What Does the Future Hold for Yellow Fever Virus? (I)
Abstract
:1. Introduction, a Historical Perspective
2. Ecology of Yellow Fever Virus
2.1. In Africa
2.2. In South America
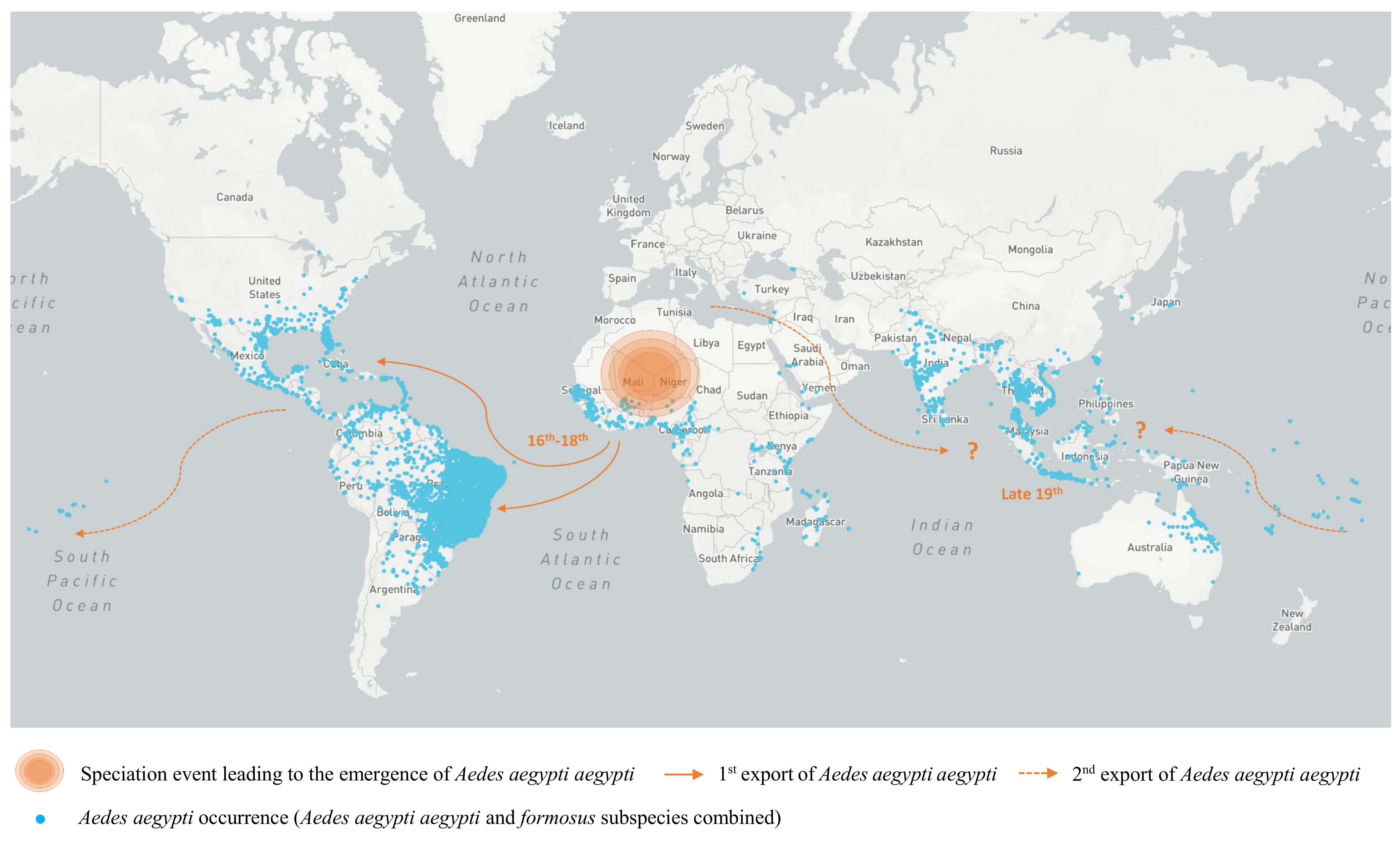
2.3. Heterogeneous Populations within the Domestic Vector Species Aedes (Stegomyia) Aegypti (Linnaeus)
3. Evolution and Dispersal of Yellow Fever Virus Strains
3.1. Phylogeny
3.2. Emergence Out of Africa
- Five genotypes of YFV have been identified in Africa [138] while two descendant lineages were reported in the Americas [116,145]. As phylogenetic studies showed broadly equivalent rates of nucleotide substitution among African and American isolates [1], the emergence of the five distinct YF genotypes in Africa must have required a longer time span than that of the two American genotypes.
- According to phylogenetic reconstructions, the deepest (i.e., most ancient) phylogenetic node corresponds to the common ancestor of the Angolan and East African lineages, further supporting an African origin for YFV [1].
- There is an association between genotypes and the number of imperfectly repeated sequences (RYFs) in the 3′ untranslated region (UTR) of YFV genomes [142,143]. The higher number of RYFs in African YFV sequences also supports the concept of evolution of South American genotypes from West African genotypes notably through the deletion of RYF(s) [142,143,152].
- Phylogenetic reconstructions based on related flaviviral sequences indicate that YFV is most closely related to Old World flaviviruses. Several evolutionary lineages diverged from YFV in Africa (Uganda S, Banzi, Jugra, Wesselsbron) [3,150,153], some of which gave rise to lineages that spread to Asia and Australia (Sepik, Edge Hill viruses) [154]. In contrast, none of these viruses has emerged in America [85].
4. Yellow Fever Virus Epidemiology: A Wide but Not Global, Circulation
4.1. In Africa
4.2. In South America
4.3. In Asia
- genetic resistance in Asian populations [2]
5. Discussion
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D. Out of Africa: A molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007, 3, e75. [Google Scholar] [CrossRef] [PubMed]
- Strode, G.K. Yellow Fever; McGraw-Hill: New York, NY, USA, 1951; pp. 77–78. [Google Scholar]
- Gould, E.A.; de Lamballerie, X.; Zanotto, P.M.; Holmes, E.C. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv Virus Res. 2003, 59, 277–314. [Google Scholar] [PubMed]
- Tabachnick, W.J. Evolutionary genetics and arthropod-borne diseases: The yellow fever mosquito, Aedes aegypti. Am. J. Entomol. 1991, 37, 14–24. [Google Scholar] [CrossRef]
- Carter, H.R. Yellow fever: An epidemiological and historical study of its place of origin. J. Am. Med. Assoc. 1931, 97, 1645. [Google Scholar]
- Findlay, G.M. John Williams and the early history of yellow fever. Br. Med J. 1948, 2, 474–476. [Google Scholar] [PubMed]
- Schotte, J.P.; Murray, J.; Scott, M. A Treatise on the Synochus Atrabiliosa, a Contagious Fever: Wich Raged at Senegal in the Year 1778, and Proved Fatal to the Greatest Part of the Europeans, and to a Number of the Natives: To Which is Prefixed, a Journal of the Weather during the Prevalence of that Disease ...; And Annexed to it, a Short Reflexion on the Gum Trade of Senegal, and the Importance of the Place on that Account; Printed for the author, by M. Scott, and sold by J. Murray: London, UK, 1782; Volume 169, p. 70. [Google Scholar]
- Eckert, J. In the days of the epidemic: The 1793 yellow fever outbreak in Philadelphia as seen by physicians. Trans Stud. Coll. Phys. Phila. 1993, 15, 31–38. [Google Scholar]
- Rush, B. Medical Inquiries and Observations; J. Conrad & Co.: Renton, WA, USA, 1796. [Google Scholar]
- Legan, M.S. Mississippi and the yellow fever epidemics of 1878–1879. J. Miss Hist. 1971, 33, 199–217. [Google Scholar] [PubMed]
- Bloom, K.J. The Mississippi Valley’s Great Yellow Fever Epidemic of 1878; Louisiana State University Press: Baton Rouge, LA, USA, 1993. [Google Scholar]
- Pascual Artiaga, M. The city in the face of contagion: Political measures and administrative dictates in the 1804 yellow fever epidemic in Alicante. Asclepio 2002, 54, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Van Heiningen, T.W. Contagiousness of yellow fever in the Netherlands between 1820 and 1825. Medical and sanitary aspects of a polymorphic disease. Hist. Sci. Med. 2006, 40, 9–22. [Google Scholar] [PubMed]
- Chastel, C. The "plague" of Barcelona. Yellow fever epidemic of 1821. Bull. Soc. Pathol. Exot. 1999, 92, 405–407. [Google Scholar] [PubMed]
- Waddell, D. Yellow fever in Europe in the early 19th century—Cadiz 1819. Rep. Proc. Scott. Soc. Hist. Med. 1990–1992, 20–34. [Google Scholar]
- Smith, C.E.; Gibson, M.E. Yellow fever in South Wales, 1865. Med. Hist. 1986, 30, 322–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, G. Tropical Medicine: An Illustrated History of The Pioneers; Elsevier: New York City, NY, USA, 2007. [Google Scholar]
- Manson, P. On the development of Filaria sanguinis Hominis, and on the mosquito considered as a nurse. J. Linn. Soc. Zool. 1879, 14, 304–311. [Google Scholar] [CrossRef]
- Manson, P. Further observations on Filaria sanguinis Hominis. Medical Reports, for the half-year ended 30th September, 1877. Med. Rep. China Imp. Marit. Cust. Shanghai 1878, 14, 1–26. [Google Scholar]
- Finlay, C.J. The mosquito hypothetically considered as the agent of transmission of yellow fever. Mil. Med. 2001, 166, 6–10. [Google Scholar] [CrossRef]
- Carter, H.R. A note on the interval between infecting and secondary cases of yellow fever from the records of the yellow fever at Orwood and Taylor, Miss., in 1898. New Orleans Med. Surg. J. 1900, 52, 617–636. [Google Scholar] [CrossRef]
- Carter, H.R. A correlation of some facts in the propagation of yellow fever, with the theory of its conveyance by the Culex fasciatus. Phila. Med. J. 1901, 7, 694–696. [Google Scholar]
- Carter, H.R. The period of incubation of yellow fever: A study from unpublished observations. Med. Rec. 1901, 59, 361–367. [Google Scholar]
- Carter, H.R. A note on the spread of yellow fever in houses. Extrinsic incubation. Med. Rec. 1901, 59, 933–937. [Google Scholar]
- Nott, J.C. The cause of yellow fever. New Orleans Med. Surg. J. 1848, 4, 563–601. [Google Scholar]
- Reed, W.; Carroll, J.; Agramonte, A.; Lazear, J.W. The etiology of yellow fever-a preliminary note. Public Health Pap. Rep. 1900, 26, 37–53. [Google Scholar] [PubMed]
- Reed, W.; Carroll, J.; Agramonte, A. Experimental yellow fever. 1901. Mil. Med. 2001, 166, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.; Carroll, J. The etiology of yellow fever: A supplemental note. 1902. Mil. Med. 2001, 166, 62–66. [Google Scholar] [CrossRef]
- Clements, A.N.; Harbach, R.E. History of the discovery of the mode of transmission of yellow fever virus. J. Vector Ecol. 2017, 42, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, A.; Bauert, J.H.; Hudson, N.P. Experimental transmission of yellow fever virus to laboratory animals. Int. J. Infect. Dis. 1997, 2, 54–59. [Google Scholar] [CrossRef]
- American Association for the Advancement of Science. The Bacillus icteroides as the cause of yellow fever. Science 1899, 10, 379–380. [Google Scholar]
- Stokes, A.; Bauer, J.H.; Hudson, N.P. The transmission of yellow fever to Macacus rhesus. JAMA 1928, 90, 253–254. [Google Scholar] [CrossRef]
- Stokes, A.; Bauer, J.H.; Hudson, N.P. Experimental transmission of yellow fever to laboratory animals. Am. J. Trop. Med. 1928, 8, 103–164. [Google Scholar] [CrossRef]
- Laigret, J. Hommage to Jean Laigret (1893–1966). Small history of the discovery of the vaccination against yellow fever. Press. Med. 1966, 74, 2441–2442. [Google Scholar] [PubMed]
- Theiler, M. Susceptibility of white mice to the virus of yellow fever. Science 1930, 71, 367. [Google Scholar] [CrossRef] [PubMed]
- Carrel, A. On the permanent life of tissues outside of the organism. J. Exp. Med. 1912, 15, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Haagen, E.; Theiler, M. Studies of yellow fever virus in tissue culture. Proc. Soc. Exp. Biol. Med. 1932, 29, 435–436. [Google Scholar] [CrossRef]
- Theiler, M.; Smith, H.H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937, 65, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Theiler, M.; Smith, H.H. The effect of prolonged cultivation in vitro upon the pathogenicity of yellow fever virus. J. Exp. Med. 1937, 65, 767–786. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.G.S. Yellow Fever Vaccine, 6th ed.; Elsevier: New York City, NY, USA, 2012; pp. 870–968. [Google Scholar]
- Holbrook, M.R. Historical perspectives on flavivirus research. Viruses 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Cornet, M.; Mouchet, J.; Herve, J.P.; Robert, V.; Camicas, J.L.; Cordellier, R.; Hervy, J.P.; Digoutte, J.P.; Monath, T.P.; et al. Sylvatic yellow fever in Africa recent advances and present approach (author’s transl). Med. Trop. 1981, 41, 31–43. [Google Scholar]
- Shannon, R.C.; Whitman, L.; Franca, M. Yellow fever virus in jungle mosquitoes. Science 1938, 88, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W.; Gillett, J.D. The cyclical transmission of yellow fever virus through the grivet monkey, Cercopithecus aethiops centralis Neumann, and the mosquito Aedes (Stegomyia) africanus Theobald. Ann. Trop. Med. Parasitol. 1950, 44, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Andral, L.; Bres, P.; Serie, C.; Casals, J.; Panthier, R. Studies on yellow fever in Ethiopia. 3. Serological and virological studies of the woodland fauna. Bull World Health Organ. 1968, 38, 855–861. [Google Scholar] [PubMed]
- Haddow, A.J. A review of the results of yellow fever protection-tests on the sera of primates from Kenya. Ann. Trop. Med. Parasitol. 1952, 46, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Haddow, A.J.; Lumsden, W.H. An outbreak of sylvan yellow fever in Uganda with Aedes (Stegomyia) africanus Theobald as principal vector and insect host of the virus. Ann. Trop. Med. Parasitol. 1949, 43, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Craven, R.B.; Adjukiewicz, A.; Germain, M.; Francy, D.B.; Ferrara, L.; Samba, E.M.; N’jie, H.; Cham, K.; Fitzgerald, S.A.; et al. Yellow fever in the Gambia, 1978–1979: Epidemiologic aspects with observations on the occurrence of orungo virus infections. Am. J. Trop. Med. Hyg. 1980, 29, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.; Monath, T.P. Epidemiology and ecology of yellow fever virus. Adv. Virus Res. 2003, 61, 291–315. [Google Scholar] [PubMed]
- Germain, M.; Francy, D.B.; Monath, T.P.; Ferrara, L.; Bryan, J.; Salaun, J.J.; Heme, G.; Renaudet, J.; Adam, C.; Digoutte, J.P. Yellow fever in the Gambia, 1978–1979: Entomological aspects and epidemiological correlations. Am. J. Trop. Med. Hyg. 1980, 29, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Carrington, C.V.; Auguste, A.J. Evolutionary and ecological factors underlying the tempo and distribution of yellow fever virus activity. Infect. Genet. Evol. 2013, 13, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Shearer, F.M.; Longbottom, J.; Browne, A.J.; Pigott, D.M.; Brady, O.J.; Kraemer, M.U.G.; Marinho, F.; Yactayo, S.; de Araújo, V.E.; da Nóbrega, A.A.; et al. Existing and potential infection risk zones of yellow fever worldwide: A modelling analysis. Lancet Glob. Health 2018, 6, e270–e278. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C.; Freitas, D.S.D. The role of monkeys, mosquitoes, and humans in the occurrence of a yellow fever outbreak in a fragmented landscape in South Brazil: Protecting howler monkeys is a matter of public health. Trop. Conserv. Sci. 2010, 3, 78–89. [Google Scholar] [CrossRef]
- Bugher, J.C. The mammalian host in yellow fever. In Yellow Fever; Strode, G.K., Ed.; McGraw Hill: New York, NY, USA, 1951; pp. 299–384. [Google Scholar]
- Rawlins, S.C.; Hull, B.; Chadee, D.D.; Martinez, R.; LeMaitre, A.; James, F.; Webb, L. Sylvatic yellow fever activity in Trinidad, 1988–1989. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 142–143. [Google Scholar] [CrossRef]
- Auguste, A.J.; Lemey, P.; Bergren, N.A.; Giambalvo, D.; Moncada, M.; Moron, D.; Hernandez, R.; Navarro, J.C.; Weaver, S.C. Enzootic transmission of yellow fever virus, Venezuela. Emerg. Infect. Dis. 2015, 21, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Wang, H.; Cabezas, C.; Ramirez, G.; Watts, D.; Russell, K.; Barrett, A. Enzootic transmission of yellow fever virus in Peru. Emerg. Infect. Dis. 2003, 9, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.R.; Barrett, A.D. The enigma of yellow fever in East Africa. Rev. Med. Virol. 2008, 18, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016, 12, e1005548. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.; Haseeb, M.A. Animals and yellow fever infection in the Anglo-Egyptian Sudan. Ann. Trop. Med. Parasitol. 1953, 47, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cornet, J.P.; Huard, M.; Camicas, J.L.; Herve, J.P.; Germain, M. Experimental transmission of the yellow fever virus by the tick Amblyomma variegatum (F.) (author’s transl). Bull. Soc. Pathol. Exot. Fil. 1982, 75, 136–140. [Google Scholar]
- Germain, M.; Saluzzo, J.F.; Cornet, J.P.; Herve, J.P.; Sureau, P.; Camicas, J.L.; Robin, Y.; Salaün, J.J.; Heme, G. Isolation of the yellow fever virus from an egg-cluster and the larvae of the tick Amblyomma variegatum. CR Seances Acad. Sci. D 1979, 289, 635–637. [Google Scholar]
- Jentes, E.S.; Poumerol, G.; Gershman, M.D.; Hill, D.R.; Lemarchand, J.; Lewis, R.F.; Staples, J.E.; Tomori, O.; Wilder-Smith, A.; Monath, T.P. The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 2011, 11, 622–632. [Google Scholar] [CrossRef]
- Germain, M. SPHJFJMJRYGBCJaVMF. Isolements du virus de la fièvre jaune à partir d’Aedes du groupe A. africanus (Theobald) en République Centrafricaine : Importance des savanes humides et semi-humides en tant que zone d’émergence du virus amaril. Cahiers ORSTOM Sér. Entomol. Méd. Parasitol. 1976, 14, 125–139. [Google Scholar]
- Herve, J.P.; Germain, M.; Geoffroy, B. Bioécologie comparée d’Aedes (Stegomyia) opok Corbet et Van Someren et A. (S.) africanus (Theobald) dans une galerie forestière du sud de l’Empire Centrafricain, I.I. Cycles saisonniers d’abondance. Cahiers ORSTOM Sér. Entomol. Méd. Parasitol. 1977, 15, 271–282. [Google Scholar]
- Huang, Y. The subgenus Stegomyia of Aedes in the Afrotropical region. I. The africanus group of species (Diptera: Culicidae). Contrib. Am. Entomol. Inst. 1990, 26, 1–90. [Google Scholar]
- World Health Organization. Dengue and Severe Dengue. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed on 4 June 2018).
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Ngoagouni, C.; Kamgang, B.; Nakoune, E.; Paupy, C.; Kazanji, M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: What consequences for emerging diseases? Parasites Vectors 2015, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Ollomo, B.; Kamgang, B.; Moutailler, S.; Rousset, D.; Demanou, M.; et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector-Borne Zoonotic Dis. 2010, 10, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Kassa Kassa, F.; Caron, M.; Nkoghe, D.; Leroy, E.M. A Chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector-Borne Zoonotic Dis. 2012, 12, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Paupy, C.; Grard, G.; Becquart, P.; Mombo, I.; Nso, B.B.; Kassa, F.; Nkoghe, D.; Leroy, E.M. Recent introduction and rapid dissemination of Chikungunya virus and Dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin. Infect. Dis. 2012, 55, e45–e53. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Miller, B.R.; Gubler, D.J. Vector competence of Aedes albopictus from Houston, Texas, for dengue serotypes 1 to 4, yellow fever and Ross River viruses. J. Am. Mosq. Control Assoc. 1987, 3, 460–465. [Google Scholar] [PubMed]
- Amraoui, F.; Vazeille, M.; Failloux, A.B. French Aedes albopictus are able to transmit yellow fever virus. Eurosurveillance 2016, 21, 30361. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.A.; Santos, F.B.D.; Vazeille, M.; da Costa Vasconcelos, P.F.; Lourenço-de-Oliveira, R.; Failloux, A.B. Potential risk of re-emergence of urban transmission of yellow fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M. Yellow Fever Expert Committee. Yellow Fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germain, M.C.; Cornet, M.; Mouchet, J.; Monath, T.P.; Hervé, J.; Salaün, J.J.; Cordellier, R.; Saluzzo, J.F.; Camicas, J.; Hervy, J.; et al. Recent advances in research regarding sylvatic yellow fever in West and Central Africa. Bull. Inst. Pasteur 1982, 80, 315–330. [Google Scholar]
- Huang, Y.M. Aedes (Stegomyia) bromeliae (Diptera: Culicidae), the yellow fever virus vector in East Africa. J. Med. Entomol. 1986, 23, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.J. Yellow fever in Central Uganda, 1964. Part I. Historical introduction. Trans. R. Soc. Trop. Med. Hyg. 1965, 59, 436–440. [Google Scholar] [CrossRef]
- Bauer, J.H. The transmission of yellow fever by mosquitoes other than Aedes aegypti. J. Am. Med. Assoc. 1928, 26, 2091–2092. [Google Scholar] [CrossRef]
- Philip, C.B. Preliminary report of further tests with yellow fever transmission by mosquitoes other than Aedes Aegypti. Am. J. Trop. Med. Hyg. 1929, 9, 267–269. [Google Scholar] [CrossRef]
- Henderson, B.E.; Metselaar, D.; Kirya, G.B.; Timms, G.L. Investigations into yellow fever virus and other arboviruses in the northern regions of Kenya. Bull. World Health Organ. 1970, 42, 787–795. [Google Scholar] [PubMed]
- Reiter, P.; Cordellier, R.; Ouma, J.O.; Cropp, C.B.; Savage, H.M.; Sanders, E.J.; Marfin, A.A.; Tukei, P.M.; Agata, N.N.; Gitau, L.G.; et al. First recorded outbreak of yellow fever in Kenya, 1992-1993. II. Entomologic investigations. Am. J. Trop. Med. Hyg. 1998, 59, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Jupp, P.G.; Kemp, A. Laboratory vector competence experiments with yellow fever virus and five South African mosquito species including Aedes aegypti. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 493–498. [Google Scholar] [CrossRef]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. 2013, 19, 292–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilay, A.; Asamene, N.; Bekele, A.; Mengesha, M.; Wendabeku, M.; Tareke, I.; Girmay, A.; Wuletaw, Y.; Adossa, A.; Ba, Y.; et al. Reemergence of yellow fever in Ethiopia after 50 years, 2013: Epidemiological and entomological investigations. BMC Infect Dis. 2017, 17, 343. [Google Scholar] [CrossRef] [PubMed]
- Cordellier, R.; Bouchité, B.; Roche, J.C.M.; Monteny, N.; Diaco, B.; Akoliba, P. Circulation selvatique du virus Dengue 2 en 1980, dans les savanes sub-soudaniennes de Côte d’Ivoire: Données entomologiques et considérations épidémiologiques. Cahiers ORSTOM Sér. Entomol. Méd. Parasitol. 1983, 21, 165–179. [Google Scholar]
- Miller, B.R.; Monath, T.P.; Tabachnick, W.J.; Ezike, V.I. Epidemic yellow fever caused by an incompetent mosquito vector. Trop. Med. Parasitol. 1989, 40, 396–399. [Google Scholar] [PubMed]
- Monath, T.P. Yellow fever as an endemic/epidemic disease and priorities for vaccination. Bull. Soc. Pathol. Exot. 2006, 99, 341–347. [Google Scholar] [PubMed]
- Cornet, M.; Robin, Y.; Heme, G. Une poussée épizootique de fièvre jaune selvatique au Sénégal oriental. Isolement du virus de lots de moustiques adultes mâles et femelles. Med. Mal. Infect. 1979, 9, 63–66. [Google Scholar] [CrossRef]
- Aitken, T.H.; Tesh, R.B.; Beaty, B.J.; Rosen, L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am. J. Trop. Med. Hyg. 1979, 28, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Tesh, R.B.; Aitken, T.H. Transovarial transmission of yellow fever virus in Stegomyia mosquitoes. Am. J. Trop. Med. Hyg. 1980, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Salaun, J.J.; Germain, M.; Robert, V.; Robin, Y.; Monath, T.P.; Camicas, J.L.; Digoutte, J.P. Yellow fever in Senegal from 1976 to 1980 (author’s transl). Med. Trop. 1981, 41, 45–51. [Google Scholar]
- Rosen, L. Transovarial transmission of arboviruses by mosquitoes (author’s transl). Med. Trop. 1981, 41, 23–29. [Google Scholar]
- Fontenille, D.; Diallo, M.; Mondo, M.; Ndiaye, M.; Thonnon, J. First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 533–535. [Google Scholar] [CrossRef]
- Diallo, M.; Thonnon, J.; Fontenille, D. Vertical transmission of the yellow fever virus by Aedes aegypti (Diptera, Culicidae): Dynamics of infection in F1 adult progeny of orally infected females. Am. J. Trop. Med. Hyg. 2000, 62, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.M.; Rambaut, A.; Pybus, O.G.; Holmes, E.C. Rates of molecular evolution in RNA viruses: A quantitative phylogenetic analysis. J. Mol. Evol. 2002, 54, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Sall, A.A.; Faye, O.; Diallo, M.; Firth, C.; Kitchen, A.; Holmes, E.C. Yellow fever virus exhibits slower evolutionary dynamics than dengue virus. J. Virol. 2010, 84, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, P.M.; Gao, G.F.; Gritsun, T.; Marin, M.S.; Jiang, W.R.; Venugopal, K.; Reid, H.W.; Gould, E.A. An arbovirus cline across the northern hemisphere. Virology 1995, 210, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Slovak, M.; Kazimirova, M.; Siebenstichova, M.; Ustanikova, K.; Klempa, B.; Gritsun, T.; Gould, E.A.; Nuttall, P.A. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick-Borne Dis. 2014, 5, 962–969. [Google Scholar] [CrossRef] [PubMed]
- McCrae, A.W.; Kirya, B.G. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 552–562. [Google Scholar] [CrossRef]
- Holzmann, I.; Agostini, I.; Areta, J.I.; Ferreyra, H.; Beldomenico, P.; Di Bitetti, M.S. Impact of yellow fever outbreaks on two howler monkey species (Alouatta guariba clamitans and A. caraya) in Misiones, Argentina. Am. J. Primatol. 2010, 72, 475–480. [Google Scholar] [PubMed]
- De Almeida, M.A.; Dos Santos, E.; da Cruz Cardoso, J.; da Fonseca, D.F.; Noll, C.A.; Silveira, V.R.; Maeda, A.Y.; Souza, R.P.D.; Kanamura, C.; Brasil, R.A. Yellow fever outbreak affecting Alouatta populations in southern Brazil (Rio Grande do Sul State), 2008–2009. Am. J. Primatol. 2012, 74, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.S.; Spinola, R.; Tengan, C.H.; Brasil, R.A.; Siciliano, M.M.; Coimbra, T.L.; Silveira, V.R.; Rocco, I.M.; Bisordi, I.; Souza, R.P.D.; et al. Yellow fever epizootics in non-human primates, Sao Paulo state, Brazil, 2008–2009. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 45–50. [Google Scholar] [CrossRef] [PubMed]
- De Rodaniche, E.; Galindo, P.; Johnson, C.M. Isolation of yellow fever virus from Haemagogus lucifer, H. equinus, H. spegazzinii falco, Sabethes chloropterus and Anopheles neivai captured in Panama in the fall of 1956. Am. J. Trop. Med. Hyg. 1957, 6, 681–685. [Google Scholar] [CrossRef] [PubMed]
- De Rodaniche, E.; Galindo, P. Isolation of yellow fever virus from Haemagogus mesodentatus, H. equinus and Sabethes chloropterus captured in Guatemala in 1956. Am. J. Trop. Med. Hyg. 1957, 6, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Dutary, B.E.; Leduc, J.W. Transovarial transmission of yellow fever virus by a sylvatic vector, Haemagogus equinus. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 128. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Sperb, A.F.; Monteiro, H.A.; Torres, M.A.; Sousa, M.R.; Vasconcelos, H.B.; Mardini, L.B. and Rodrigues, S.G. Isolations of yellow fever virus from Haemagogus leucocelaenus in Rio Grande do Sul State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 60–62. [Google Scholar] [CrossRef]
- Cardoso Jda, C.; de Almeida, M.A.; dos Santos, E.; da Fonseca, D.F.; Sallum, M.A.; Noll, C.A.; Monteiro, H.A.D.O.; Cruz, A.C.; Carvalho, V.L.; Pinto, E.V.; et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, southern Brazil, 2008. Emerg. Infect. Dis. 2010, 16, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Soper, F.L. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. Am. J. Public Health Nations Health 1963, 53, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Slosek, J. Aedes aegypti mosquitoes in the Americas: A review of their interactions with the human population. Soc. Sci. Med. 1986, 23, 249–257. [Google Scholar] [CrossRef]
- Le Prince, J.A.A.; Orenstein, A.J. Mosquito Control in Panama; the Eradication of Malaria and Yellow Fever in Cuba and Panama; Putnam: New York, NY, USA, 1916. [Google Scholar]
- PAHO. Control of YFV, Field Guide, 2005. 2005. Available online: http://www.paho.org/english/ad/fch/im/fieldguide_yellowfever.pdf (accessed on 4 June 2018).
- Vasconcelos, P.F. Yellow fever in Brazil: Thoughts and hypotheses on the emergence in previously free areas. Rev. Saúde Pública 2010, 44, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, P.N.; Aldighieri, S.; Machado, G.; Leonel, D.G.; Vilca, L.M.; Uriona, S.; Schneider, M.C. Geographic patterns and environmental factors associated with human yellow fever presence in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005897. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.; Bryant, J.E.; da Rosa, T.P.; Tesh, R.B.; Rodrigues, S.G.; Barrett, A.D. Genetic divergence and dispersal of yellow fever virus, Brazil. Emerg. Infect. Dis. 2004, 10, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, P.F. Genetical aspects of the Aedes aegypti problem. I. Taxonom: And bionomics. Ann. Trop. Med. Parasitol. 1957, 51, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, P.F. Taxonomy of Aedes aegypti and related species. Bull. World Health Organ. 1967, 36, 552–554. [Google Scholar] [PubMed]
- Brown, J.E.; Evans, B.R.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 2014, 68, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Gloria-Soria, A.; Ayala, D.; Bheecarry, A.; Calderon-Arguedas, O.; Chadee, D.D.; Chiappero, M.; Coetzee, M.; Elahee, K.B.; Fernandez-Salas, I.; Kamal, H.A.; et al. Global genetic diversity of Aedes aegypti. Mol. Ecol. 2016, 25, 5377–5395. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.L.; Shija, F.; Linton, Y.M.; Misinzo, G.; Kaddumukasa, M.; Djouaka, R.; Anyaele, O.; Harris, A.; Irish, S.; Hlaing, T. Historical environmental change in Africa drives divergence and admixture of Aedes aegypti mosquitoes: A precursor to successful worldwide colonization? Mol. Ecol. 2016, 25, 4337–4354. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; McBride, C.S.; Johnson, P.; Ritchie, S.; Paupy, C.; Bossin, H.; Lutomiah, J.; Fernandez-Salas, I.; Ponlawat, A.; Cornel, A.J. Worldwide patterns of genetic differentiation imply multiple ’domestications’ of Aedes aegypti, a major vector of human diseases. Proc. Biol. Sci. 2011, 278, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Le Goff, G.; Brengues, C.; Guerra, M.; Revollo, J.; Barja Simon, Z.; Hervé, J.P.; Fontenille, D. Genetic structure and phylogeography of Aedes aegypti, the dengue and yellow-fever mosquito vector in Bolivia. Infect. Genet. Evol. 2012, 12, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Curtin, T.J. Status of Aedes aegypti in the Eastern Mediterranean. J. Med. Entomol. 1967, 4, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Holstein, M. Dynamics of Aedes aegypti distribution, density and seasonal prevalence in the Mediterranean area. Bull. World Health Organ. 1967, 36, 541–543. [Google Scholar] [PubMed]
- Gubler, D.J. The changing epidemiology of yellow fever and dengue, 1900 to 2003: Full circle? Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Linss, J.G.; Brito, L.P.; Garcia, G.A.; Araki, A.S.; Bruno, R.V.; Lima, J.B.; Valle, D.; Martins, A.J. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasites Vectors 2014, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.A.; Schama, R.; Shama, R.; Martins, A.J.; Gloria-Soria, A.; Brown, J.E.; Powell, J.R. Genetic diversity of Brazilian Aedes aegypti: Patterns following an eradication program. PLoS Negl. Trop. Dis. 2014, 8, e3167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z. Adaptive diversification between yellow fever virus west african and south american lineages: A genome-wide study. Am. J. Trop. Med. Hyg. 2017, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Cardosa, J.; Hanley, K.A.; Holmes, E.C.; Weaver, S.C. Fever from the forest: Prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011, 9, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Abrao, E.P.; da Fonseca, B.A. Infection of mosquito cells (C6/36) by Dengue-2 virus interferes with subsequent infection by yellow fever virus. Vector Borne Zoonotic Dis. 2016, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ruckert, C.; Weger-Lucarelli, J.; Garcia-Luna, S.M.; Young, M.C.; Byas, A.D.; Murrieta, R.A.; Fauver, J.R.; Ebel, G.D. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 2017, 8, 15412. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer Paploski, I.A.; Souza, R.L.; Tauro, L.B.; Cardoso, C.W.; Mugabe, V.A.; Pereira Simoes Alves, A.B.; de Jesus Gomes, J.; Kikuti, M.; Campos, G.S.; Sardi, S.; et al. Epizootic outbreak of yellow fever virus and risk for human disease in Salvador, Brazil. Ann. Intern. Med. 2017, 165, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Monath, T.P. Responding to the threat of urban yellow fever outbreaks. Lancet Infect. Dis. 2017, 17, 248–250. [Google Scholar] [CrossRef]
- Possas, C.; Martins, R.M.; Oliveira, R.L.; Homma, A. Urgent call for action: Avoiding spread and re-urbanisation of yellow fever in Brazil. Mem. Inst. Oswaldo Cruz 2018, 113, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, J.P.; Wang, H.; Li, L.; Bryant, J.E.; Barrett, A.D. Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. J. Virol. 2001, 75, 6999–7008. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Lemey, P.; Pybus, O.G.; Suchard, M.A.; Salas, R.A.; Adesiyun, A.A.; Barrett, A.D.; Tesh, R.B.; Weaver, S.C.; Carrington, C.V. Yellow fever virus maintenance in Trinidad and its dispersal throughout the Americas. J. Virol. 2010, 84, 9967–9977. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.J.; Cropp, B.C.; Kinney, R.M.; Trent, D.W.; Gubler, D.J. Nucleotide sequence variation of the envelope protein gene identifies two distinct genotypes of yellow fever virus. J. Virol. 1995, 69, 5773–5780. [Google Scholar] [PubMed]
- Lepiniec, L.; Dalgarno, L.; Huong, V.T.; Monath, T.P.; Digoutte, J.P.; Deubel, V. Geographic distribution and evolution of yellow fever viruses based on direct sequencing of genomic cDNA fragments. J. Gen. Virol. 1994, 75, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutebi, J.P.; Rijnbrand, R.C.; Wang, H.; Ryman, K.D.; Wang, E.; Fulop, L.D.; Titball, R.; Barrett, A.D. Genetic relationships and evolution of genotypes of yellow fever virus and other members of the yellow fever virus group within the Flavivirus genus based on the 3’ noncoding region. J. Virol. 2004, 78, 9652–9665. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Weaver, S.C.; Shope, R.E.; Tesh, R.B.; Watts, D.M.; Barrett, A.D. Genetic variation in yellow fever virus: Duplication in the 3’ noncoding region of strains from Africa. Virology 1996, 225, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Guzman, H.; Li, L.; Ellis, B.; Tesh, R.B.; Barrett, A.D. Phylogeographic reconstruction of African yellow fever virus isolates indicates recent simultaneous dispersal into east and west Africa. PLoS Negl. Trop. Dis. 2013, 7, e1910. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.P.; Foster, P.G.; Sallum, M.A.; Coimbra, T.L.; Maeda, A.Y.; Silveira, V.R.; Moreno, E.S.; da Silva, F.G.; Rocco, I.M.; Ferreira, I.B.; et al. Detection of a new yellow fever virus lineage within the South American genotype I in Brazil. J. Med. Virol. 2010, 82, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mir, D.; Delatorre, E.; Bonaldo, M.; Lourenco-de-Oliveira, R.; Vicente, A.C.; Bello, G. Phylodynamics of yellow fever virus in the Americas: New insights into the origin of the 2017 Brazilian outbreak. Sci. Rep. 2017, 7, 7385. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.M.; Abreu, F.V.S.; Santos, A.A.C.D.; Mello, I.S.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; de Miranda, R.M.; de Castro, M.G.; Ribeiro, M.S.; et al. Genomic and structural features of the yellow fever virus from the 2016-2017 Brazilian outbreak. J. Gen. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Soto, A.; Torres, M.C.; Lima de Mendonca, M.C.; Mares-Guia, M.A.; Damasceno Dos Santos Rodrigues, C.; Fabri, A.; dos Santos, C.C.; Araújo, E.M.; Fischer, C.; Nogueira, R.R.; et al. Evidence for multiple sylvatic transmission cycles during the 2016-2017 yellow fever virus outbreak, Brazil. Clin. Microbiol. Infect. 2018. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.A.; de Lamballerie, X.; Zanotto, P.M.; Holmes, E.C. Evolution, epidemiology, and dispersal of flaviviruses revealed by molecular phylogenies. Adv. Virus Res. 2001, 57, 71–103. [Google Scholar] [PubMed]
- Zanotto, P.M.; Gould, E.A.; Gao, G.F.; Harvey, P.H.; Holmes, E.C. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc. Natl. Acad. Sci. USA 1996, 93, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Vasconcelos, P.F.; Rijnbrand, R.C.; Mutebi, J.P.; Higgs, S.; Barrett, A.D. Size heterogeneity in the 3’ noncoding region of South American isolates of yellow fever virus. J. Virol. 2005, 79, 3807–3821. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Holmes, E.C. A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: Flaviviridae) and the evolution of vector transmission. Arch. Virol. 2006, 151, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Poidinger, M.; Mackenzie, J.S.; Russell, R.C.; Doggett, S.; Broom, A.K.; Phillips, D.; Potamski, J.; Gard, G.; Whelan, P.; et al. Molecular phylogeny of edge hill virus supports its position in the yellow fever virus group and identifies a new genetic variant. Evol. Bioinform. 2010, 6, 91–96. [Google Scholar] [CrossRef]
- Morillon, M.; Mafart, B.; Matton, T. Yellow fever in Europe in 19th century. In Ecological Aspects of Past Settlement in Europe; European Anthropological Association: Budapest, Hungary, 2002; pp. 1–23. [Google Scholar]
- WHO. Yellow Fever 2016. Available online: http://www.who.int/mediacentre/factsheets/fs100/en/ (accessed on 4 June 2018).
- WHO. Immunization, Vaccines and Biologicals. National Programmes and Systems. 2017. Available online: http://www.who.int/immunization/programmes_systems/en/ (accessed on 4 June 2018).
- Shearer, F.M.; Moyes, C.L.; Pigott, D.M.; Brady, O.J.; Marinho, F.; Deshpande, A.; Longbottom, J.; Browne, A.J.; Kraemer, M.U.; O’Reilly, K.M.; et al. Global yellow fever vaccination coverage from 1970 to 2016: An adjusted retrospective analysis. Lancet Infect. Dis. 2017, 17, 1209–1217. [Google Scholar] [CrossRef]
- World Health Organization. Progress in the control of yellow fever in Africa. Wkly Epidemiol. Rec. 2005, 80, 50–55. [Google Scholar]
- Ahmed, Q.A.; Memish, Z.A. Yellow fever from Angola and Congo: A storm gathers. Trop. Dr. 2017, 47, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Faria, N.R.; Reiner, R.C., Jr.; Golding, N.; Nikolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.R.; et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–2016: A modelling study. Lancet Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef]
- PAHO. 24 May 2017: Yellow fever—Epidemiological update. Available online: http://www.paho.org/hq/index.php?option=com_topics&view=article&id=69&Itemid=40784&lang=fr (accessed on 4 June 2018).
- Wamala, J.F.; Malimbo, M.; Okot, C.L.; Atai-Omoruto, A.D.; Tenywa, E.; Miller, J.R.; Balinandi, S.; Shoemaker, T.; Oyoo, C.; Omony, E.O.; et al. Epidemiological and laboratory characterization of a yellow fever outbreak in northern Uganda, October 2010–January 2011. Int. J. Infect. Dis. 2012, 16, e536–e542. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Pan, Y.; Lyu, Y.; Liang, Z.; Li, J.; Sun, Y.; Dou, X.; Tian, L.; Huo, D.; Chen, L.; et al. Detection of yellow fever virus genomes from four imported cases in China. Int. J. Infect. Dis. 2017, 60, 93–95. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Yellow fever in Africa and the Americas, 2016. Wkly Epidemiol. Rec. 2017, 92, 442–452. [Google Scholar]
- Chen, Z.; Liu, L.; Lv, Y.; Zhang, W.; Li, J.; Zhang, Y.; Di, T.; Zhang, S.; Liu, J.; Li, J.; et al. A fatal yellow fever virus infection in China: Description and lessons. Emerg. Microbes Infect. 2016, 5, e69. [Google Scholar] [CrossRef] [PubMed]
- WHO. Emergencies: Q&A: Yellow Fever Outbreak in Angola and Democratic Republic of the Congo; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Grobbelaar, A.A.; Weyer, J.; Moolla, N.; Jansen van Vuren, P.; Moises, F.; Paweska, J.T. Resurgence of yellow fever in Angola, 2015–2016. Emerg. Infect. Dis. 2016, 22, 1854–1855. [Google Scholar] [CrossRef] [PubMed]
- Control NNCfd. An update of yellow fever outbreak in Nigeria for Week 11. 2018. Available online: https://ncdc.gov.ng/diseases/sitreps/?cat=10&name=An%20update%20of%20Yellow%20Fever%20outbreak%20in%20Nigeria (accessed on 4 June 2018).
- Gardner, C.L.; Ryman, K.D. Yellow fever: A reemerging threat. Clin. Lab. Med. 2010, 30, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.A.; Cardoso Jda, C.; Dos Santos, E.; da Fonseca, D.F.; Cruz, L.L.; Faraco, F.J.; Bercini, M.A.; Vettorello, K.C.; Porto, M.A.; Mohrdieck, R.; et al. Surveillance for yellow fever virus in non-human primates in southern Brazil, 2001–2011: A tool for prioritizing human populations for vaccination. PLoS Negl. Trop. Dis. 2014, 8, e2741. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.P.; Costa, Z.G.; Ramos, D.G.; Andrade, M.A.; Jayme Vde, S.; Almeida, M.A.; Vettorello, K.C.; Mascheretti, M.; Flannery, B. Yellow fever outbreaks in unvaccinated populations, Brazil, 2008–2009. PLoS Negl. Trop. Dis. 2014, 8, e2740. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Epidemiological Update: Yellow Fever. 10 July 2017; Pan-American Health Organization: Washington, DC, USA, 2017. [Google Scholar]
- Saude, M.D. Monitoramento do Período Sazonal da Febre Amarela Brasil—2017/2018. 2018. Available online: portalarquivos2.saude.gov.br/images/pdf/2018/.../Informe-FA.pdf (accessed on 4 June 2018).
- PAHO. Epidemiological Update: Yellow Fever. 20 March 2018; Pan-American Health Organization: Washington, DC, USA, 2018. [Google Scholar]
- Bonaldo, M.C.; Gómez, M.M.; Dos Santos, A.A.; Abreu, F.V.S.; Ferreira-de-Brito, A.; Miranda, R.M.; Castro, M.G.D.; Lourenço-de-Oliveira, R. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil reveals polymorphisms. Mem. Inst. Oswaldo Cruz 2017, 112, 447–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.; de Jesus, J.G.; de Aguiar, R.S.; Iani, F.C.; Xavier, J.; Quick, J.; de Plessis, L.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. BioRxiv 2018, doi. BioRxiv 2018. [Google Scholar] [CrossRef]
- Rogers, D.J.; Wilson, A.J.; Hay, S.I.; Graham, A.J. The global distribution of yellow fever and dengue. Adv. Parasitol. 2006, 62, 181–220. [Google Scholar] [PubMed]
- Amaku, M.; Coutinho, F.A.; Massad, E. Why dengue and yellow fever coexist in some areas of the world and not in others? Biosystems 2011, 106, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, J.P.; Barrett, A.D. The epidemiology of yellow fever in Africa. Microbes Infect. 2002, 4, 1459–1468. [Google Scholar] [CrossRef]
- Tabachnick, W.J.; Wallis, G.P.; Aitken, T.H.; Miller, B.R.; Amato, G.D.; Lorenz, L.; Powell, J.R.; Beaty, B.J. Oral infection of Aedes aegypti with yellow fever virus: Geographic variation and genetic considerations. Am. J. Trop. Med. Hyg. 1985, 34, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Aitken, T.H.G. In vitro transmission of yellow fever virus by geographic strains of Aedes aegypti. Mosq. News 1979, 39, 232–238. [Google Scholar]
- Henderson, B.E.; Cheshire, P.P.; Kirya, G.B.; Lule, M. Immunologic studies with yellow fever and selected African group B arboviruses in rhesus and vervet monkeys. Am. J. Trop. Med. Hyg. 1970, 19, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Theiler, M.; Anderson, C.R. The relative resistance of dengue-immune monkeys to yellow fever virus. Am. J. Trop. Med. Hyg. 1975, 24, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, S.; Tambyah, P.A.; Lim, P.L. Yellow fever cases in Asia: Primed for an epidemic. Int. J. Infect. Dis. 2016, 48, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.; Monath, T.P. Yellow fever remains a potential threat to public health. Vector Borne Zoonotic Dis. 2016, 16, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.E.; Hull, B.P.; Tomori, O.; Bele, O.; LeDuc, J.W.; Esteves, K. Yellow fever: A decade of reemergence. JAMA 1996, 276, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.Y.; Guzman, H.; da Rosa, A.P.; Zhu, H.B.; Tesh, R.B. Alteration of clinical outcome and histopathology of yellow fever virus infection in a hamster model by previous infection with heterologous flaviviruses. Am. J. Trop. Med. Hyg. 2003, 68, 695–703. [Google Scholar] [PubMed]
- Wilder-Smith, A.; Leong, W.Y. Importation of yellow fever into China: Assessing travel patterns. J. Travel Med. 2017, 24. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klitting, R.; Gould, E.A.; Paupy, C.; De Lamballerie, X. What Does the Future Hold for Yellow Fever Virus? (I). Genes 2018, 9, 291. https://doi.org/10.3390/genes9060291
Klitting R, Gould EA, Paupy C, De Lamballerie X. What Does the Future Hold for Yellow Fever Virus? (I). Genes. 2018; 9(6):291. https://doi.org/10.3390/genes9060291
Chicago/Turabian StyleKlitting, Raphaëlle, Ernest A. Gould, Christophe Paupy, and Xavier De Lamballerie. 2018. "What Does the Future Hold for Yellow Fever Virus? (I)" Genes 9, no. 6: 291. https://doi.org/10.3390/genes9060291




