Water-Soluble Ionic Composition of Aerosols at Urban Location in the Foothills of Himalaya, Pokhara Valley, Nepal
Abstract
:1. Introduction
2. Sampling and Analysis
2.1. Overview of the Study Area
2.2. Sampling and Laboratory Analysis
2.3. Data Quality
3. Results and Discussion
3.1. Summary of Concentrations of Aerosol Mass and Water-Soluble Inorganic Ions
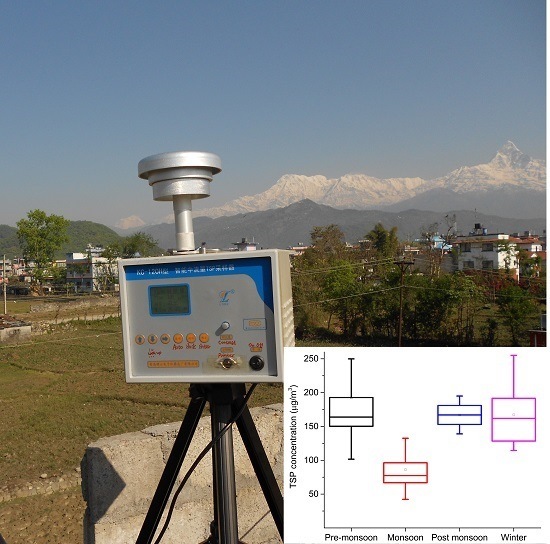
3.2. Seasonal Variations of TSP and Water-Soluble Inorganic Ions
3.3. Ionic Balance
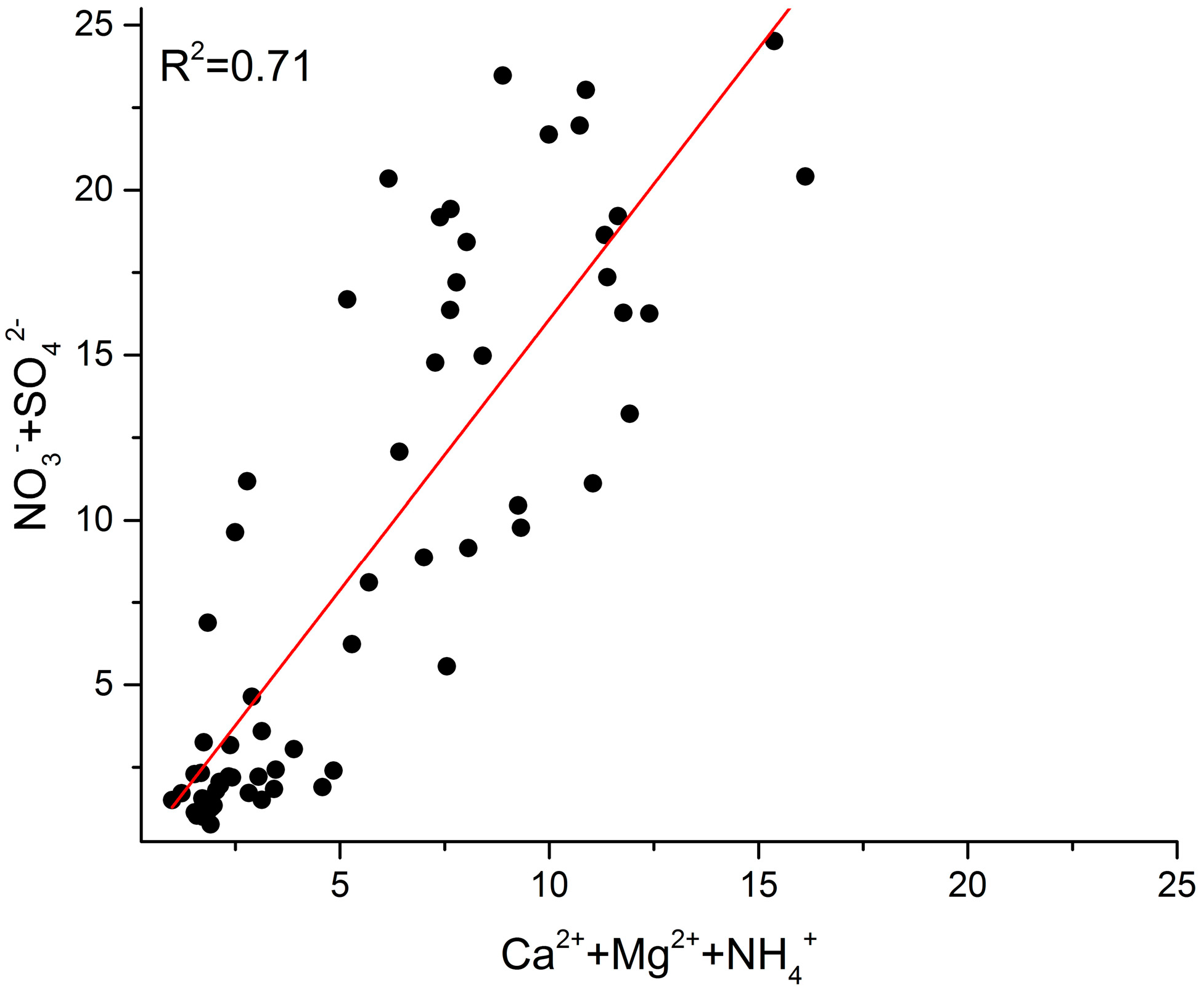
3.4. Neutralization Factors
3.5. Sources of Water-Soluble Inorganic Ions (WSIIs) in Pokhara Valley
3.5.1. Determination of Non-Sea Salt Aerosols
3.5.2. Principal Component Analysis (PCA)
3.5.3. Source Identification by NO3−/SO42− Ratio
3.5.4. HYSPLIT Back Trajectory Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Safai, P.D.; Rao, P.S.P.; Momin, G.A.; Ali, K.; Chate, D.M.; Praveen, P.S.; Devara, P.C.S. Variation in the chemistry of aerosols in two different winter seasons at Pune and Sinhagad, India. Aerosol Air Qual. Res. 2005, 5, 115–126. [Google Scholar]
- Xiao, H.-Y.; Liu, C.-Q. Chemical characteristics of water-soluble components in tsp over guiyang, SW China, 2003. Atmos. Environ. 2004, 38, 6297–6306. [Google Scholar] [CrossRef]
- Charlson, R.J.; Schwartz, S.; Hales, J.; Cess, R.D.; Coakley, J.; Hansen, J.; Hofmann, D. Climate forcing by anthropogenic aerosols. Science 1992, 255, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.M.; Pandis, S.N.; Seinfeld, J.H. Aerosol production and growth in the marine boundary layer. J. Geophys. Res. 1994, 99, 20989–21003. [Google Scholar] [CrossRef]
- Schichtel, B.A.; Husar, R.B.; Falke, S.R.; Wilson, W.E. Haze trends over the united states, 1980–1995. Atmos. Environ. 2001, 35, 5205–5210. [Google Scholar] [CrossRef]
- Lee, C.-G.; Yuan, C.-S.; Chang, J.-C.; Yuan, C. Effects of aerosol species on atmospheric visibility in Kaohsiung city, Taiwan. J. Air Waste Manag. 2005, 55, 1031–1041. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, J.; Arimoto, R.; Han, Z.; Zhang, R.; Han, Y.; Liu, S.; Okuda, T.; Nakao, S.; Tanaka, S. Ionic composition of tsp and PM2.5 during dust storms and air pollution episodes at Xi’an, China. Atmos. Environ. 2009, 43, 2911–2918. [Google Scholar] [CrossRef]
- Deshmukh, D.K. Characterization of dicarboxylates and inorganic ions in urban PM10 aerosols in the Eastern Central India. Aerosol Air Qual. Res. 2012, 12, 592–607. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, A.H.; Verma, A.K.; Singh, K.A.; Mathur, N.; Kisku, G.C.; Barman, S.C. Seasonal trends of PM2.5 and PM10 in ambient air and their correlation in ambient air of Lucknow City, India. Bull. Environ. Contam. Toxicol. 2012, 88, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Carrico, C.M.; Bergin, M.H.; Shrestha, A.B.; Dibb, J.E.; Gomes, L.; Harris, J.M. The importance of carbon and mineral dust to seasonal aerosol properties in the Nepal Himalaya. Atmos. Environ. 2003, 37, 2811–2824. [Google Scholar] [CrossRef]
- Decesari, S.; Facchini, M.C.; Carbone, C.; Giulianelli, L.; Rinaldi, M.; Finessi, E.; Fuzzi, S.; Marinoni, A.; Cristofanelli, P.; Duchi, R.; et al. Chemical composition of PM10 and PM1 at the high-altitude Himalayan station nepal climate observatory-pyramid (NCO-P) (5079 m a.S.L.). Atmos. Chem. Phys. 2010, 10, 4583–4596. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.B.; Wake, C.P.; Dibb, J.E. Chemical composition of aerosol and snow in the high Himalaya during the summer monsoon season. Atmos. Environ. 1997, 31, 2815–2826. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Wake, C.P.; Dibb, J.E.; Mayewski, P.A.; Whitlow, S.I.; Carmichael, G.R.; Ferm, M. Seasonal variations in aerosol concentrations and compositions in the Nepal Himalaya. Atmos. Environ. 2000, 34, 3349–3363. [Google Scholar] [CrossRef]
- Shakya, K.M. Characteristics and sources of carbonaceous, ionic, and isotopic species of wintertime atmospheric aerosols in Kathmandu valley, Nepal. Aerosol Air Qual. Res. 2010, 10, 219–230. [Google Scholar] [CrossRef]
- Sigdel, M.; Ma, Y. Evaluation of future precipitation scenario using statistical downscaling model over humid, subhumid, and arid region of Nepal—A case study. Theor. Appl. Climatol. 2016, 123, 453–460. [Google Scholar] [CrossRef]
- Kansakar, S.R.; Hannah, D.M.; Gerrard, J.; Rees, G. Spatial pattern in the precipitation regime of Nepal. Int. J. Climatol. 2004, 24, 1645–1659. [Google Scholar] [CrossRef]
- Chen, P.; Li, C.; Kang, S.; Yan, F.; Zhang, Q.; Ji, Z.; Tripathee, L.; Rupakheti, D.; Rupakheti, M.; Qu, B. Source apportionment of particle-bound polycyclic aromatic hydrocarbons in Lumbini, Nepal by using the positive matrix factorization receptor model. Atmos. Res. 2016, 182, 46–53. [Google Scholar] [CrossRef]
- Rupakheti, D.; Adhikary, B.; Praveen, P.S.; Rupakheti, M.; Kang, S.; Mahata, K.S.; Naja, M.; Zhang, Q.; Panday, A.K.; Lawrence, M.G. Pre-monsoon air quality over Lumbini, a world heritage site along the himalayan foothills. Atmos. Chem. Phys. Discuss. 2016. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Yu, G.; Qin, X.; Ren, J.; Qin, D. Characteristics of water soluble ionic species in fine particles from a high altitude site on the northern boundary of tibetan plateau: Mixture of mineral dust and anthropogenic aerosol. Atmos. Res. 2014, 143, 43–56. [Google Scholar] [CrossRef]
- Cong, Z.; Kang, S.; Kawamura, K.; Liu, B.; Wan, X.; Wang, Z.; Gao, S.; Fu, P. Carbonaceous aerosols on the south edge of the Tibetan Plateau: Concentrations, seasonality and sources. Atmos. Chem. Phys. 2015, 15, 1573–1584. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Cao, J.; Li, L.; Arimoto, R.; Cheng, Y.; Huebert, B.; Wang, D. Characterization of atmospheric aerosol over Xi’an in the south margin of the Loess Plateau, China. Atmos. Environ. 2002, 36, 4189–4199. [Google Scholar] [CrossRef]
- Deshmukh, D.K.; Tsai, Y.I.; Deb, M.K.; Zarmpas, P. Characteristics and sources of water-soluble ionic species associated with PM10 particles in the ambient air of central India. Bull. Environ. Contam. Toxicol. 2012, 89, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Tripathee, L.; Kang, S.; Huang, J.; Sillanpaa, M.; Sharma, C.M.; Luthi, Z.L.; Guo, J.; Paudyal, R. Ionic composition of wet precipitation over the southern slope of central Himalayas, Nepal. Environ. Sci. Pollut. Res. Int. 2014, 21, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, A.; Pachauri, T.; Singla, V.; Lakhani, A.; Kumari, K.M. Water soluble ionic species in atmospheric aerosols: Concentrations and sources at Agra in the indo-gangetic plain (IGP). Aerosol Air Qual. Res. 2013, 13, 1877–1889. [Google Scholar] [CrossRef]
- Chelani, A.; Gajghate, D.; ChalapatiRao, C.; Devotta, S. Particle size distribution in ambient air of Delhi and its statistical analysis. Bull. Environ. Contam. Toxicol. 2010, 85, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhuang, G.; Sun, Y.; An, Z. The variation of characteristics and formation mechanisms of aerosols in dust, haze, and clear days in Beijing. Atmos. Environ. 2006, 40, 6579–6591. [Google Scholar] [CrossRef]
- Huang, T.; Chen, J.; Zhao, W.; Cheng, J.; Cheng, S. Seasonal variations and correlation analysis of water-soluble inorganic ions in PM2.5 in Wuhan, 2013. Atmosphere 2016, 7, 49. [Google Scholar] [CrossRef]
- Tripathee, L.; Kang, S.; Huang, J.; Sharma, C.M.; Sillanpää, M.; Guo, J.; Paudyal, R. Concentrations of trace elements in wet deposition over the central Himalayas, Nepal. Atmos. Environ. 2014, 95, 231–238. [Google Scholar] [CrossRef]
- Kang, S.; Chen, P.; Li, C.; Liu, B.; Cong, Z. Atmospheric aerosol elements over the inland Tibetan Plateau: Concentration, seasonality, and transport. Aerosol Air Qual. Res. 2016, 16, 789–800. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.J.; Tie, X.X.; Shen, Z.X.; Liu, S.X.; Ding, H.; Han, Y.M.; Wang, G.H.; Ho, K.F.; Qiang, J.; et al. Water-soluble ions in atmospheric aerosols measured in Xi’an, China: Seasonal variations and sources. Atmos. Res. 2011, 102, 110–119. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Zhou, P. Ion chemistry and individual particle analysis of atmospheric aerosols over Mt. Bogda of eastern Tianshan mountains, central Asia. Environ. Monit. Assess. 2011, 180, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, T.; Azechi, S.; Somada, Y.; Ijyu, M.; Nakaema, F.; Hitomi, Y.; Handa, D.; Oshiro, Y.; Miyagi, Y.; Tsuhako, A.; et al. Spatial and temporal variations of chemicals in the tsp aerosols simultaneously collected at three islands in Okinawa, Japan. Atmos. Environ. 2014, 97, 479–485. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, J.; Shen, Z.; Xu, B.; Zhu, C.; Chen, L.W.A.; Su, X.; Liu, S.; Han, Y.; Wang, G.; et al. Aerosol particles at a high-altitude site on the southeast Tibetan Plateau, China: Implications for pollution transport from South Asia. J. Geophys. Res. 2013, 118, 11,360–11,375. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, S. Seasonal variations in water soluble inorganic ions, oc and ec in pm10 and PM > 10 aerosols over delhi: Influence of sources and meteorological factors. Aerosol Air Qual. Res. 2016, 16, 1165–1178. [Google Scholar] [CrossRef]
- Wang, H.; Shooter, D. Water soluble ions of atmospheric aerosols in three new zealand cities: Seasonal changes and sources. Atmos. Environ. 2001, 35, 6031–6040. [Google Scholar] [CrossRef]
- Dewan, N.; Wang, Y.-Q.; Zhang, Y.-X.; Zhang, Y.; He, L.-Y.; Huang, X.-F.; Majestic, B.J. Effect of pollution controls on atmospheric pm2. 5 composition during universiade in Shenzhen, China. Atmosphere 2016, 7. [Google Scholar] [CrossRef]
- Migliavacca, D.; Teixeira, E.; Wiegand, F.; Machado, A.; Sanchez, J. Atmospheric precipitation and chemical composition of an urban site, guaiba hydrographic basin, Brazil. Atmos. Environ. 2005, 39, 1829–1844. [Google Scholar]
- Deng, X.L.; Shi, C.E.; Wu, B.W.; Yang, Y.J.; Jin, Q.; Wang, H.L.; Zhu, S.; Yu, C. Characteristics of the water-soluble components of aerosol particles in Hefei, China. J. Environ. Sci. 2016, 42, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.; Bahramifar, N.; Younesi, H.; Taghavi, S.M.; Givehchi, R. Dry deposition, seasonal variation and source interpretation of ionic species at Abali, Firouzkouh and Varamin, Tehran province, Iran. Atmos. Res. 2015, 157, 74–90. [Google Scholar] [CrossRef]
- Park, S.-M.; Seo, B.-K.; Lee, G.; Kahng, S.-H.; Jang, Y.W. Chemical composition of water soluble inorganic species in precipitation at Shihwa Basin, Korea. Atmosphere 2015, 6, 732–750. [Google Scholar] [CrossRef]
- Arimoto, R.; Duce, R.A.; Savoie, D.L.; Prospero, J.M.; Talbot, R.; Cullen, J.D.; Tomza, U.; Lewis, N.F.; Ray, B.J. Relationships among aerosol constituents from asia and the north pacific during PEM-WEST A. J. Geophys. Res. 1996, 101, 2011–2023. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Tang, A.; Yuan, H.; Sun, Y.; Chen, S.; Zheng, A. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Kuniyal, J.C. Water soluble ionic components in particulate matter (PM10) during high pollution episode days at Mohal and Kothi in the north-western Himalaya, INDIA. Aerosol Air Qual. Res. 2015, 15, 529–543. [Google Scholar] [CrossRef]
- Chen, P.; Kang, S.; Bai, J.; Sillanpää, M.; Li, C. Yak dung combustion aerosols in the Tibetan Plateau: Chemical characteristics and influence on the local atmospheric environment. Atmos. Res. 2015, 156, 58–66. [Google Scholar] [CrossRef]
- Tiwari, S.; Srivastava, A.K.; Bisht, D.S.; Bano, T.; Singh, S.; Behura, S.; Srivastava, M.K.; Chate, D.M.; Padmanabhamurty, B. Black carbon and chemical characteristics of PM10 and PM2.5 at an urban site of north India. J. Atmos. Chem. 2010, 62, 193–209. [Google Scholar] [CrossRef]
- Kumar, V.; Sarkar, C.; Sinha, V. Influence of post harvest crop residue fires on surface ozone mixing ratios in the NW IGP analyzed using two years of continuous in-situ trace gas measurements. J. Geophys. Res. Atmos. 2016, 121, 3619–3633. [Google Scholar] [CrossRef]





| Species | TSP | Cl− | NO3− | SO42− | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Total Ions | Total Cations | Total Anions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 135.50 | 0.51 | 1.94 | 4.23 | 1.87 | 1.17 | 1.05 | 0.33 | 8.39 | 19.54 | 12.84 | 6.69 |
| SD | 62.91 | 0.53 | 1.44 | 3.39 | 1.28 | 1.24 | 0.93 | 0.23 | 7.13 | 13.85 | 9.83 | 4.81 |
| Min | 42.26 | 0.052 | 0.41 | 0.28 | 0.39 | 0.005 | 0.08 | 0.04 | 0.71 | 3.32 | 1.33 | 1.22 |
| Max | 355.02 | 2.24 | 10.02 | 14.71 | 4.32 | 4.62 | 4.03 | 0.91 | 24.80 | 45.61 | 32.05 | 22.68 |
| Study Area | Size Fraction | Study Period | Cl− | NO3− | SO42− | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pokhara, Nepal | TSP | April 2013–April 2014 | 0.51 | 1.94 | 4.23 | 1.87 | 1.17 | 1.06 | 0.33 | 8.39 | This Study |
| Agra, India | TSP | January 2009–December 2010 | 4.6 | 6.7 | 5.9 | 4 | 2.7 | 3.5 | 1.4 | 6.7 | [24] |
| Raipur, India | PM10 | July 2009–June 2010 | 3.46 | 5.52 | 9.98 | 1.75 | 2.92 | 0.76 | 0.84 | 3.02 | [8] |
| Durg, India | PM10 | July 2009–June 2010 | 3.23 | 5.63 | 8.88 | 1.76 | 5.18 | 0.87 | 0.8 | 2.53 | [22] |
| Delhi, India | PM10 | September 2005–January 2006 | 8.23 | 15.13 | 16.74 | 5.76 | 6.06 | 4.11 | 1.3 | 6.82 | [25] |
| Xi’an, China | TSP | October 2006–Sepyember 2007 | 4.6 | 16.1 | 34 | 1.4 | 8.3 | 2.3 | 0.9 | 11.4 | [7] |
| Beijing, China | TSP | Spring 2001–2004 | 8.22 | 22.76 | 27.25 | 2.05 | 12.4 | 1.84 | 0.8 | 12.16 | [26] |
| Wuhan, China | PM2.5 | January 2013–December 2013 | 1.24 | 11.28 | 16.78 | 0.24 | 9.67 | 1.08 | 0.14 | 0.54 | [27] |
| Species | Factors | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Cl− | 0.82 | −0.07 | 0.21 | 0.39 |
| NO3− | 0.09 | 0.17 | 0.93 | 0.25 |
| SO42− | 0.37 | 0.59 | 0.66 | 0.05 |
| Na+ | 0.89 | 0.35 | 0.04 | 0.13 |
| NH4+ | 0.11 | 0.89 | 0.19 | 0.23 |
| K+ | 0.61 | 0.52 | 0.46 | 0.23 |
| Mg2+ | 0.48 | 0.51 | 0.37 | 0.56 |
| Ca2+ | 0.46 | 0.36 | 0.29 | 0.73 |
| % of Variance | 30.63 | 24.80 | 23.01 | 14.94 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathee, L.; Kang, S.; Rupakheti, D.; Zhang, Q.; Huang, J.; Sillanpää, M. Water-Soluble Ionic Composition of Aerosols at Urban Location in the Foothills of Himalaya, Pokhara Valley, Nepal. Atmosphere 2016, 7, 102. https://doi.org/10.3390/atmos7080102
Tripathee L, Kang S, Rupakheti D, Zhang Q, Huang J, Sillanpää M. Water-Soluble Ionic Composition of Aerosols at Urban Location in the Foothills of Himalaya, Pokhara Valley, Nepal. Atmosphere. 2016; 7(8):102. https://doi.org/10.3390/atmos7080102
Chicago/Turabian StyleTripathee, Lekhendra, Shichang Kang, Dipesh Rupakheti, Qianggong Zhang, Jie Huang, and Mika Sillanpää. 2016. "Water-Soluble Ionic Composition of Aerosols at Urban Location in the Foothills of Himalaya, Pokhara Valley, Nepal" Atmosphere 7, no. 8: 102. https://doi.org/10.3390/atmos7080102