In BPS1 Downregulated Roots, the BYPASS1 Signal Disrupts the Induction of Cortical Cell Divisions in Bean-Rhizobium Symbiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Rhizobium Inoculation
2.2. Sequence Identification, Alignment and Phylogenetic Analysis
2.3. Plasmid Construction and Composite Plants
2.4. Physiological Analysis
2.5. Expression Analysis
2.6. Fluridone Treatment
2.7. Microscopy
3. Results
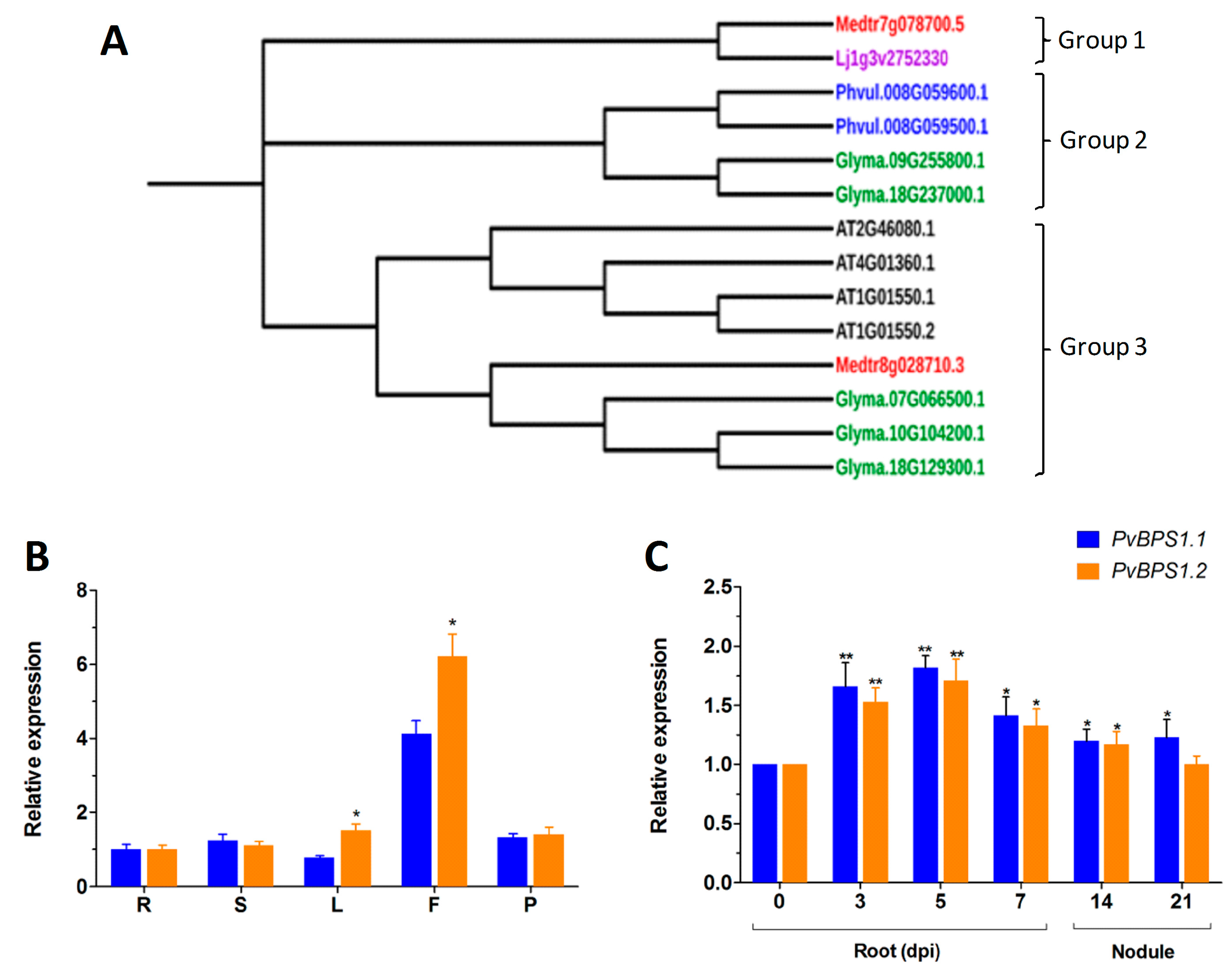
3.1. The BPS1 Gene Has Multiple Members
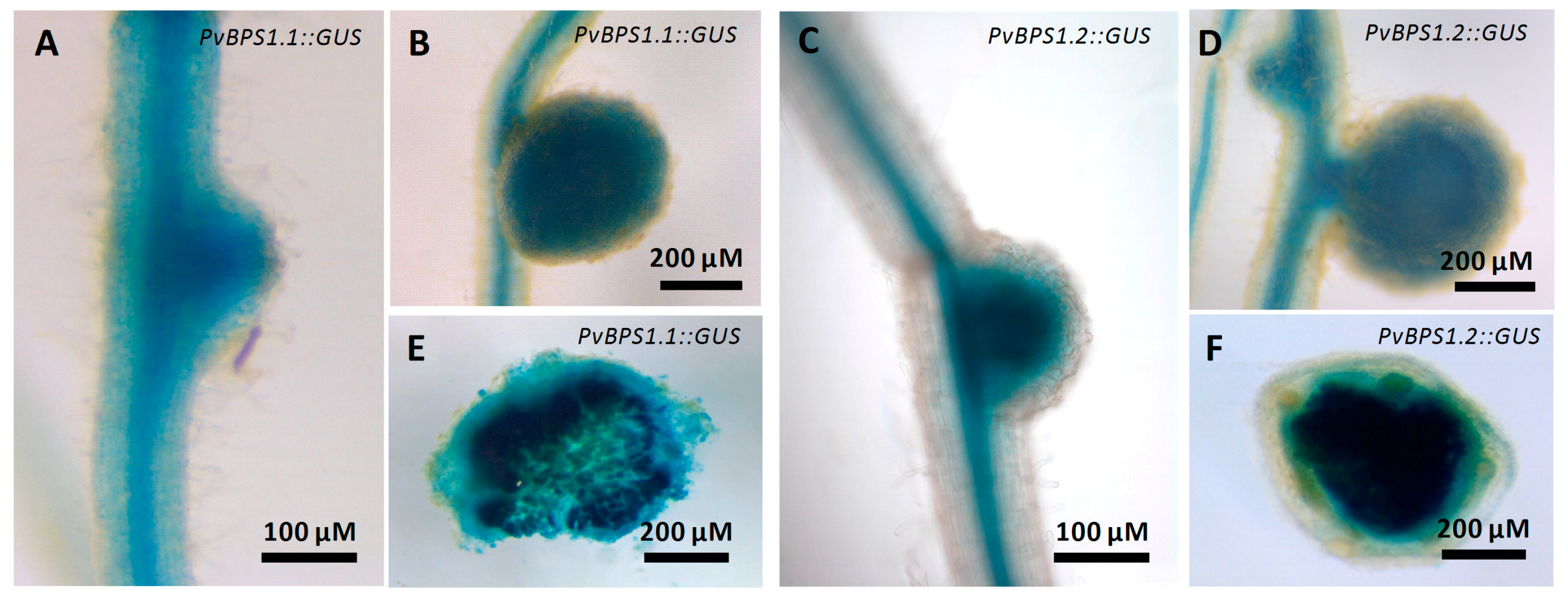
3.2. Phaseolus vulgaris BPS1 Express during Root Nodule Symbiosis
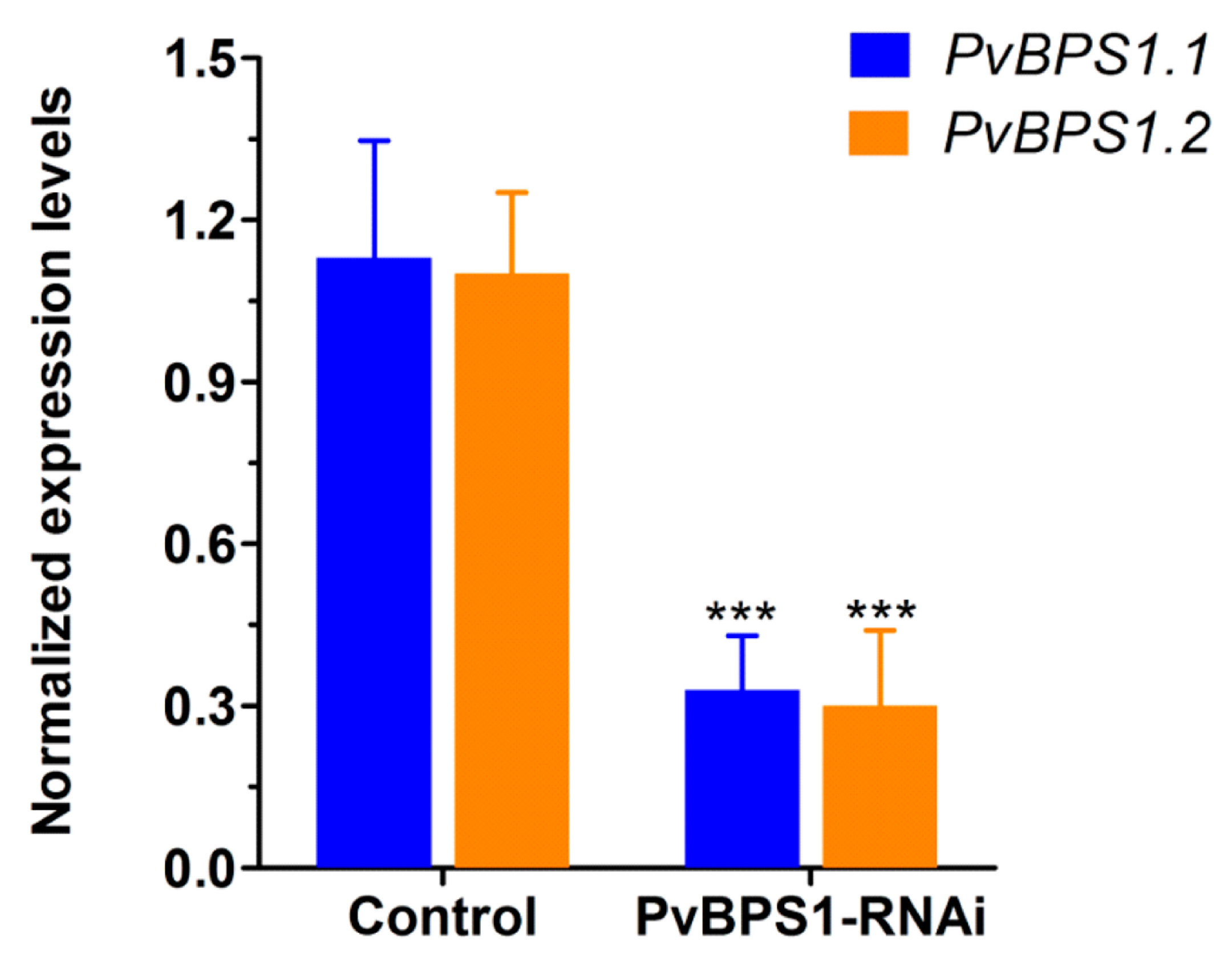
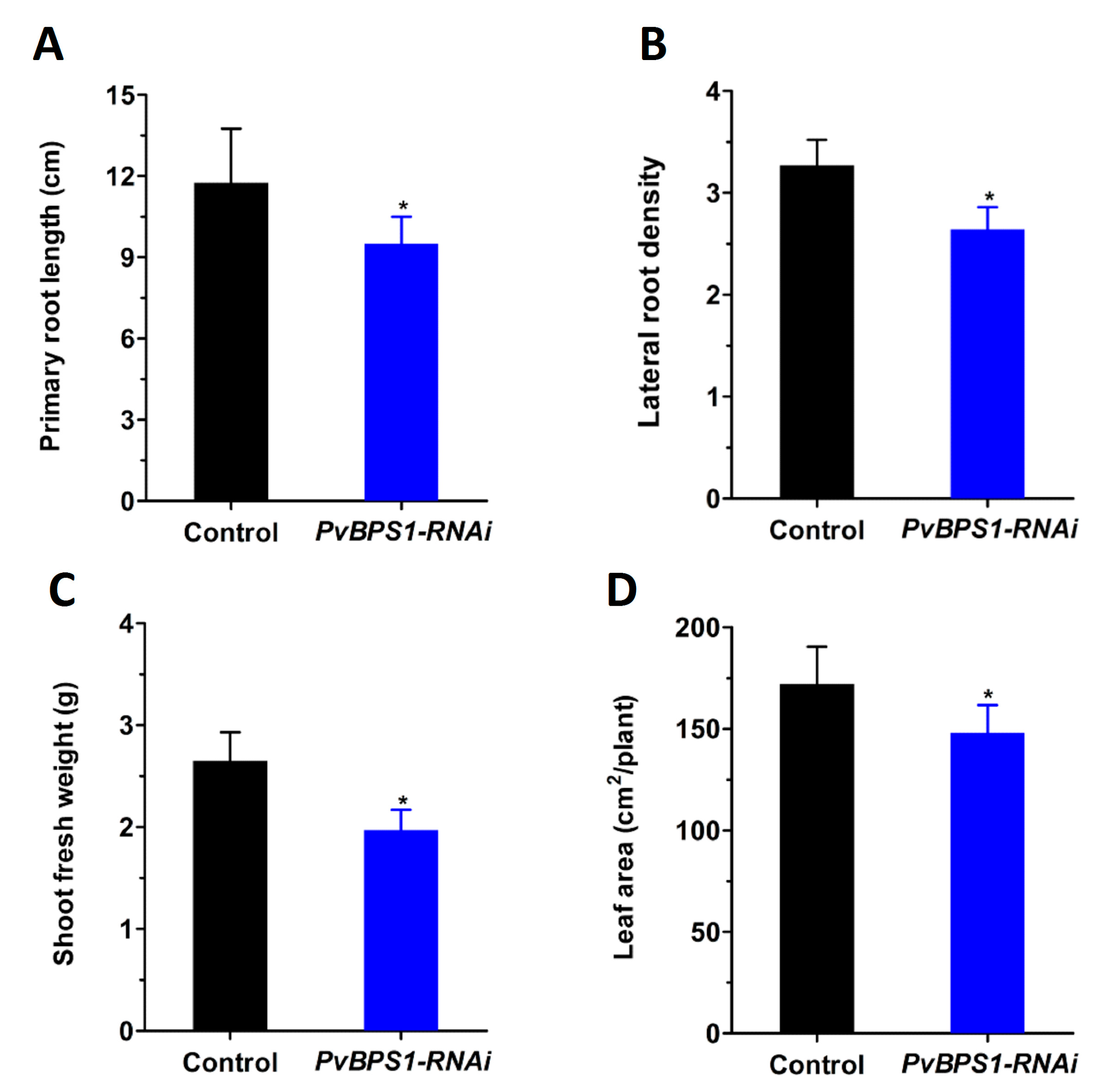
3.3. Silencing of PvBPS1 Transcripts Affects Shoot and Root Growth
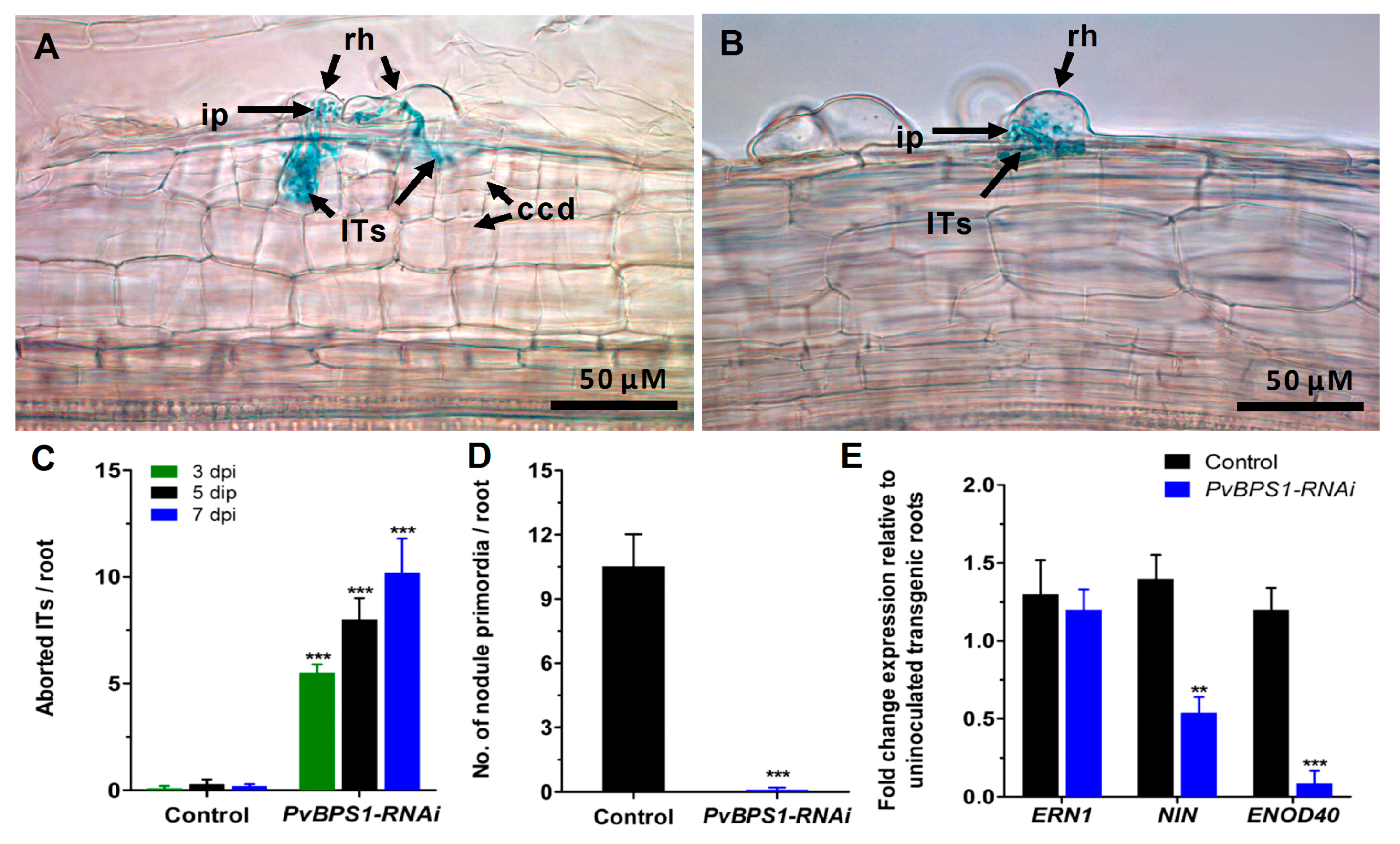
3.4. Symbiotic Phenotype in PvBPS1-RNAi Plants
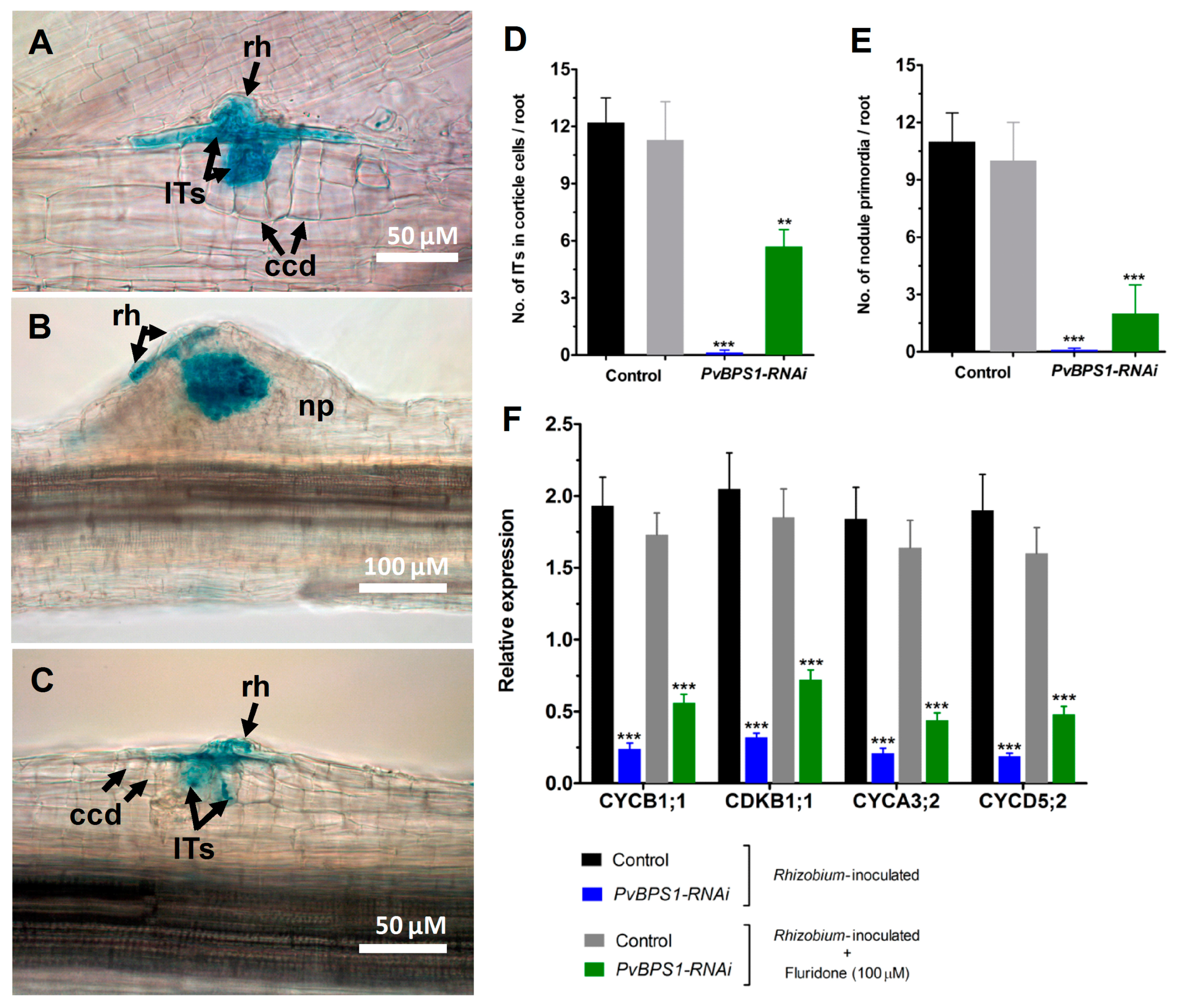
3.5. Rescue of the Symbiosis Phenotype with Fluridone
4. Discussion
4.1. BPS1 is Conserved in Legumes
4.2. Ubiquitous Effect of PvBPS1 Silencing in Phaseolus
4.3. PvBPS1 Signal Suppresses Cortical Cell Divisions during Root Nodule Symbiosis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, L. Carotenoid metabolism: Biosynthesis, regulation, and beyond. J. Integr. Plant Biol. 2008, 50, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Bjorkman, O.; Grossman, A.R. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef] [PubMed]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McC-arty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Fester, T. Isoprenoid metabolism and plastid reorganization in arbuscular mycorrhizal roots. New Phytol. 2006, 172, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; McCourt, P. Strigolactones: A new hormone with a past. Curr. Opin. Plant Biol. 2009, 12, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Floss, D.S.; Strack, D. Apocarotenoids: Hormones, mycorrhizal metabolites and aroma volatiles. Planta 2010, 232, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Furutani, M.; Vernoux, T.; Traas, J.; Kato, T.; Tasaka, M.; Aida, M. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 2004, 131, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 2007, 449, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benková, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010, 61, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ruyter-Spira, C.; Al-Babili, S.; van der Krol, S.; Bouwmeester, H. The biology of strigolactones. Trends Plant Sci. 2013, 18, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; García-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Yoneyama, K.; Hugill, C.; Quittenden, L.; Reid, J. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Novero, M.; Charnikhova, T.; Ferrandino, A.; Schubert, A.; Ruyter Spira, C.; Bonfante, P.; Lovisolo, C.; Bouwmeester, H.J.; Cardinale, F. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013, 64, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Van Norman, J.M.; Murphy, C.; Adhikari, E.; Reed, J.W.; Sieburth, L.E. In the absence of BYPASS1-related gene function, the bps signal disrupts embryogenesis by an auxin-independent mechanism. Development 2012, 139, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, J.M.; Frederick, R.L.; Sieburth, L.E. BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Curr. Biol. 2004, 14, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, J.M.; Murphy, C.; Sieburth, L.E. BYPASS1: Synthesis of the mobile root-derived signal requires active root growth and arrests early leaf development. BMC Plant Biol. 2011, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, J.M.; Sieburth, L.E. Dissecting the biosynthetic pathway for the bypass1 root-derived signal. Plant J. 2007, 49, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.; Lee, D.K.; Giavalisco, P.; Sieburth, L.E. Long-distance signaling in bypass1 mutants: Bioassay development reveals the bps signal to be a metabolite. Mol. Plant 2013, 6, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, A.; Nagata, M.; Futsuki, K.; Abe, H.; Uchiumi, T.; Abe, M.; Kucho, K.; Hashiguchi, M.; Akashi, R.; Hirsch, A.; et al. Effect of abscisic acid on symbiotic nitrogen fixation activity in the root nodules of Lotus japonicus. Plant Signal Behav. 2010, 5, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Nanjareddy, K.; Arthikala, M.K.; Gómez, B.M.; Blanco, L.; Lara, M. Differentially expressed genes in mycorrhized and nodulated roots of common bean are associated with defense, cell wall architecture, N metabolism, and P metabolism. PLoS ONE 2017, 12, e0182328. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; Dilworth, M.J. Control of leghemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.M.; Taly, J.F.; Notredame, C. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef] [PubMed]
- Timothy, L.; Mikael Bodén, B.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Nanjareddy, K.; Arthikala, M.K.; Aguirre, A.L.; Gómez, B.M.; Lara, M. Plant promoter analysis: Identification and characterization of root nodule specific promoter in common bean. J. Vis. Exp. 2017, 130, e56140. [Google Scholar] [CrossRef]
- Karimi, M.; Inze, D.; Depicker, A. Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Valdeés-López, O.; Arenas-Huertero, C.; Ramírez, M.; Girard, L.; Sánchez, F.; Vance, C.P.; Luis Reyes, J.; Hernández, G. Essential role of MYB transcription factor: PvPHR1 and microRNA: PvmiR399 in phosphorus deficiency signalling in common bean roots. Plant Cell Environ. 2008, 31, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, J.G.; Gambetta, G.A.; Hernández-Barrera, A.; Shishkova, S.; González, I. Lateral root initiation in Arabidopsis: Developmental window, spatial patterning, density and predictability. Ann. Bot. 2006, 97, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Arthikala, M.K.; Montiel, J.; Nava, N.; Santana, O.; Sánchez-López, R.; Cárdenas, L.; Quinto, C. PvRbohB negatively regulates Rhizophagus irregularis colonization in Phaseolus vulgaris. Plant Cell Physiol. 2013, 54, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van-Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal reference genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, R.A. Assaying chimeric genes in plants, the GUS gene fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Vinuesa, P.; Neumann-Silkow, F.; Pacios-Bras, C.; Spaink, H.P.; Martínez-Romero, E.; Werner, D. Genetic analysis of a pH-regulated operon from Rhizobium tropici CIAT899 involved in acid tolerance and nodulation competitiveness. Mol. Plant Microbe Interact. 2003, 16, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nutman, P.S. Physiological studies on nodule formation. 1. The relation between nodulation and lateral root formation in red clover. Ann. Bot. 1948, 12, 81–96. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Larue, T.A.; Doyle, J. Is the legume nodule a modified root or stem or an organ sui generis? Crit. Rev. Plant Sci. 1997, 16, 361–392. [Google Scholar] [CrossRef]
- Mathesius, U.; Weinman, J.J.; Rolfe, B.G.; Djordjevic, M.A. Rhizobia can induce nodules in white clover by “hijacking” mature cortical cells activated during lateral root development. Mol. Plant Microbe Interact. 2000, 13, 170–182. [Google Scholar] [CrossRef] [PubMed]
- De Billy, F.; Grosjean, C.; May, S.; Bennett, M.; Cullimore, J.V. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol. Plant Microbe Interact. 2001, 14, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Roudier, F.; Fedorova, E.; Lebris, M.; Lecomte, P.; Györgyey, J.; Vaubert, D.; Horvath, G.; Abad, P.; Kondorosi, A.; Kondorosi, E. The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiol. 2003, 131, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Bright, L.J.; Liang, Y.; Mitchell, D.M.; Harris, J.M. The LATD gene of Medicago truncatula is required for both nodule and root development. Mol. Plant Microbe Interact. 2005, 18, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, G.J.; Stougaard, J. Root nodulation: A paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Stahl, Y.; Simon, R. Plant primary meristems: Shared functions and regulatory mechanisms. Curr. Opin. Plant Biol. 2010, 13, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.K.; Ekman, D.; Light, S.; Frey-Skott, J.; Elofsson, A. Domain rearrangements in protein evolution. J. Mol. Biol. 2005, 353, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Osipova, M.A.; Dolgikh, E.A.; Lutova, L.A. Features of the expression of a meristem-specific WOX5 gene during nodule organogenesis in legumes. Ontogenez 2011, 42, 264–275. [Google Scholar] [PubMed]
- Osipova, M.A.; Mortier, V.; Demchenko, K.N.; Tsyganov, V.E.; Tikhonovich, I.A.; Lutova, L.A.; Dolgikh, E.A.; Goormachtig, S. WUSCHEL-RELATED HOMEOBOX5 gene expression and interaction of CLE peptides with components of the systemic control add two pieces to the puzzle of autoregulation of nodulation. Plant Physiol. 2012, 158, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Roux, B.; Rodde, N.; Jardinaud, M.F.; Timmers, T.; Sauviac, L.; Cottret, L.; Carrère, S.; Sallet, E.; Courcelle, E.; Moreau, S.; et al. An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to RNA sequencing. Plant J. 2014, 77, 817–837. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthikala, M.-K.; Nanjareddy, K.; Lara, M. In BPS1 Downregulated Roots, the BYPASS1 Signal Disrupts the Induction of Cortical Cell Divisions in Bean-Rhizobium Symbiosis. Genes 2018, 9, 11. https://doi.org/10.3390/genes9010011
Arthikala M-K, Nanjareddy K, Lara M. In BPS1 Downregulated Roots, the BYPASS1 Signal Disrupts the Induction of Cortical Cell Divisions in Bean-Rhizobium Symbiosis. Genes. 2018; 9(1):11. https://doi.org/10.3390/genes9010011
Chicago/Turabian StyleArthikala, Manoj-Kumar, Kalpana Nanjareddy, and Miguel Lara. 2018. "In BPS1 Downregulated Roots, the BYPASS1 Signal Disrupts the Induction of Cortical Cell Divisions in Bean-Rhizobium Symbiosis" Genes 9, no. 1: 11. https://doi.org/10.3390/genes9010011





