWP, DUF3544 | Transcription regulator, DNA damage | Nu | [129] | |
| VIII | SMARCA4 | 1 | SnAC, Helicase_C, SNF2_N, BRK, HAS, QLQ | Chromatin remodeler | Nu | [130] |
| SMARCA2 | 1 | Same as above | Chromatin remodeler, splicing | Nu | [131] | |
| PB1 | 6 | HMG_box, BAH | Chromatin remodeler | Nu, Cyt | [132] | |
| ASH1L | 1 | BAH, SET | Methyl transferase | Nu, Cyt | [133] |
| Family | Class | Members | Tissue Distribution | Subcellular Localization | Catalytic Site | Substrates | References |
|---|---|---|---|---|---|---|---|
| Classic (Zn-dependent) | I | HDAC1 | Pervasive | Nucleus | 1 | STAT3, E2F1, MyoD, p53, SHP, androgen receptor | [149] |
| HDAC2 | Pervasive | Nucleus | 1 | STAT3, BCL6, YY1, glucocorticoid receptor | [150] | ||
| HDAC3 | Pervasive | Nucleus | 1 | MEF2D, STAT3, RELA, GATA1, YY1, SHP | [151] | ||
| HDAC8 | Pervasive? | Cytoplasm/nucleus | 1 | - | [151] | ||
| IIA | HDAC4 | Brain, skeletal muscle, heart | Cytoplasm/nucleus | 1 | HP1, GATA1, GCMA | [152] | |
| HDAC5 | Brain, skeletal muscle, heart | Cytoplasm/nucleus | 1 | HP1, SMAD7, GCMA | [152] | ||
| HDAC7 | Placenta, skeletal muscle, heart, pancreas | Cytoplasm/nucleus/mitochondria | 1 | PLAG2, PLAG1 | [153] | ||
| HDAC9 | Skeletal muscle, brain | Cytoplasm/nucleus | 1 | - | [153] | ||
| IIB | HDAC6 | Placenta, kidney, liver, heart | Cytoplasm (mostly) | 2 | SMAD7, SHP, HSP90, α-tubulin | [154] | |
| HDAC10 | Kidney, spleen, liver | Cytoplasm (mostly) | 1 | - | [151] | ||
| IV | HDAC11 | Kidney, skeletal muscle, heart, brain | Cytoplasm/nucleus | 2 | - | [151] | |
| Modern (NAD+-dependent) | III | Mammalian sirtuins (SIRT 1–7) | - | - | - | - | [155] |
| Yeast Sir2 | - | - | - | - | [155] |
| Family Name | Complex Member | Substrate | Function | References |
|---|---|---|---|---|
| Lysine-associated HMTs | ||||
| hSET1 | HCF1/ASH2/SET1 | H3 (K4) | Transcription activation | [185] |
| MLL4 | MENIN, SET1 | Same as above | Same as above | [186] |
| MLL1 | Same as above | Same as above | Cell proliferation, transcription activation | [185] |
| SET7/9 | Same as above | Silencing, transcription activation | [187] | |
| SMYD3 | Same as above | Same as above | [188] | |
| SET8 | H4 (K20) | Heterochromatin, cell cycle | [189] | |
| SUV39H1/2 | E2F1/4 | H3 (K9) | Heterochromatin, transcription repression | [190] |
| DOT1L | H3 (K79) | Silencing, transcription activation | [191] | |
| EZH2 | EDD-EZH2 | H3 (K9, 27) | Silencing, transcription repression | [192] |
| G9a | Same as above | Same as above | [193] | |
| SETDB1 | He (K9) | Silencing, heterochromatin | [194] | |
| Arginine-associated HMTs | ||||
| PRMT5 | Methylosome | SMN, H4, H2A | Heterochromatin, cell cycle | [195] |
| PRMT4 | NUMAC, P300, NCOA2, PCAF, AR | H3 (R1T, 26), PAB1, CBP, TARP | Transcription coactivation | [196] |
| PRMT1 | H4 (R3), HNRPA2B1, ETOILE, ILE3 | Transcription activation | [197] | |
| Protein Name | Interaction Partner | Function | References |
|---|---|---|---|
| MeCP2 | HDACs, SIN3a | Transcription repression | [229] |
| NCOR, c-SKI | Same as above | [246] | |
| HDAC2, Sin3B | Same as above | [247] | |
| Methyltransferase (H3K9) | Same as above | [248] | |
| BRM (SWI/SNF complex) | Same as above | [249] | |
| HP1 | Transcription repression during myogenic differentiation | [250] | |
| CoREST complex | Neural genes repression | [251] | |
| HMGB1 | Unknown | [252] | |
| DNMT1 | DNA methylation maintenance targeting | [253] | |
| ATRX | Neural development epigenetic regulation | [254] | |
| YB-1 | Alternative splicing | [255] | |
| CREB1 | Transcription activation | [256] | |
| MBD1 | SUV39H1-HP1 | Transcription repression | [257] |
| MPG | DNA repairing | [258] | |
| HDAC3, PML-RARα | Silencing | [259] | |
| CAF-1 p150, SETDB1, MCAF1, MCAF2 | Epigenetic inheritance, transcription repression | [260] | |
| MBD2 | RFP | Transcription repression enhancement | [261] |
| GCNF | Silencing of Oct-4 | [262] | |
| DNMT1 | DNA methylation maintenance | [263] | |
| pCAF, HATs, TACC3 | Transcription activation | [264] | |
| TAX | Same as above | [265] | |
| SIN3A | Transcription repression | [266] | |
| MEP50, PRMT5, DOC-1, RbAp46/48, HDAC1/2, P66α/β, MTA1-3, Mi-2 (NuRD complex) | Same as above | [267] | |
| MBD3 | GCNF, CDK2AP1 | Silencing of Oct-4 | [268] |
| Dnmt1 | DNA methylation maintenance | [263] | |
| MEP50, PRMT5, DOC-1, RbAp46/48, HDAC1/2, P66α/β, MTA1-3, Mi-2 (NuRD complex) | Transcription repression | [237,269] | |
| MBD4 | RFP | Transcription repression enhancement | [261] |
| MLH1 | DNA repair | [270] | |
| FADD | Apoptosis | [271] | |
| HDAC1, SIN3A | Transcription repression | [272] | |
| Kaiso | NCOR | Transcription repression | [273] |
| P120 | Wnt signaling | [274] | |
| TCF3 | Wnt signaling suppression | [275] |
| Name of Drug | Cancer Type | Phase | Reference |
|---|---|---|---|
| HDAC inhibitors | |||
| Panobinostat | Cutaneous T cell lymphoma | III | [305] |
| Abexinostat | Follicular lymphoma | II | [306] |
| Belinostat | Thymic malignancies | II | [307] |
| Chidamide | Solid lymphomas and tumors | II | [308] |
| Entinostat | Melanoma | II | [309] |
| Givinostat | Myeloproliferative neoplasms | II | [310] |
| Mocetinostat | B-cell malignancies | II | [311] |
| Practinostat | Prostate cancer | II | [312] |
| Quisinostat | Cutaneous T cell lymphoma | II | [313] |
| Valporate | Myelodysplasia | II | [314] |
| Vorinostat | Acute myeloid leukemia | II | [315] |
| AR-42 | Hematological malignancies | I | [316] |
| CHR-3996 | Solid tumors | I | [317] |
| Methylation inhibitors | |||
| 5-azacytidine | Myelodysplasia | III | [318] |
| 5-aza-2′-deoxycytidine (decitabine) | Myelodysplasia | III | [319] |
| MG98 | Leukemia | II | [320] |
| 5-Fluorodeoxycytidine | Leukemia | I | [321] |
| Zebularine | Acute myeloid leukemia | Preclinical | [322] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javaid, N.; Choi, S. Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets. Genes 2017, 8, 196. https://doi.org/10.3390/genes8080196
Javaid N, Choi S. Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets. Genes. 2017; 8(8):196. https://doi.org/10.3390/genes8080196
Chicago/Turabian StyleJavaid, Nasir, and Sangdun Choi. 2017. "Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets" Genes 8, no. 8: 196. https://doi.org/10.3390/genes8080196





