Satellite DNA: An Evolving Topic
Abstract
:1. Introduction
2. Changing Methods
3. Changing Concepts
3.1. Nucleotide Sequence Composition and Repeat Organization
3.2. Defining satellite DNA
3.3. Principle of the Equilocal Distribution of Heterochromatin
3.4. Satellite DNA Evolution
3.4.1. The “Library” Hypothesis
3.4.2. Concerted Evolution
3.4.3. Factors Influencing Satellite DNA Evolution
3.4.3.1. Chromosomal Location, Organization, and Repeat-Copy Number
3.4.3.2. Population and Evolutionary Factors
3.4.3.3. Biological Factors
3.4.3.4. Functional Constraints
3.5. Satellite DNA Function
3.5.1. Centromeres and Pericentromeric Heterochromain
3.5.2. Subtelomeric Heterochromatin
3.5.3. Heterochromatin Assembly
3.5.4. Gene Regulation
4. Concluding Remarks and Perspectives
Acknowledgments
Conflicts of Interest
References
- López-Flores, I.; Garrido-Ramos, M.A. The repetitive DNA content of eukaryotic genomes. Genome Dyn. 2012, 7, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, M.A.; Olmo, E.; Heslop-Harrison, J.S. Repetitive DNA in eukaryotic genomes. Chromosome Res. 2015, 23, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.R. Genome size evolution in animals. In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier: Burlington, NJ, USA, 2005; pp. 3–87. [Google Scholar]
- Jaillon, O. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 2004, 431, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.D.; Leitch, I.J. Genome size evolution in plants. In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier: Burlington, NJ, USA, 2005; pp. 89–162. [Google Scholar]
- Piegu, B.; Guyot, R.; Picault, N.; Roulin, A.; Saniyal, A.; Kim, H.; Collura, K.; Brar, D.S.; Jackson, S.; Win, R.A.; et al. Doubling genome size without polyploidization: Dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res. 2006, 16, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.T.; Pattyn, P.; Bakker, E.G.; Cao, J.; Cheng, J.-F.; Clark, R.M.; Fahlgren, N.; Fawcett, J.A.; Grimwood, J.; Gundlach, H.; et al. The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat. Genet. 2011, 43, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Piednoël, M.; Aberer, A.J.; Schneeweiss, G.M.; Macas, J.; Novak, P.; Gundlach, H.; Temsch, E.M.; Renner, S.S. Next-generation sequencing reveals the impact of repetitive DNA across phylogenetically closely related genomes of Orobanchaceae. Mol. Biol. Evol. 2012, 29, 3601–3611. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.H.C.; Grenier, J.K.; Barbash, D.A.; Clark, A.G. Correlated variation and population differentiation in satellite DNA abundance among lines of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2014, 111, 18793–18798. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Wakefield, M.J.; Aken, B.; Amemiya, C.T.; Chang, J.L.; Duke, S.; Garber, M.; Gentles, A.J.; Goodstadt, L.; Heger, A.; et al. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature 2007, 447, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Macas, J.; Neumann, P.; Navratilova, A. Repetitive DNA in the pea (Pisum sativum L.) genome: Comprehensive characterization using 454 sequencing and comparison to soybean and Medicago truncatula. BMC Genom. 2007, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruano, F.J.; López-León, M.D.; Cabrero, J.; Camacho, J.P.M. High-throughput analysis of the satellitome illuminates satellite DNA evolution. Sci. Rep. 2016, 6, 28333. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Sutton, G.; Ng, P.C.; Feuk, L.; Halpern, A.L.; Walenz, B.P.; Axelrod, N.; Huang, J.; Kirkness, E.F.; Denisov, G.; et al. The diploid genome sequence of an individual human. PLoS Biol. 2007, 5, e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miga, K.H. Completing the human genome: The progress and challenge of satellite DNA assembly. Chromosome Res. 2015, 23, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, N.; Plohl, M.; Mravinac, B.; Ugarković, D. Evolution of satellite DNAs from the genus Palorus-experimental evidence for the “library” hypothesis. Mol. Biol. Evol. 1998, 15, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Mravinac, B.; Plohl, M.; Meštrović, N.; Ugarković, D. Sequence of PRAT satellite DNA “frozen” in some Coleopteran species. J. Mol. Evol. 2002, 54, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Mravinac, B.; Plohl, M.; Ugarković, D. Preservation and high sequence conservation of satellite DNAs suggest functional constraints. J. Mol. Evol. 2005, 61, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Ugarković, D.; Podnar, M.; Plohl, M. Satellite DNA of the red flour beetle Tribolium castaneum-comparative study of satellites from the genus Tribolium. Mol. Biol. Evol. 1996, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Feliciello, I.; Chinali, G.; Ugarković, Đ. Structure and evolutionary dynamics of the major satellite in the red flour beetle Tribolium castaneum. Genetica 2011, 139, 999–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, L.; Venanzetti, F.; Sbordoni, V. Characterization of a species- specific satellite DNA family of Dolichopoda schiavazzii (Orthoptera, Rhaphidophoridae) cave crickets. J. Mol. Evol. 1994, 39, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, L.; Venanzetti, F.; Johnsen, A.; Sbordoni, V.; Bachmann, L. Molecular evolution of the pDo500 satellite DNA family in Dolichopoda cave crickets (Rhaphidophoridae). BMC Evol. Biol. 2009, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafasso, D.; Chinali, G. An ancient satellite DNA has maintained repetitive units of the original structure in most species of the living fossil plant genus Zamia. Genome 2014, 57, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Pérez, R.; de la Herrán, R.; Jamilena, M.; Lozano, R.; Ruiz Rejón, C.R.; Ruiz Rejón, M.; Garrido-Ramos, M.A. Reduced rates of sequence evolution of Y-linked satellite DNA in Rumex (Polygonaceae). J. Mol. Evol. 2005, 60, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Pérez, R.; Quesada del Bosque, M.E.; Garrido-Ramos, M.A. Effect of location, organization and repeat-copy number in satellite-DNA evolution. Mol. Genet. Gen. 2009, 282, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Pérez, R.; Schwarzacher, T.; Ruiz Rejón, M.; Garrido-Ramos, M.A. Characterization of RUSI, a telomere-associated satellite DNA, in the genus Rumex (Polygonaceae). Cytogenet. Genome Res. 2009, 124, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Pérez, R.; Schwarzacher, T.; Ruiz Rejón, M.; Garrido-Ramos, M.A. Molecular cytogenetic characterization of Rumex papillaris, a dioecious plant with an XX/XY1Y2 sex chromosome system. Genetica 2009, 135, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A.; de la Herran, R.; Ruiz Rejón, M.; Ruiz Rejón, C. A satellite DNA of the Sparidae family (Pisces, Perciformes) associated with telomeric sequences. Cytogenet. Cell Genet. 1998, 83, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A.; Jamilena, M.; Lozano, R.; Ruiz Rejón, C.; Ruiz Rejón, M. The EcoRI centromeric satellite DNA of the Sparidae family (Pisces, Perciformes) contains a sequence motive common to other vertebrate centromeric satellite DNAs. Cytogenet. Cell. Genet. 1995, 71, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A.; de la Herran, R.; Jamilena, M.; Lozano, R.; Ruiz Rejón, C.; Ruiz Rejón, M. Evolution of centromeric satellite-DNA and its use in phylogenetic studies of the Sparidae family (Pisces, Perciformes). Mol. Phyl. Evol. 1999, 12, 200–204. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán, R.; Ruiz Rejón, C.; Ruiz Rejón, M.; Garrido-Ramos, M.A. The molecular phylogeny of the Sparidae (Pisces, Perciformes) based on two satellite DNA families. Heredity 2001, 87, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Robles, F.; de la Herrán, R.; Ludwig, A.; Ruiz Rejón, C.; Ruiz Rejón, M.; Garrido-Ramos, M.A. Evolution of ancient satellite DNAs in sturgeon genomes. Gene 2004, 338, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Árnason, Ú.; Widegren, B. Pinniped phylogeny enlightened by molecular hybridizations using highly repetitive DNA. Mol. Biol. Evol. 1986, 3, 356–365. [Google Scholar]
- Arnason, U. Phylogeny of marine mammals-evidence from chromosomes and DNA. Chromosomes Today 1990, 10, 267–278. [Google Scholar]
- Arnason, U.; Grettarsdottir, S.; Widegren, B. Mysticete (baleen whale) relationships based upon the sequence of the common cetacean DNA satellite. Mol. Biol. Evol. 1992, 9, 1018–1028. [Google Scholar] [PubMed]
- Gretarsdottir, G.; Arnason, U. Evolution of the common cetacean highly repetitive DNA component and the systematic position of Orcaella brevirostris. J. Mol. Evol. 1992, 34, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sujiwattanarat, P.; Thapana, W.; Srikulnath, K.; Hirai, Y.; Hirai, H.; Koga, A. Higher-order repeat structure in alpha satellite DNA occurs in New World monkeys and is not confined to hominoids. Sci. Rep. 2015, 5, 10315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, S.; Goyal, V. Repetitive sequences in plant nuclear DNA: Types, Distribution, Evolution and Function. Genom. Proteom. Bioinform. 2014, 12, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Petraccioli, A.; Odierna, G.; Capriglione, T.; Barucca, M.; Forconi, M.; Olmo, E.; Biscotti, M.A. A novel satellite DNA isolated in Pecten jacobaeus shows high sequence similarity among mollusks. Mol. Genet. Genom. 2015, 290, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A. Satellite DNA in Plants: More than Just Rubbish. Cytogenet. Genome Res. 2015, 146, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Čížková, J.; Hřibová, A.; Humplíková, L.; Christelová, P.; Suchánková, P.; Doležel, J. Molecular analysis and genomic organization of major DNA satellites in banana (Musa spp.). PLoS ONE 2013, 8, e54808. [Google Scholar] [CrossRef] [PubMed]
- Hribová, E.; Neumann, P.; Matsumoto, T.; Roux, N.; Macas, J.; Dolezel, J. Repetitive part of the banana (Musa acuminata) genome investigated by low-depth 454 sequencing. BMC Plant Biol. 2010, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Macas, J.; Požárková, D.; Navrátilová, A.; Nouzová, M.; Neumann, P. Two new families of tandem repeats isolated from genus Vicia using genomic self-priming PCR. Mol. Gen. Genet. 2000, 263, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Ambrožová, K.; Mandáková, T.; Bureš, P.; Neumann, P.; Leitch, I.J.; Koblížková, A.; Macas, J.; Lysak, M.A. Diverse retrotransposon families and an AT-rich satellite DNA revealed in giant genomes of Fritillaria lilies. Ann. Bot. 2011, 107, 255–268. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán, R.; Robles, F.; Cuñado, N.; Santos, J.L.; Ruiz Rejón, M.; Garrido-Ramos, M.A.; Ruiz Rejón, C. A heterochromatic satellite DNA is highly amplified in a single chromosome of Muscari (Hyacinthaceae). Chromosoma 2001, 110, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Emadzade, K.; Jang, T.S.; Macas, J.; Kovařík, A.; Novák, P.; Parker, J.; Weiss-Schneeweiss, H. Differential amplification of satellite PaB6 in chromosomally hypervariable Prospero autumnale complex (Hyacinthaceae). Ann. Bot. 2014, 114, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Subirana, J.A.; Messeguer, X. A Satellite explosion in the genome of holocentric nematodes. PLoS ONE 2013, 8, e62221. [Google Scholar] [CrossRef] [PubMed]
- Subirana, J.A.; Albà, M.; Messeguer, X. High evolutionary turnover of satellite families in Caenorhabditis. BMC Evol. Biol. 2015, 15, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Ramos, M.A.; Jamilena, M.; Lozano, R.; Ruiz Rejón, C.; Ruiz Rejón, M. Cloning and characterization of a fish centromeric satellite DNA. Cytogenet. Cell Genet. 1994, 65, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kopecna, O.; Kubickova, S.; Cernohorska, H.; Cabelova, K.; Vahala, J.; Martinkova, N.; Rubes, J. Tribe-specific satellite DNA in non-domestic Bovidae. Chromosome Res. 2014, 22, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Steflova, P.; Tokan, V.; Vogel, I.; Lexa, M.; Macas, J.; Novák, P.; Hobza, R.; Vyskot, B.; Kejnovsky, E. Contrasting patterns of transposable element and satellite distribution on sex chromosomes (XY1Y2) in the dioecious plant Rumex acetosa. Genome Biol. Evol. 2013, 5, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.F. Highly rRepeated sequences in mammalian genomes. Int. Rev. Cytol. 1982, 76, 67–112. [Google Scholar] [PubMed]
- Kit, S. Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J. Mol. Biol. 1961, 3, 711–716. [Google Scholar] [CrossRef]
- Sueoka, N. Variation and heterogeneity of base composition of deoxyribonucleic acids: A compilation of old and new data. J. Mol. Biol. 1961, 3, 31–40. [Google Scholar] [CrossRef]
- Waring, M.; Britten, R.J. Nucleotide sequence repetition: A rapidly reassociating fraction of mouse DNA. Science 1966, 154, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.J.; Kohne, D.E. Repeated sequences in DNA. Science 1968, 161, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.J.; Graham, D.E.; Neufeld, B.R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974, 29, 363–405. [Google Scholar] [PubMed]
- Britten, R.J.; Davidson, E.H. Hybridisation strategy. In Nucleic Acid Hybridization; Hames, B.D., Higgins, S.J., Eds.; IRL Press: Washington, DC, USA, 1986; pp. 3–15. [Google Scholar]
- Peterson, D.G.; Schulze, S.R.; Sciara, E.B.; Lee, S.A.; Bowers, J.E.; Nagel, A.; Jiang, N.; Tibbitts, D.C.; Wessler, S.R.; Paterson, A.H. Integration of Cot Analysis, DNA Cloning, and High-Throughput sequencing facilitates genome characterization and gene discovery. Genome Res. 2002, 12, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Horz, W.; Zachau, H.G. Characterization of distinct segments in mouse satellite DNA by restriction nucleases. Eur. J. Biochem. 1977, 73, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A.; Jamilena, M.; Lozano, R.; Cárdenas, S.; Ruiz Rejón, C.; Ruiz Rejón, M. Phylogenetic relationships of the Sparidae family (Pisces, Perciformes) inferred from satellite DNA. Hereditas 1995, 122, 1–6. [Google Scholar] [CrossRef]
- Pardue, M.L.; Gall, J.G. Chromosomal localization of mouse satellite DNA. Science 1970, 168, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.G. The origin of in situ hybridization—A personal history. Methods 2016, 98, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Schwarzacher, T.; Heslop-Harrison, P. Practical In Situ Hybridization; BIOS Scientific Publishers Ltd.: Oxford, UK, 2000. [Google Scholar]
- Feliciello, I.; Picariello, O.; Chinali, G. The first characterisation of the overall variability of repetitive units in a species reveals unexpected features of satellite DNA. Gene 2005, 349, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Feliciello, I.; Picariello, O.; Chinali, G. Intra-specific variability and unusual organization of the repetitive units in a satellite DNA from Rana dalmatina: Molecular evidence of a new mechanism of DNA repair acting on satellite DNA. Gene 2006, 383, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada del Bosque, M.E.; López-Flores, I.; Suárez-Santiago, V.N.; Garrido-Ramos, M.A. Differential spreading of HinfI satellite DNA variants during radiation in Centaureinae. Ann. Bot. 2013, 112, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Luchetti, A.; Meštrović, N.; Mantovani, B. Satellite DNAs between selfishness and functionality: Structure, genomics and evolution of tandem repeats in centromeric (hetero)chromatin. Gene 2008, 409, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Meštrović, N.; Mravinac, B. Satellite DNA evolution. Genome Dyn. 2012, 7, 126–152. [Google Scholar] [PubMed]
- Plohl, M.; Meštrović, N.; Mravinac, B. Centromere identity from the DNA point of view. Chromosoma 2014, 123, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Pezer, Z.; Brajković, J.; Feliciello, I.; Ugarković, Đ. Satellite DNA-Mediated Effects on Genome Regulation. Genome Dyn. 2012, 7, 153–169. [Google Scholar] [PubMed]
- Weiss-Schneeweiss, H.; Leitch, A.R.; McCann, J.; Jang, T.S.; Macas, J. Employing next generation sequencing to explore the repeat landscape of the plant genome. In Next Generation Sequencing in Plant Systematics Regnum Vegetabile; Hörandl, E., Appelhans, M., Eds.; Koeltz Scientific Books: Königstein, Germany, 2015; pp. 155–179. [Google Scholar]
- Novák, P.; Neumann, P.; Macas, J. Graph-based clustering and characterization of repetitive sequences in next-generation sequencing data. BMC Bioinform. 2010, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Neumann, P.; Pech, J.; Steinhaisl, J.; Macas, J. RepeatExplorer: A Galaxy-based web server for genome-wide characterization of eukaryotic repetitive elements from next generation sequence reads. Bioinformatics 2013, 29, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Pagan, H.J.T.; Macas, J.; Novak, P.; McCulloch, E.S.; Stevens, R.D.; Ray, D.A. Survey sequencing reveals elevated DNA transposon activity, novel elements, and variation in repetitive landscapes among bats. Genome Biol. Evol. 2012, 4, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Melters, D.P.; Bradnam, K.R.; Young, H.A.; Telis, N.; May, M.R.; Ruby, J.G.; Sebra, R.; Peluso, P.; Eid, J.; Rank, D.; et al. Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution. Genome Biol. 2013, 14, R10. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M.; Ruiz-Ruano, F.J.; Martín-Blázquez, R.; López-León, M.D.; Cabrero, J.; Lorite, P.; Cabral-de-Mello, D.C.; Bakkali, M. A step to the gigantic genome of the desert locust: Chromosome sizes and repeated DNAs. Chromosoma 2015, 124, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Hribova, E.; Neumann, P.; Koblizkova, A.; Dolezel, J.; Macas, J. Genome-wide analysis of repeat diversity across the family Musaceae. PLoS ONE 2014, 9, e98918. [Google Scholar] [CrossRef] [PubMed]
- Pita, S.; Panzera, F.; Mora, P.; Vela, J.; Cuadrado, Á.; Sánchez, A.; Palomeque, T.; Lorite, P. Comparative repeatome analysis on Triatoma infestans Andean and Non-Andean lineages, main vector of Chagas disease. PLoS ONE 2017, 12, e0181635. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Ávila Robledillo, P.; Koblížková, A.; Vrbová, I.; Neumann, P.; Macas, J. TAREAN: A computational tool for identification and characterization of satellite DNA from unassembled short reads. Nucleic Acids Res. 2017, 45, e111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Oh, J.H.; McIver, L.J.; Rashkovetsky, E.; Michalak, K.; Garner, H.R.; Kang, L.; Nevo, E.; Korol, A.B.; Michalak, P. Divergence of Drosophila melanogaster repeatomes in response to a sharp microclimate contrast in Evolution Canyon, Israel. Proc. Natl. Acad. Sci. USA 2014, 111, 10630–10635. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.G.; Kwoh, C.K.; Hsu, Y.L.; Wirawan, A. Review of tandemrepeat search tools: A systematic approach to evaluating algorithmic performance. Brief. Bioinform. 2012, 14, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Monjo, T.; Hoang, P.H.; Yoshimura, J.; Yurino, H.; Mitsui, J.; Ishiura, H.; Takahashi, Y.; Ichikawa, Y.; Goto, J.; et al. Rapid detection of expanded short tandem repeats in personal genomics using hybrid sequencing. Bioinformatics 2014, 30, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Pavlek, M.; Gelfand, Y.; Plohl, M.; Meštrović, N. Genome wide analysis of tandem repeats in Tribolium castaneum genome reveals abundant and highly dynamic tandem repeat families with satellite DNA features in euchromatic chromosomal arms. DNA Res. 2015, 22, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, Y.; Rodriguez, A.; Benson, G. TRDB—The Tandem Repeats Database. Nucleic Acids Res. 2006, 35, D80–D87. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.; Igo-Kemenes, T.; Zachau, H.G. Nucleotide sequence of a highly repetitive component of rat DNA. Nucleic Acids Res. 1979, 7, 417–432. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán, R.; Fontana, F.; Lanfredi, M.; Congiu, L.; Leis, M.; Rossi, R.; Ruiz Rejón, C.; Ruiz Rejón, M.; Garrido-Ramos, M.A. Slow rates of evolution and sequence homogenization in an ancient satellite DNA family of sturgeons. Mol. Biol. Evol. 2001, 18, 432–436. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán, R.; Robles, F.; Navas, J.I.; López-Flores, I.; Herrera, M.; Hachero, I.; Garrido-Ramos, M.A.; Ruiz Rejón, C.; Ruiz Rejón, M. The centromeric satellite of the wedge sole (Dicologoglossa cuneata, Pleuronectiformes) is composed mainly of a sequence motif conserved in other vertebrate centromeric DNAs. Cytogenet. Genome Res. 2008, 121, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Macas, J.; Navrátilová, A.; Koblížková, A. Sequence homogenization and chromosomal localization of VicTR-B satellites differ between closely related Vicia species. Chromosoma 2006, 115, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Stupar, R.M.; Song, J.; Tek, A.L.; Cheng, Z.; Dong, F.; Jiang, J. Highly condensed potato pericentromeric heterochromatin contains rDNA-related tandem repeats. Genetics 2002, 162, 1435–1444. [Google Scholar] [PubMed]
- Willard, H.F.; Waye, J.S. Hierarchical order in chromosome-specific human alpha satellite DNA. Trends Genet. 1987, 3, 192–198. [Google Scholar] [CrossRef]
- Warburton, P.E.; Willard, H.F. Genomic analysis of sequence variation in tandemly repeated DNA. Evidence for localized homogeneous sequence domains within arrays of alpha-satellite DNA. J. Mol. Biol. 1990, 216, 3–16. [Google Scholar] [CrossRef]
- Miga, K.H.; Newton, Y.; Jain, M.; Altemose, N.; Willard, H.F.; Kent, W.J. Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res. 2014, 24, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Schueler, M.G.; Higgins, A.W.; Rudd, M.K.; Gustashaw, K.; Willard, H.F. Genomic and genetic definition of a functional human centromere. Science 2001, 294, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rudd, M.K.; Wray, G.A.; Willard, H.F. The evolutionary dynamics of alpha-satellite. Genome Res. 2006, 16, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Cacheux, L.; Ponger, L.; Gerbault-Seureau, M.; Richard, F.A.; Escudé, C. Diversity and distribution of alpha satellite DNA in the genome of an Old World monkey: Cercopithecus solatus. BMC Genom. 2016, 17, 916. [Google Scholar] [CrossRef] [PubMed]
- Shepelev, V.A.; Alexandrov, A.A.; Yurov, Y.B.; Alexandrov, I.A. The evolutionary origin of man can be traced in the layers of defunct ancestral alpha satellites flanking the active centromeres of human chromosome. PLoS Genet. 2009, 5, e1000641. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, I.A.; Mitkevich, S.P.; Yurov, Y.B. The phylogeny of human chromosome specific alpha satellites. Chromosoma 1988, 96, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Cellamare, A.; Catacchio, C.R.; Alkan, C.; Giannuzzi, G.; Antonacci, F.; Cardone, M.F.; Della Valle, G.; Malig, M.; Rocchi, M.; Eichler, E.E.; et al. New insights into centromere organization and evolution from the white-cheeked gibbon and marmoset. Mol. Biol. Evol. 2009, 26, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Hirai, Y.; Hirai, H.; Koga, A. Higher-order repeat structure in alpha satellite DNA is an attribute of hominoids rather than hominids. J. Hum. Genet. 2013, 58, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Koga, A.; Hirai, Y.; Terada, S.; Jahan, I.; Baicharoen, S.; Arsaithamkul, V.; Hirai, H. Evolutionary Origin of Higher-Order Repeat Structure in Alpha-Satellite DNA of Primate Centromeres. DNA Res. 2014, 21, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.M.; Carlisle, A.; Newell, C.; Hong, S.B.; Musich, P.R. Sequence and evolution of rhesus monkey alphoid DNA. J. Mol. Evol. 1986, 23, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Ventura, M.; Archidiacono, N.; Rocchi, M.; Sahinalp, S.C.; Eichler, E.E. Organization and evolution of primate centromeric DNA from whole-genome shotgun sequence data. PLoS Comput. Biol. 2007, 3, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.; Seuánez, H.N.; Fanning, T. Alpha satellite DNA in neotropical primates (Platyrrhini). Chromosoma 1994, 103, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Palomeque, T.; Lorite, P. Satellite DNA in insects—A review. Heredity 2008, 100, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Vlahović, I.; Glunčić, M.; Rosandić, M.; Ugarković, Đ.; Paar, V. Regular higher order repeat structures in beetle Tribolium castaneum genome. Genome Biol. Evol. 2016, evw174. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, N.; Mravinac, B.; Pavlek, M.; Vojvoda-Zeljko, T.; Šatović, E.; Plohl, M. Structural and functional liaisons between transposable elements and satellite DNAs. Chromosome Res. 2015, 23, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Cornudella, L. Characterization of a complex satellite DNA in the mollusc Donax trunculus: Analysis of sequence variations and divergence. Gene 1996, 169, 157–164. [Google Scholar] [CrossRef]
- López-Flores, I.; de la Herrán, R.; Garrido-Ramos, M.A.; Boudry, P.; Ruiz-Rejón, C.; Ruiz-Rejón, M. The molecular phylogeny of oysters based on a satellite DNA related to transposons. Gene 2004, 339, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Petrovic, V.; Luchetti, A.; Ricci, A.; Satovic, E.; Passamonti, M.; Mantovani, B. Long-term conservation vs high sequence divergence: The case of an extraordinarily old satellite DNA in bivalve mollusks. Heredity 2010, 104, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Macas, J.; Koblizkova, A.; Navratilova, A.; Neumann, P. Hypervariable 3′ UTR region of plant LTR-retrotransposons as a source of novel satellite repeats. Gene 2009, 448, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Šatović, E.; Plohl, M. Tandem repeat-containing MITEs in the clam Donax trunculus. Genome Biol. Evol. 2013, 5, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Satović, E.; Zeljko, T.V.; Luchetti, A.; Mantovani, B.; Plohl, M. Adjacent sequences disclose potential for intra-genomic dispersal of satellite DNA repeats and suggest a complex network with transposable elements. BMC Genom. 2016, 17, 997. [Google Scholar] [CrossRef] [PubMed]
- Schueler, M.G.; Dunn, J.M.; Bird, C.P.; Ross, M.T.; Viggiano, L.; NISC Comparative Sequencing Program; Rocchi, M.; Willard, H.F.; Green, E.D. Progressive proximal expansion of the primate X chromosome centromere. Proc. Natl. Acad. Sci. USA 2005, 102, 10563–10568. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Navrátilová, A.; Koblížková, A.; Kejnovský, E.; Hřibová, E.; Hobza, R.; Widmer, A.; Doležel, J.; Macas, J. Plant centromeric retrotransposons: A structural and cytogenetic perspective. Mob. DNA 2011, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhang, W.; Yang, Q.; Zhang, Y.; Han, B.; Gu, M.; Xue, Y.; Cheng, Z. Diversity of centromeric repeats in two closely related wild rice species, Oryza officinalis and Oryza rhizomatis. Mol. Genet. Genom. 2006, 275, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Choulet, F.; Heng, Y.; Hao, W.; Paux, P.; Liu, Z.; Yue, W.; Jin, W.; Feuillet, C.; Zhang, X. Wheat centromeric retrotransposons: The new ones take a major role in centromeric structure. Plant J. 2013, 73, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, K.; Neumann, P.; Zhang, D.F.; Ouyang, S.; Buell, C.R.; Cheng, Z.K.; Jiang, J.M. Structure, divergence, and distribution of the CRR centromeric retrotransposon family in rice. Mol. Biol. Evol. 2005, 22, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Khost, D.E.; Eickbush, D.G.; Larracuente, A.M. Single-molecule sequencing resolves the detailed structure of complex satellite DNA loci in Drosophila melanogaster. Genome Res. 2017, 27, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Friero, E.; de Bustos, A.; Jouve, N.; Cuadrado, A. Cytogenetic diversity of SSR motifs within and between Hordeum species carrying the H genome: H. vulgare L. and H. bulbosum L. Theor. Appl. Genet. 2013, 126, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Carmona, A.; Jouve, N. Chromosomal characterization of the three subgenomes in the polyploids of Hordeum murinum L.: New insight into the evolution of this complex. PLoS ONE 2013, 8, e81385. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Jouve, N. The nonrandom distribution of long clusters of all possible classes of trinucleotide repeats in barley chromosomes. Chromosome Res. 2007, 15, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Ananiev, E.V.; Chamberlin, M.A.; Klaiber, J.; Svitashev, S. Microsatellite megatracts in the maize (Zea mays L.) genome. Genome 2005, 48, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.; Frommert, M.; Paul, C.; Vincent, P.C. Sequence relationships of three human satellite DNAs. J. Mol. Riol. 1986, 187, 145–155. [Google Scholar] [CrossRef]
- Kuhn, G.S.C.; Küttler, H.; Moreira-Filho, O.; Heslop-Harrison, J.S. The 1.688 Repetitive DNA of Drosophila: Concerted Evolution at Different Genomic Scales and Association with Genes. Mol. Biol. Evol. 2012, 29, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Brajković, J.; Feliciello, I.; Bruvo-Mađarić, B.; Ugarković, Ð. Satellite DNA-like elements associated with genes within euchromatin of the beetle Tribolium castaneum. G3 Genes Genomes Genet. 2012, 2, 931. [Google Scholar] [CrossRef] [PubMed]
- Larracuente, A.M. The organization and evolution of the Responder satellite in species of the Drosophila melanogaster group: Dynamic evolution of a target of meiotic drive. BMC Evol. Biol. 2014, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Feliciello, I.; Akrap, I.; Ugarković, Đ. Satellite DNA Modulates Gene Expression in the Beetle Tribolium castaneum after Heat Stress. PLoS Genet 2015, 11, e1005466. [Google Scholar]
- De Lima, L.G.; Svartman, M.; Kuhn, G.C.S. Dissecting the satellite DNA landscape in three cactophilic Drosophila sequenced genomes. G3 Genes Genomes Genet. 2017. [Google Scholar] [CrossRef] [PubMed]
- John, B.; King, M.; Schweizer, D.; Mendelak, M. Equilocality of heterochromatin distribution and heterochromatin heterogeneity in acridid grasshoppers. Chromosoma 1985, 91, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Guenatri, M.; Bailly, D.; Maison, C.; Almouzni, G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J. Cell. Biol. 2004, 166, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, D.; Loidl, J. A model for heterochromatin dispersion and the evolution of C-band patterns. In Chromosomes Today; Hayman, D.L., Rofe, R.H., Sharp, P.J., Eds.; Allen and Unwin: London, UK, 1987; Volume 9, pp. 61–74. [Google Scholar]
- Cuadrado, A.; Jouve, N. Evolutionary trends of different repetitive DNA sequences during speciation in the genus Secale. J. Hered. 2002, 93, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, M.; Hamilton, B.; Mayr, B.; Schweizer, D. Meiotic chromosome behaviour reflects levels of sequence divergence in Sus scrofa domestica satellite DNA. Chromosoma 1990, 99, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 2002, 88, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Filatov, D.A.; Moneger, F.; Negrutiu, I.; Charlesworth, D. Low variability in a Y-linked plant gene and its implications for Y-chromosome evolution. Nature 2000, 404, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, M.; Steinemann, S. The enigma of the Y chromosome degeneration: TRAM, a novel retrotransposon is preferentially located on the neo-Y chromosome of Drosophila miranda. Genetics 1997, 145, 261–266. [Google Scholar] [PubMed]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, J.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Hobza, R.; Kubat, Z.; Cegan, R.; Jesionek, W.; Vyskot, B.; Kejnovsky, E. Impact of repetitive DNA on sex chromosome evolution in plants. Chromosome Res. 2015, 23, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Vyskot, B.; Hobza, R. The genomics of plant sex chromosomes. Plant Sci. 2015, 236, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, H.; Ross, L.; Bachtrog, D. Sex determination, sex chromosomes, and karyotype evolution in insects. J. Hered. 2017, 108, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Gimenez, O.M.; Dias, G.B.; de Lima, L.G.; Kuhn, G.C.E.S.; Ramos, É.; Martins, C.; Cabral-de-Mello, D.C. High-throughput analysis of the satellitome revealed enormous diversity of satellite DNAs in the neo-Y chromosome of the cricket Eneoptera surinamensis. Sci. Rep. 2017, 7, 6422. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D. Plant Sex Chromosomes. Annu. Rev. Plant Biol. 2016, 67, 397–420. [Google Scholar] [CrossRef] [PubMed]
- Shibata, F.; Hizume, M.; Kurori, Y. Chromosome painting of Y chromosomes and isolation of a Y chromosome-specific repetitive sequence in the dioecious plant Rumex acetosa. Chromosoma 1999, 108, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Shibata, F.; Hizume, M.; Kurori, Y. Differentiation and the polymorphic nature of the Y chromosomes revealed by repetitive sequences in the dioecious plant, Rumex acetosa. Chromosome Res. 2000, 8, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Pérez, R.; Schwarzacher, T.; de la Herrán, R.; Ruiz Rejón, C.; Ruiz Rejón, M.; Garrido-Ramos, M.A. The origin and evolution of the variability in a Y-specific satellite-DNA of Rumex acetosa and its relatives. Gene 2006, 368, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cuñado, N.; Navajas-Pérez, R.; de la Herrán, R.; Ruiz Rejón, C.; Ruiz Rejón, M.; Santos, J.L.; Garrido-Ramos, M.A. The evolution of sex chromosomes in the genus Rumex (Polygonaceae): Identification of a new species with heteromorphic sex chromosomes. Chromosome Res. 2007, 15, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, B.; Navajas-Pérez, R.; Lozano, R.; Parker, J.S.; de la Herrán, R.; Ruiz Rejón, C.; Ruiz Rejón, M.; Garrido-Ramos, M.A.; Jamilena, M. Cloning and characterization of dispersed repetitive DNA derived from microdissected sex chromosomes of Rumex acetosa. Genome 2006, 49, 114–121. [Google Scholar] [PubMed]
- Mariotti, B.; Manzano, S.; Kejnovský, E.; Vyskot, B.; Jamilena, M. Accumulation of Y-specific satellite DNAs during the evolution of Rumex acetosa sex chromosomes. Mol. Genet. Genom. 2009, 281, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kejnovský, E.; Michalovova, M.; Steflova, P.; Kejnovska, I.; Manzano, S.; Hobza, R.; Kubat, Z.; Kovarik, J.; Jamilena, M.; Vyskot, B. Expansion of microsatellites on evolutionary young Y chromosome. PLoS ONE 2013, 8, e45519. [Google Scholar] [CrossRef] [PubMed]
- Kubat, Z.; Hobza, R.; Vyskot, B.; Kejnovsky, E. Microsatellite accumulation on the Y chromosome in Silene latifolia. Genome 2008, 51, 350–356. [Google Scholar] [PubMed]
- Hobza, R.; Lengerova, M.; Svoboda, J.; Kubekova, H.; Kejnovsky, E.; Vyskot, B. An accumulation of tandem DNA repeats on the Y chromosome in Silene latifolia during early stages of sex chromosome evolution. Chromosoma 2006, 115, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Macas, J.; Kejnovský, E.; Neumann, P.; Novák, P.; Koblížková, A.; Vyskot, B. Next generation sequencing-based analysis of repetitive DNA in the model dioceous plant Silene latifolia. PLoS ONE 2011, 6, e27335. [Google Scholar] [CrossRef]
- Hobza, R.; Kubat, Z.; Cegan, R.; Jesione, W.; Kejnovsky, E.; Vyskot, B. Sex chromosome evolution in plants—Insight into the Y chromosome formation. Submitted.
- Klemme, S.; Banaei-Moghaddam, A.M.; Macas, J.; Wicker, T.; Novák, P.; Houben, A. High-copy sequences reveal distinct evolution of the rye B chromosome. New Phytol. 2013, 199, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M. B chromosomes. In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier: Burlington, NJ, USA, 2005; pp. 223–285. [Google Scholar]
- Perfectti, F.; Werren, J.H. The interspecific origin of B chromosomes: Experimental evidence. Evolution 2001, 55, 1069–1073. [Google Scholar] [CrossRef]
- Tosta, V.C.; Marthe, J.B.; Tavares, M.G.; Fernandes-Salomão, T.M.; Pompolo, S.G.; Recco-Pimentel, S.M.; Perfectti, F.; Campos, L.A.; Camacho, J.P. Possible introgression of B chromosomes between bee species (genus Partamona). Cytogenet. Genome Res. 2014, 144, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruano, F.J.; Cabrero, J.; López-León, M.D.; Camacho, J.P.M. Satellite DNA content illuminates the ancestry of a supernumerary (B) chromosome. Chromosoma 2017, 126, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Banaei-Moghaddama, A.M.; Martis, M.M.; Macas, J.; Gundlach, H.; Himmelbach, A.; Altschmied, L.; Mayer, K.F.X.; Houben, A. Genes on B chromosomes: Old questions revisited with new tools. Biochim. Biophys. Acta 2015, 1849, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Carchilan, M.; Kumke, K.; Mikolajewski, S.; Houben, A. Rye B chromosomes are weakly transcribed and might alter the transcriptional activity of a chromosome sequences. Chromosoma 2009, 118, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Estevez, M.; Lopez-Leon, M.D.; Cabrero, J.; Camacho, J.P.M. B-chromosome ribosomal DNA is functional in the grasshopper Eyprepocnemis plorans. PLoS ONE 2012, 7, e36600. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, V.A.; Dementyeva, P.V.; Larkin, D.M.; O’Brien, P.C.; Perelman, P.L.; Yang, F.; Ferguson-Smith, M.A.; Graphodatsky, A.S. Transcription of a protein-coding gene on B chromosomes of the Siberian roe deer (Capreolus pygargus). BMC Biol. 2013, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.T.; Conte, M.A.; Fantinatti, B.E.; Cabral-de-Mello, D.C.; Carvalho, R.F.; Vicari, M.R.; Kocher, T.D.; Martins, C. Origin and evolution of B chromosomes in the cichlid fish Astatotilapia latifasciata based on integrated genomic analyses. Mol. Biol. Evol. 2014, 31, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Schmutzer, T.; Scholz, U.; Houben, A. Origin and evolution of B chromosomes—What did we learn from next generation sequencing data analysis? Submitted.
- Shibata, F.; Hizume, M.; Kurori, Y. Molecular cytogenetic analysis of supernumerary heterochromatic segments in Rumex acetosa. Genome 2000, 43, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M.; Cabrero, J. New hypotheses about the origin of supernumerary chromosome segments in grasshoppers. Heredity 1987, 58, 341–343. [Google Scholar] [CrossRef]
- Lopez-Leon, M.D.; Cabrero, J.; Camacho, J.P.M. Male and female segregation distortion for heterochromatic supernumerary segments on the B8 chromosome of the grasshopper Chortippus Jacobsi. Chromosoma 1992, 101, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Uno, Y.; Srikulnath, K.; Seki, R.; Nishida, N.; Matsuda, Y. Molecular cloning and characterization of satellite DNA sequences from constitutive heterochromatin of the habu snake (Protobothrops flavoviridis, Viperidae) and the Burmese python (Python bivittatus, Pythonidae). Chromosoma 2015, 124, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lanfredi, M.; Congiu, L.; Garrido-Ramos, M.A.; dela Herrán, M.; Leis, M.; Chicca, M.; Rossi, R.; Tagliavini, J.; Ruiz Rejón, C.; Ruiz Rejón, M.; et al. Chromosomal location and evolution of a satellite DNA family in seven sturgeons species. Chromosome Res. 2001, 9, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G.C.S.; Franco, F.F.; Manfrin, M.H.; Moreira-Filho, O.; Sene, F.M. Low rates of homogenization of the DBC-150 satellite DNA family restricted to a single pair of microchromosomes in species from the Drosophila buzzatii cluster. Chromosome Res. 2007, 15, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Fry, K.; Salser, W. Nucleotide sequences of HS- alpha satellite DNA from kangaroo rat Dipodomys ordii and characterization of similar sequences in other rodents. Cell 1977, 12, 1069–1084. [Google Scholar] [CrossRef]
- Pons, J.; Bruvo, B.; Petitpierre, E.; Plohl, M.; Ugarkovic, D.; Juan, C. Complex structural features of satellite DNA sequences in the genus Pimelia (Coleoptera: Tenebrionidae): Random differential amplification from a common ‘satellite DNA library’. Heredity 2004, 92, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Luchetti, A.; Passamonti, M.; Scali, V.; Mantovani, B. Polymerase chain reaction amplification of the Bag320 satellite family reveals the ancestral library and past gene conversion events in Bacillus rossius (Insecta Phasmatodea). Gene 2003, 312, 289–295. [Google Scholar] [CrossRef]
- Quesada del Bosque, M.E.; Navajas-Pérez, R.; Panero, J.L.; Fernández-González, A.; Garrido-Ramos, M.A. A satellite DNA evolutionary analysis in the North American endemic dioecious plant Rumex hastatulus (Polygonaceae). Genome 2011, 54, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Samoluk, S.S.; Robledo, G.; Bertioli, D.; Seijo, J.G. Evolutionary dynamics of an AT-rich satellite DNA and its contribution to karyotype differentiation in wild diploid Arachis species. Mol. Genet. Genom. 2017, 292, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Agmon, N.; Yacobi, K.; Mislovati, M.; Segal, D. Evidence for rolling circle replication of tandem genes in Drosophila. Nucleic Acids Res. 2005, 33, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Agmon, N.; Sobol, O.; Segal, D. Extrachromosomal circles of satellite repeats and 5S ribosomal DNA in human cells. Mob. DNA 2010, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.B.; Svartman, M.; Delprat, A.; Ruiz, A.; Kuhn, G.C.S. Tetris is a foldback transposon that provided the building blocks for an emerging satellite DNA of Drosophila virilis. Genome Biol. Evol. 2014, 6, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, A. terMITEs: Miniature inverted-repeat transposable elements (MITEs) in the termite genome (Blattodea: Termitoidae). Mol. Genet. Genom. 2015, 290, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Santiago, V.N.; Blanca, G.; Ruiz-Rejón, M.; Garrido-Ramos, M.A. Satellite-DNA evolutionary patterns under a complex evolutionay scenario: The case of Acrolophus subgroup (Centaurea L., Compositae) from the western Mediterranean. Gene 2007, 404, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Quesada del Bosque, M.E.; López-Flores, I.; Suárez-Santiago, V.N.; Garrido-Ramos, M.A. Satellite-DNA diversification and the evolution of major lineages in Cardueae (Carduoideae, Asteraceae). J. Plant Res. 2014, 127, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.N.; Sharma, S.; Sasakuma, T.; Kishii, M.; Vaishnavi, S. Novel repeated DNA sequences in safflower (Carthamus tinctorius L.) (Asteraceae): Cloning, sequencing, and physical mapping by fluorescence in situ hybridization. J. Hered. 2005, 96, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Dover, G. Molecular drive: A cohesive mode of species evolution. Nature 1982, 299, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dover, G. Molecular drive. Trends Genet. 2002, 18, 587–589. [Google Scholar] [CrossRef]
- Elder, J.F., Jr.; Turner, B.J. Concerted evolution at the population level: Pupfish HindIII satellite DNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Feliciello, I.; Akrap, I.; Brajković, J.; Zlatar, I.; Ugarković, Đ. Satellite DNA as a driver of population divergence in the red flour beetle Tribolium castaneum. Genome Biol. Evol. 2014, 7, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gutiérrez, M.A.; Suárez-Santiago, V.N.; López-Flores, I.; Romero, A.T.; Garrido-Ramos, M.A. Concerted evolution of satellite DNA in Sarcocapnos: A matter of time. Plant Mol. Biol. 2012, 78, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Strachan, T.; Webb, D.; Dover, G. Transition stages of molecular drive in multiple-copy DNA families in Drosophila. EMBO J. 1985, 4, 1701–1708. [Google Scholar] [PubMed]
- Bachmann, L.; Sperlich, D. Gradual evolution of a specific satellite DNA family in Drosophila ambigua, D. tristis, and D. obscura. Mol. Biol. Evol. 1993, 10, 647–659. [Google Scholar] [PubMed]
- Navajas-Pérez, R.; Ruiz Rejón, M.; Garrido-Ramos, M.A.; Aznarte, J.L.; Rubio-Escudero, C. SatDNA Analyzer: A computing tool for satellite-DNA evolutionary analysis. Bioinformatics 2007, 23, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Strachan, T.; Coen, E.; Webb, D.; Dover, G. Modes and rates of change of complex DNA families of Drosophila. J. Mol. Biol. 1982, 158, 37–54. [Google Scholar] [CrossRef]
- Luchetti, A.; Cesari, M.; Carrara, G.; Cavicchi, S.; Passamonti, M.; Scali, V.; Mantovani, B. Unisexualityand molecular drive: Bag320 sequence diversity in Bacillus taxa (Insecta Phasmatodea). J. Mol. Evol. 2003, 56, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, A.; Marini, M.; Mantovani, B. Non-concerted evolution of RET76 satellite DNA family in Reticulitermes taxa (Insecta, Isoptera). Genetica 2006, 128, 123–132. [Google Scholar]
- Lorite, P.; Muñoz-López, M.; Carrillo, J.A.; Sanllorente, O.; Vela, J.; Mora, P.; Tinaut, A.; Torres, M.I.; Palomeque, T. Concerted evolution, a slow process for ant satellite DNA: Study of the satellite DNA in the Aphaenogaster genus (Hymenoptera, Formicidae). Org. Divers Evol. 2017. [Google Scholar] [CrossRef]
- Ruiz Rejón, C.; Jamilena, M.; Garrido-Ramos, M.A.; Parker, J.S.; Ruiz Rejón, M. Cytogenetic and molecular analysis of the multiple sex chromosome system of Rumex acetosa. Heredity 1994, 72, 209–215. [Google Scholar] [CrossRef]
- McAllister, B.F.; Werren, J.H. Evolution of tandemly repeated sequences: What happens at the end of an array? J. Mol. Evol. 1999, 48, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Mravinac, B.; Plohl, M. Satellite DNA junctions identify the potential origin of new repetitive elements in the beetle Tribolium madens. Gene 2007, 394, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Ugarković, D. Analysis of divergence of Alphitobius diaperinus satellite DNA—Roles of recombination, replication slippage and gene conversion. Mol. Gen. Genet. 1994, 242, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.E.; Heslop-Harrison, J.S. Centromeric repetitive DNA sequences in the genus Brassica. Theor. Appl. Genet. 1995, 90, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Petitpierre, E.; Juan, C. Evolutionary dynamics of satellite DNA family PIM357 in species of the genus Pimelia (Tenebrionidae, Coleoptera). Mol. Biol. Evol. 2002, 19, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Gillespie, R.G. Common origin of the satellite DNAs of the Hawaiian spiders of the genus Tetragnatha: Evolutionary constraints on the length and nucleotide composition of the repeats. Gene 2003, 313, 169–177. [Google Scholar] [CrossRef]
- Vasil’ev, V.P. Polyploidization by reticular speciation in Acipenseriform evolution: A working hypothesis. J. Appl. Ichthyol. 1999, 15, 29–31. [Google Scholar] [CrossRef]
- Lorite, P.; Carrillo, J.A.; Tinaut, A.; Palomeque, T. Evolutionary dynamics of satellite DNA in species of the genus Formica (Hymenoptera, Formicidae). Gene 2004, 332, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.S.; Henikoff, S. Adaptive evolution of Cid, a centromere specific histone in Drosophila. Genetics 2001, 157, 1293–1298. [Google Scholar] [PubMed]
- Henikoff, S.; Ahmad, K.; Malik, H.S. The centromere paradox: Stable inheritance with rapidly evolving DNA. Science 2001, 293, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Ferree, P.M.; Barbash, D.A. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 2009, 7, e1000234. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, N.; Castagnone-Sereno, P.; Plohl, M. Interplay of selective pressure and stochastic events directs evolution of the MEL172 satellite DNA library in root-knot nematodes. Mol. Biol. Evol. 2006, 23, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.S.; Henikoff, S. Major Evolutionary Transitions in Centromere Complexity. Cell 2009, 138, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Mravinac, B.; Plohl, M. Parallelism in evolution of highly repetitive DNAs in sibling species. Mol. Biol. Evol. 2010, 27, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Djupedal, I.; Kos-Braun, I.C.; Mosher, R.A.; Söderholm, N.; Simmer, F.; Hardcastle, T.J.; Fender, A.; Heidrich, N.; Kagansky, A.; Bayne, E.; et al. Analysis of small RNA in fission yeast; centromeric siRNAs are potentially generated through a structured RNA. EMBO J. 2009, 28, 3832–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haaf, T.; Mater, A.G.; Wienberg, J.; Ward, D.C. Presence and abundance of CENP-B box sequences in great ape subsets of primate-specific alpha-satellite DNA. J. Mol. Evol. 1995, 41, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Masukata, H.; Muro, Y.; Nozaki, N.; Okazaki, T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J. Cell Biol. 1989, 109, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Muro, Y.; Masumoto, H.; Yoda, K.; Nozaki, N.; Ohashi, M.; Okazaki, T. Centromere protein B assembles human centromeric alpha-satellite DNA at the 17-bp sequence, CENP-B box. J. Cell Biol. 1992, 116, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Nakano, M.; Ohzeki, J. The role of CENP-B and alpha-satellite DNA: De novo assembly and epigenetic maintenance of human centromeres. Chromosome Res. 2004, 12, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Bulazel, K.; Metcalfe, C.; Ferreri, G.C.; Yu, J.; Eldridge, M.D.; O’Neill, R.J. Cytogenetic and molecular evaluation of centromereassociated DNA sequences from a marsupial (Macropodidae: Macropus rufogriseus) X chromosome. Genetics 2006, 172, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Cardone, M.F.; Catacchio, C.R.; Antonacci, F.; O’Brien, S.J.; Ryder, O.A.; Purgato, S.; Zoli, M.; Della Valle, G.; Eichler, E.E.; et al. Genome-wide characterization of centromeric satellites from multiple mammalian genomes. Genome Res. 2011, 21, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Schueler, M.G.; Swanson, W.; Thomas, P.J. NISC Comparative Sequencing Program & Green, E.D. Adaptive evolution of foundation kinetochore proteins in primates. Mol. Biol. Evol. 2010, 27, 1585–1597. [Google Scholar] [PubMed]
- Fachinetti, D.; Han, J.S.; McMahon, M.A.; Ly, P.; Abdullah, A.; Wong, A.J.; Cleveland, D.W. DNA sequence-specific binding of CENP-B enhances the fidelity of human centromere function. Dev. Cell 2015, 33, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Kugou, K.; Hirai, H.; Masumoto, H.; Koga, A. Formation of functional CENP-B boxes at diverse locations in repeat units of centromeric DNA in New World monkeys. Sci. Rep. 2016, 6, 27833. [Google Scholar] [CrossRef] [PubMed]
- Graur, D.; Zheng, Y.; Price, N.; Azevedo, R.B.R.; Zufall, R.A.; Elhaik, E. On the immortality of television sets: “function” in the human genome according to the evolution-free gospel of ENCODE. Genome Biol. Evol. 2013, 5, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Graur, D.; Zheng, Y.; Azevedo, R.B.R. An Evolutionary Classification of Genomic Function. Genome Biol. Evol. 2015, 7, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, W.F. Is junk DNA bunk? A critique of ENCODE. Proc. Natl. Acad. Sci. USA 2013, 110, 5294–5300. [Google Scholar] [CrossRef] [PubMed]
- Varley, J.M.; Macgregor, H.C.; Erba, H.P. Satellite DNA is transcribed on lampbrush chromosomes. Nature 1980, 283, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Varley, J.M.; Macgregor, H.C.; Nardi, I.; Andrews, C.; Erba, H.P. Cytological evidence of transcription of highly repeated DNA sequences during the lampbrush stage in Triturus cristatus carnifex. Chromosoma 1980, 80, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.O.; Barsacchi-Pilone, G.; Mahon, K.A.; Gall, J.G. Transcripts from both DNA strands of a satellite DNA occur on lampbrush chromosome loops of the newt Notophthalmus. Cell 1981, 24, 649–659. [Google Scholar] [CrossRef]
- Gall, J.G.; Stephenson, E.C.; Erba, H.P.; Diaz, M.O.; Barsacchi-Pilone, G. Histone genes are located at the sphere loci of newt lampbrush chromosomes. Chromosoma 1981, 84, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.M.; Mahon, K.A.; Gall, J.G. Transcription of a satellite DNA in the newt. J. Cell Biol. 1986, 103, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Solovei, I.V.; Joffe, B.I.; Gaginskaya, E.R.; Macgregor, H.C. Transcription of lampbrush chromosomes of a centromerically localized highly repeated DNA in pigeon (Columba) relates to sequence arrangement. Chromosome Res. 1996, 4, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Suzuki, Y.; Solovei, I.; Saitoh, Y.; Hutchison, N.; Ikeda, J.E.; Macgregor, H.; Mizuno, S. Characterization of DNA sequences constituting the terminal heterochromatin of the chicken Z chromosome. Chromosome Res. 1996, 4, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, M.A.; Canapa, A.; Forconi, M.; Olmo, E.; Barucca, M. Transcription of tandemly repetitive DNA: Functional roles. Chromosome Res. 2015, 23, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Grenfell, A.W.; Strzelecka, M.; Heald, R. Transcription brings the complex(ity) to the centromere. Cell Cycle 2017, 16, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Verdaasdonk, J.S.; Bloom, K. Centromeres: Unique chromatin structures that drive chromosome segregation. Nat. Rev. Mol. Cell Biol. 2011, 12, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, T.; Earnshaw, W.C. The Centromere: Chromatin Foundation for the Kinetochore Machinery. Dev. Cell 2014, 30, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.A.; Henikoff, S. Diversity in the organization of centromeric chromatin. Curr. Opin. Genet. Dev. 2015, 31, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bierhoff, H.; Postepska-Igielska, A.; Grummt, I. Noisy silence: Non-coding RNA and heterochromatin formation at repetitive elements. Epigenetics 2013, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bersani, F.; Lee, E.; Kharchenko, P.V.; Xu, A.W.; Liu, M.; Xega, K.; MacKenzie, O.C.; Brannigan, B.W.; Wittner, B.S.; Jung, H.; et al. Pericentromeric satellite repeat expansions through RNA-derived DNA intermediates in cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 15148–15153. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, X.; Jin, W. An overview of plant centromeres. J. Genet. Genom. 2009, 36, 529–537. [Google Scholar] [CrossRef]
- Neumann, P.; Navratilova, A.; Schroeder-Reiter, E.; Koblizkova, A.; Steinbauerova, V.; Chocholova, E.; Novak, P.; Wanner, G.; Macas, J. Stretching the rules: Monocentric chromosomes with multiple centromere domains. PLoS Genet. 2012, 8, e1002777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.; Wu, Y.; Koblízková, A.; Torres, G.A.; Wang, K.; Iovene, M.; Neumann, P.; Zhang, W.; Novák, P.; Buell, C.R.; et al. Repeatless and repeat-based centromeres in potato: Implications for centromere evolution. Plant Cell 2012, 24, 3559–3574. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Tek, A.L.; Richard, M.M.S.; Abernathy, B.; Fonseca, A.; Schmutz, J.; Chen, N.W.G.; Thareau, V.; Magdelenat, G.; Li, Y.; et al. Identification and characterization of functional centromeres of the common bean. Plant J. 2013, 76, 47–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, C.M.; Giulotto, E.; Sigurdsson, S.; Zoli, M.; Gnerre, S.; Imsland, F.; Lear, T.L.; Adelson, D.L.; Bailey, E.; Bellone, R.R.; et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 2009, 326, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.H.; Hori, T.; Toyoda, A.; Kato, J.; Popendorf, K.; Sakakibara, Y.; Fujiyama, A.; Fukagawa, T. Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res. 2010, 20, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Locke, D.P.; Hillier, L.W.; Warren, W.C.; Worley, K.C.; Nazareth, L.V.; Muzny, D.M.; Yang, S.P.; Wang, Z.; Chinwalla, A.T.; Minx, P.; et al. Comparative and demographic analysis of orangutan genomes. Nature 2011, 469, 529–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerutti, F.; Gamba, R.; Mazzagatti, A.; Piras, F.M.; Cappelletti, E.; Belloni, E.; Nergadze, S.G.; Raimondi, E.; Giulotto, E. The major horse satellite DNA family is associated with centromere competence. Mol. Cytogenet. 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Hasson, D.; Cheung, F.; Warburton, P.E. A paucity of heterochromatin at functional human neocentromeres. Epigenet. Chromatin 2010, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Talbertc, P.B.; Zhang, W.; Wua, Y.; Yang, Z.; Henikoff, J.G.; Henikoff, S.; Jianga, J. The CentO satellite confers translational and rotational phasing on cenH3 nucleosomes in rice centromeres. Proc. Natl. Acad. Sci. USA 2013, 110, E4875–E4883. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fu, S.; Dong, Q.; Han, F.; Birchler, J.A. Inactivation of a centromere during the formation of a translocation in maize. Chromosome Res. 2011, 19, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lv, Z.; Pang, J.; Liu, Y.; Guo, X.; Fu, S.; Li, J.; Dong, Q.; Wu, H.-J.; Gao, Z.; et al. Formation of a functional maize centromere after loss of centromeric sequences and gain of ectopic sequences. Plant Cell 2013, 25, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Gao, Z.; Birchler, J.; Han, F. Dicentric chromosome formation and epigenetics of centromere formation in plants. J. Genet. Genom. 2012, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schueler, M.G.; Sullivan, B.A. Structural and functional dynamics of human centromeric chromatin. Annu. Rev. Genom. Hum. Genet. 2006, 7, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lee, H.R.; Koo, D.H.; Jiang, J. Epigenetic modification of centromeric chromatin: Hypomethylation of DNA sequences in the CENH3-associated chromatin in Arabidopsis thaliana and maize. Plant Cell 2008, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gent, J.I.; Dawe, R.K. RNA as a structural and regulatory component of the centromere. Annu. Rev. Genet. 2012, 46, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Marshall, O.J.; Saffery, R.; Kim, B.W.; Earle, E.; Choo, K.H.; Wong, L.H. Active transcription and essential role of RNA polymerase II at the centromere during mitosis. Proc. Natl. Acad. Sci. USA 2012, 109, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.H.; Jakubsche, J.N.; Martins, N.M.; Kagansky, A.; Nakano, M.; Kimura, H.; Kelly, D.A.; Turner, B.M.; Masumoto, H.; Larionov, V.; et al. Epigenetic engineering: Histone H3K9 acetylation is compatible with kinetochore structure and function. J. Cell Sci. 2012, 125, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Pezer, Z.; Ugarković, D. Role of non-coding RNA and heterochromatin in aneuploidy and cancer. Semin. Cancer Biol. 2008, 18, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Frescas, D.; Guardavaccaro, D.; Kuchay, S.M.; Kato, H.; Poleshko, A.; Basrur, V.; Elenitoba-Johnson, K.S.; Katz, R.A.; Pagano, M. KDM2A represses transcription of centromeric satellite repeats and maintains the heterochromatic state. Cell Cycle 2008, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.H.; Brettingham-Moore, K.H.; Chan, L.; Quach, J.M.; Anderson, M.A.; Northrop, E.L.; Hannan, R.; Saffery, R.; Shaw, M.L.; Williams, E.; et al. Centromere RNA is a key component for the assembly of nucleoproteins at the nucleolus and centromere. Genome Res. 2007, 17, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Saffery, R.; Sumer, H.; Hassan, S.; Wong, L.H.; Craig, J.M.; Todokoro, K.; Anderson, M.; Stafford, A.; Choo, K.H. Transcription within a functional human centromere. Mol. Cell 2003, 12, 509–516. [Google Scholar] [CrossRef]
- Quénet, D.; Dalal, Y. A long non-coding RNA is required for targeting centromeric protein A to the human centromere. Elife 2014, 3, e03254. [Google Scholar] [CrossRef] [PubMed]
- Grenfell, A.W.; Heald, R.; Strzelecka, M. Mitotic noncoding RNA processing promotes kinetochore and spindle assembly in Xenopus. J. Cell Biol. 2016, 214, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rošic, S.; Köhler, F.; Erhardt, S. Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J. Cell Biol. 2014, 207, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Neumann, P.; Macas, J.; Jiang, J. Transcription and evolutionary dynamics of the centromeric satellite repeat CentO in rice. Mol. Biol. Evol. 2006, 23, 2505–2520. [Google Scholar] [CrossRef] [PubMed]
- Carone, D.M.; Longo, M.S.; Ferreri, G.C.; Hall, L.; Harris, M.; Shook, N.; Bulazel, K.V.; Carone, B.R.; Obergfell, C.; O’Neill, M.J.; et al. A new class of retroviral and satellite encoded small RNAs emanates from mammalian centromeres. Chromosoma 2009, 118, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Pezer, Z.; Ugarković, D. Transcription of pericentromeric heterochromatin in beetles—Satellite DNAs as active regulatory elements. Cytogenet. Genome Res. 2009, 124, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Bouzinba-Segard, H.; Guais, A.; Francastel, C. Accumulation of small murine minor satellite transcripts leads to impaired centromeric architecture and function. Proc. Natl. Acad. Sci. USA 2006, 103, 8709–8714. [Google Scholar] [CrossRef] [PubMed]
- Melters, D.P.; Paliulis, L.V.; Korf, I.F.; Chan, S.W. Holocentric chromosomes: Convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Res. 2012, 20, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Cuacos, M.; Franklin, F.C.H.; Heckmann, S. Atypical centromeres in plants—What they can tell us. Front. Plant Sci. 2015, 6, 913. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Pavlíková, Z.; Koblížková, A.; Fuková, I.; Jedlicková, V.; Novák, P.; Macas, J. Centromeres off the hook: Massive changes in centromere size and structure following duplication of CenH3 gene in Fabeae species. Mol.Biol. Evol. 2015, 32, 1862–1879. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Schubert, V.; Fuková, I.; Manning, J.E.; Houben, A.; Macas, J. Epigenetic Histone Marks of Extended Meta-Polycentric Centromeres of Lathyrus and Pisum Chromosomes. Front. Plant Sci. 2016, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Ribeiro, T.; Neumann, P.; Macas, J.; Novák, P.; Schubert, V.; Pellino, M.; Fuchs, J.; Ma, W.; Kuhlmann, M.; et al. Holocentromeres in Rhynchospora are associated with genome-wide centromere-specific repeat arrays interspersed among euchromatin. Proc. Natl. Acad. Sci. USA 2015, 112, 13633–13638. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Marques, A.; Novák, P.; Schubert, V.; Vanzela, A.L.L.; Macas, J.; Houben, A.; Pedrosa-Harand, A. Centromeric and non-centromeric satellite DNA organization differs in holocentric Rhynchospora species. Chromosoma 2017, 126, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, S.; Macas, J.; Kumke, K.; Fuchs, J.; Schubert, V.; Ma, L.; Novak, P.; Neumann, P.; Taudien, S.; Platzer, M.; et al. The holocentric species Luzula elegans shows an interplay between centromere and large-scale genome organization. Plant J. 2013, 73, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Drinnenberg, I.A.; deYoung, D.; Henikoff, S.; Malik, H.S. Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife 2014, 3, e03676. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, R.; Rechtsteiner, A.; Yuen, K.W.; Muroyama, A.; Egelhofer, T.; Gaydos, L.; Barron, F.; Maddox, P.; Essex, A.; Monen, J.; et al. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature 2012, 484, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.A.; Henikoff, S. Holocentromeres are dispersed point centromeres localized at transcription factor hotspots. Elife 2014, 3, e02025. [Google Scholar] [CrossRef] [PubMed]
- Zedek, F.; Bureš, P. CenH3 evolution reflects meiotic symmetry as predicted by the centromere drive model. Sci. Rep. 2016, 6, 33308. [Google Scholar] [CrossRef] [PubMed]
- Dawe, R.K.; Henikoff, S. Centromeres put epigenetics in the driver’s seat. Trends Biochem. Sci. 2006, 31, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ugarković, Ð. Evolution of Alpha-Satellite DNA; eLS. John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Talbert, P.B.; Bayes, J.J.; Henikoff, S. Evolution of centromeres and kinetochores: A two-part fugue. In The Kinetochore; De Wulf, P., Earnshaw, W.C., Eds.; Springer: Berlin, Germany, 2008; pp. 193–230. [Google Scholar]
- Zedek, F.; Bureš, P. Evidence for Centromere Drive in the Holocentric Chromosomes of Caenorhabditis. PLoS ONE 2012, 7, e30496. [Google Scholar] [CrossRef] [PubMed]
- Zedek, F.; Bureš, P. Absence of positive selection on CenH3 in Luzula suggests that holokinetic chromosomes may suppress centromere drive. Ann. Bot. 2016, 118, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Finseth, F.R.; Dong, Y.; Saunders, A.; Fishman, L. Duplication and Adaptive Evolution of a Key Centromeric Protein in Mimulus, a Genus with Female Meiotic Drive. Mol. Biol. Evol. 2015, 32, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Rogers, K. Phylogenetic Analysis of Fungal Centromere H3 Proteins. Genetics 2006, 174, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Bureš, P.; Zedek, F.; Marková, M. Holocentric chromosomes. In Plant Genome Diversity. Vol. 2. Physical Structure of Plant Genomes; Wendel, J., Greilhuber, J., Doležel, J., Leitch, I.J., Eds.; Springer: Heidelberg, Germany, 2013; pp. 187–208. [Google Scholar]
- Martínez, P.; Blasco, M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 2011, 11, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, D.C.; Londoño-Vallejo, A. Telomere dynamics in mammals. Genome Dyn. 2012, 7, 29–45. [Google Scholar] [PubMed]
- Mravinac, B.; Meštrović, N.; Čavrak, V.V.; Plohl, M. TCAGG, an alternative telomeric sequence in insects. Chromosoma 2011, 120, 367–376. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán, R.; Cuñado, N.; Navajas-Pérez, R.; Santos, J.L.; Ruiz Rejón, C.; Garrido-Ramos, M.A.; Ruiz Rejón, M. The controversial telomeres of lily plants. Cytogenet. Genome Res. 2005, 109, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, E.; Lim, K.Y.; Kunická, Z.; Chase, M.W.; Bennett, M.D.; Fajkus, J.; Leitch, A.R. Telomere variability in the monocotyledonous plant order Asparagales. Proc. R. Soc. Lond. B 2003, 270, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Peška, V.; Fajkus, P.; Fojtová, M.; Dvořáčková, M.; Hapala, J.; Dvořáček, V.; Polanská, P.; Leitch, A.R.; Sýkorová, E.; Fajkus, J. Characterisation of an unusual telomere motif (TTTTTTAGGG)n in the plant Cestrum elegans (Solanaceae), a species with a large genome. Plant J. 2015, 82, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Fulnečková, J.; Sěvčíková, T.; Fajkus, J.; Lukešová, A.; Lukeš, M.; Vlček, C.; Lang, B.F.; Kim, E.; Eliáš, M.; Sýkorová, E. A broad phylogenetic survey unveils the diversity and evolution of telomeres in eukaryotes. Genome Biol. Evol. 2013, 5, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sousa, R.; López-Panadès, E.; Casacuberta, E. Drosophila telomeres: An example of co- evolution with transposable Elements. Genome Dyn. 2012, 7, 46–67. [Google Scholar] [PubMed]
- Sýkorová, E.; Fajkus, J.; Meznikova, M.; Lim, K.Y.; Neplechova, K.; Blattner, F.R.; Chase, M.W.; Leitch, A.R. Minisatellite telomeres occur in the family Alliaceae but are lost in Allium. Am. J. Bot. 2006, 93, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Pich, U.; Schubert, I. Terminal heterochromatin and alternative telomeric sequences in Allium cepa. Chromosome Res. 1998, 6, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.J.; Vershinin, A.V. Chromosome ends: Different sequences may provide conserved functions. Bioessays 2005, 27, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Riethman, H.; Ambrosini, A.; Castaneda, C.; Finklestein, J.; Hu, X.L.; Mudunuri, U.; Paul, S.; Wei, J. Mapping and initial analysis of human subtelomeric sequence assemblies. Genome Res. 2004, 14, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Riethman, H.; Ambrosini, A.; Paul, S. Human subtelomere structure and variation. Chromosome Res. 2005, 13, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Jouve, N. Mapping and organization of highly-repeated DNA sequences by means of simultaneous and sequential FISH and C-banding in 6x-triticale. Chromosome Res. 1994, 2, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Jouve, N. Fluorescent in situ hybridization and C-banding analyses of highly repetitive DNA sequences in the heterochromatin of rye (Secale montanum Guss.) and wheat incorporating S. montanum chromosome segments. Genome 1995, 38, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Vershinin, A.V.; Heslop-Harrison, J.S. Comparative analysis of the nucleosomal structure of rye, wheat and their relatives. Plant Mol. Biol. 1998, 36, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Contento, A.; Heslop-Harrison, J.S.; Schwarzacher, T. Diversity of a major repetitive DNA sequence in diploid and polyploid Triticeae. Cytogenet. Genome Res. 2005, 109, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.A.; Gong, Z.; Iovene, M.; Hirsch, C.D.; Buell, C.R.; Bryan, G.J.; Novák, P.; Macas, J.; Jiang, J. Organization and evolution of subtelomeric satellite repeats in the potato genome. GE Genes Genomes Genet. 2011, 1, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Dos Santos, K.G.; Richard, M.M.; Sévignac, M.; Thareau, V.; Geffroy, V.; Pedrosa-Harand, A. Evolutionary dynamics of satellite DNA repeats from Phaseolus beans. Protoplasma 2017, 254, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.M.S.; Chen, N.W.G.; Thareau, V.; Pflieger, E.; Blanchet, S.; Pedrosa-Harand, A.; Iwata, A.; Chavarro, C.; Jackson, S.A.; Geffroy, V. The subtelomeric khipu satellite repeat from Phaseolus vulgaris: Lessons learned from the genome analysis of the Andean genotype G19833. Front. Plant Sci. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazama, Y.; Sugiyama, R.; Suto, Y.; Uchida, W.; Kawano, S. The clustering of four subfamilies of satellite DNA at individual chromosome ends in Silene latifolia. Genome 2006, 49, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A.; de la Herrán, R.; Ruiz Rejón, M.; Ruiz Rejón, C. A subtelomeric satellite DNA family isolated from the genome of the dioecious plant Silene latifolia. Genome 1999, 42, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, E.; Cartagena, J.; Horáková, M.; Fukui, K.; Fajkus, J. Characterization of telomere-subtelomere junctions in Silene latifolia. Mol. Gen. Genom. 2003, 269, 13–20. [Google Scholar]
- Lim, K.Y.; Kovarik, A.; Matyášek, R.; Chase, M.W.; Knapp, S.; McCarthy, E.; Clarkson, J.J.; Leitch, A.R. Comparative genomics and repetitive sequence divergence in the species of diploid Nicotiana section Alatae. Plant J. 2006, 48, 907–919. [Google Scholar] [PubMed]
- He, L.; Liu, J.; Torres, G.A.; Zhang, H.; Jiang, J.; Xie, C. Interstitial telomeric repeats are enriched in the centromeres of chromosomes in Solanum species. Chromosome Res. 2013, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Feuerbach, F.; Galy, V.; Trelles-Sticken, E.; Fromont-Racine, M.; Jacquier, A.; Gilson, E.; Olivo-Marin, J.C.; Scherthan, H.; Nehrbass, U. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat. Cell Biol. 2002, 4, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005, 579, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Hebden, A.K.; Nakamura, T.M.; Miller, K.M.; Cooper, J.P. HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature 2010, 467, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Lundblad, V. Telomere maintenance without telomerase. Oncogene 2002, 21, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, A.; Garcia, F.; Azzalin, C.; Giulotto, E.; Egozcue, J.; Ponsa, M.; Garcia, M. Distribution of intrachromosomal telomeric sequences (ITS) on Macaca fascicularis (Primates) chromosomes and their implication for chromosome evolution. Hum. Genet. 2002, 110, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Cusanelli, E.; Chartrand, P. Telomeric repeat-containing RNA TERRA: A noncoding RNA connecting telomere biology to genome integrity. Front. Genet. 2015, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Norseen, J.; Wiedmer, A.; Riethman, H.; Lieberman, P.M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell 2009, 35, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, N.; VanBeneden, A.; Decottignies, A. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1 alpha. Nat. Struct. Mol. Biol. 2012, 19, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Panza, A.; Redon, S.; Iglesias, N.; Li, Z.; Lingner, J. The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell 2008, 32, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Vrbsky, J.; Akimcheva, S.; Watson, J.M.; Turner, T.L.; Daxinger, L.; Vyskot, B.; Aufsatz, W.; Riha, K. siRNA-mediated methylation of Arabidopsis telomeres. PLoS Genet. 2010, 6, e1000986. [Google Scholar] [CrossRef] [PubMed]
- Eymery, A.; Horard, B.; El Atifi-Borel, M.; Fourel, G.; Berger, F.; Vitte, A.L.; Van den Broeck, A.; Brambilla, E.; Fournier, A.; Callanan, M.; et al. A transcriptomic analysis of human centromeric and pericentric sequences in normal and tumor cells. Nucleic Acids Res. 2009, 37, 6340–6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, T.A.; Kidner, C.; Hall, I.M.; Teng, G.; Grewal, S.I.S.; Martienssen, R. Regulation of heterochromatic silencing and histone H3 lysine- 9 methylation by RNAi. Science 2002, 297, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Holoch, D.; Moazed, D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015, 16, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.L.; Straight, A.F. RNA-mediated regulation of heterochromatin. Curr. Opin. Cell Biol. 2017, 46, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, F.; Weisshaar, B.; Fuchs, J.; Bannack, E.; Minoche, A.E.; Dohm, J.C.; Himmelbauer, H.; Schmidt, T. Epigenetic profiling of heterochromatic satellite DNA. Chromosoma 2011, 120, 409–422. [Google Scholar] [CrossRef] [PubMed]
- May, B.P.; Lippman, Z.B.; Fang, Y.; Spector, D.L.; Martienssen, R.A. Differential regulation of strandspecific transcripts from Arabidopsis centromeric satellite repeat. PLoS Genet. 2005, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, T.; Nogami, M.; Yoshikawa, M.; Ikeno, M.; Okazaki, T.; Takami, Y.; Nakayama, T.; Oshimura, M. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat. Cell Biol. 2004, 6, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Grishok, A.; Tabara, H.; Mello, C.C. Genetic requirements for inheritance of RNAi in C. elegans. Science 2000, 287, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Pal-Bhadra, M.; Leibovitch, B.; Gandhi, S.; Rao, M.; Bhadra, U.; Birchler, J.; Elgin, S. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 2004, 303, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulou, C.; Muljo, S.; Kung, A.; Ganesan, S.; Drapkin, R.; Jenuwein, T.; Livingston, D.; Rajewsky, K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005, 19, 489. [Google Scholar] [CrossRef] [PubMed]
- Saksouk, N.; Simboeck, E.; Déjardin, J. Constitutive heterochromatin formation and transcription in mammals. Epigenet. Chromatin 2015, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Maida, Y.; Yasukawa, M.; Okamoto, N.; Ohka, S.; Kinoshita, K.; Totoki, Y.; Ito, T.K.; Minamino, T.; Nakamura, H.; Yamaguchi, S.; et al. Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol. Cell Biol. 2014, 34, 1576–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pao, G.M.; Huynh, A.M.; Suh, H.; Tonnu, N.; Nederlof, P.M.; Gage, F.H.; Verma, I.M. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature 2011, 477, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.T.; Lipson, D.; Paul, S.; Brannigan, B.W.; Akhavanfard, S.; Coffman, E.J.; Contino, G.; Deshpande, V.; Iafrate, A.J.; Letovsky, S.; et al. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science 2011, 331, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.P.; Meles, S.; Escudeiro, A.; Mendes-da-Silva, A.; Adega, F.; Chaves, R. Satellite Noncoding RNAs: The emerging players in cells, cellular pathways and cancer. Chromosome Res. 2015, 23, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Suzuki, K.; Kakizawa, N.; Fukui, T.; Takayama, Y.; Watanabe, F.; Kato, T.; Saito, M.; Futsuhara, K.; Rikiyama, T.; et al. Overexpression of satellite alpha transcript in breast cancer patients to impair specific chromosomes. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Ichida, K.; Suzuki, K.; Takayama, Y.; Muto, Y.; Fukui, T.; Kato, T.; Saito, M.; Watanabe, F.; Kakizawa, N.; Imoto, H.; et al. Detection of satellite alpha transcript in sera, as a surrogate marker for the risk of development of multiple cancers in colorectal cancer patients. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Kakizawa, N.; Suzuki, K.; Yoshizawa, A.; Ichida, K.; Saito, M.; Futsuhara, K. Toshiki Rikiyama Satellite alpha transcripts as a biomarker to predict the development of breast cancer with bilateral or multiple primary malignancies. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Ugarković, Đ. Functional elements residing within satellite DNAs. EMBO Rep. 2005, 6, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Valgardsdottir, R.; Chiodi, I.; Giordano, M.; Cobianchi, F.; Riva, S.; Biamonti, G. Structural and functional characterization of noncoding repetitive RNAs transcribed in stressed human cells. Mol. Biol. Cell 2005, 16, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Col, E.; Hoghoughi, N.; Dufour, S.; Penin, J.; Koskas, S.; Faure, V.; Ouzounova, M.; Hernandez-Vargash, H.; Reynoird, N.; Daujat, S.; et al. Bromodomain factors of BET family are new essential actors of pericentric heterochromatin transcriptional activation in response to heat shock. Sci. Rep. 2017, 7, 5418. [Google Scholar] [CrossRef] [PubMed]
- Jehan, Z.; Vallinayagam, S.; Tiwari, S.; Pradhan, S.; Singh, L.; Suresh, A.; Reddy, H.M.; Ahuja, Y.R.; Jesudasan, R.A. Novel noncoding RNA from human Y distal heterochromatic block (Yq12) generates testis specific chimeric CDC2L2. Genome Res. 2007, 17, 433–440. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, R.; Baumann, C.; Viveiros, M.M. ATRX contributes to epigenetic asymmetry and silencing of major satellite transcripts in the maternal genome of the mouse embryo. Development 2015, 142, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.M.; Maze, I.; Zhao, D.; Xiang, B.; Wenderski, W.; Lewis, P.W.; Shen, L.; Li, H.; Allis, C.D. ATRX tolerates activitydependent histone H3 methyl/phos switching to maintain repetitive element silencing in neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Pezer, Z.; Ugarkovic, D. Satellite DNA-associated siRNAs as mediators of heat shock response in insects. RNA Biol. 2012, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Ferree, P.M. Sex Differences: Satellite DNA Directs Male-Specific Gene Expression. Curr. Biol. 2017, 27, 1866. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.U.; Coarfa, C.; Xiao, W.; Gunaratne, P.H.; Meller, V.H. siRNAs from an X-linked satellite repeat promote X-chromosome recognition in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2014, 111, 16460–16465. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Meller, V.H. Satellite Repeats Identify X Chromatin for Dosage Compensation in Drosophila melanogaster males. Curr. Biol. 2017, 27, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.L.; Byron, M.; Carone, D.M.; Whitfield, T.W.; Pouliot, G.P.; Fischer, A.; Jones, P.; Lawrence, J.B. Demethylated HSATII DNA and HSATII RNA foci sequester PRC1 and MeCP2 into cancer-specific nuclear bodies. Cell Reports 2017, 18, 2943–2956. [Google Scholar] [CrossRef] [PubMed]
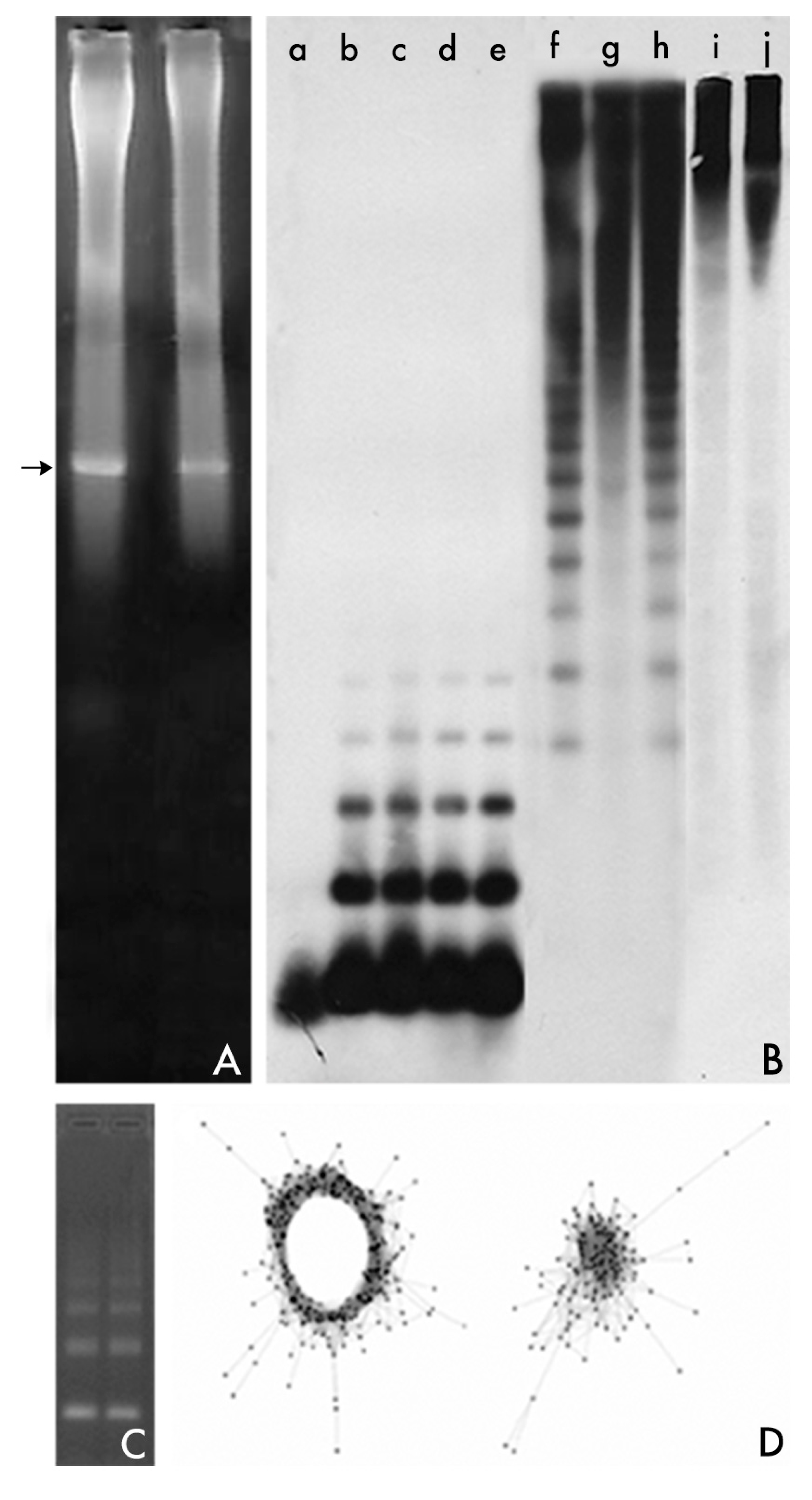
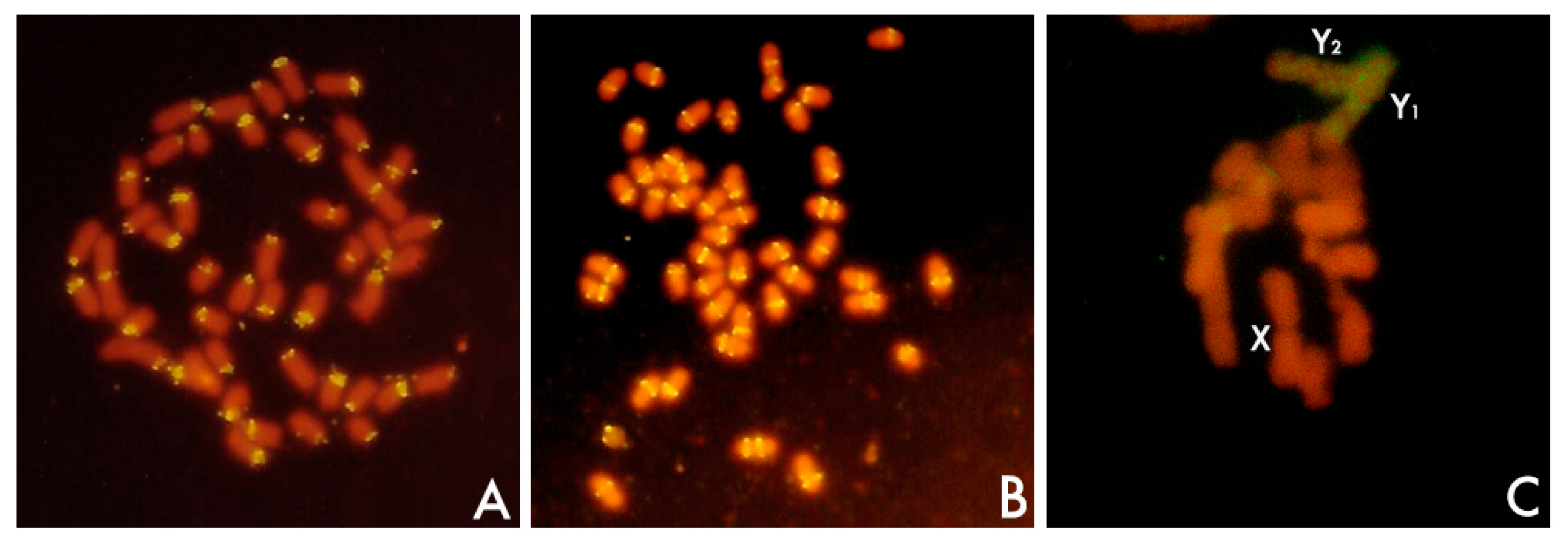
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Ramos, M.A. Satellite DNA: An Evolving Topic. Genes 2017, 8, 230. https://doi.org/10.3390/genes8090230
Garrido-Ramos MA. Satellite DNA: An Evolving Topic. Genes. 2017; 8(9):230. https://doi.org/10.3390/genes8090230
Chicago/Turabian StyleGarrido-Ramos, Manuel A. 2017. "Satellite DNA: An Evolving Topic" Genes 8, no. 9: 230. https://doi.org/10.3390/genes8090230




