Characterization of Atmospheric Iron Speciation and Acid Processing at Metropolitan Newark on the US East Coast
Abstract
:1. Introduction
2. Methodology
2.1. Sampling
2.2. Lab Analysis
2.2.1. Iron Speciation Analysis
2.2.2. IC Analysis
3. Meteorological Data
4. Results and Discussion
4.1. Iron Speciation
4.2. Particle Size Distribution
4.2.1. Fe(II) and Fe(TD)
4.2.2. NH4+, NO3−, SO42−, Oxalate
4.3. Acid Processing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sunda, W. Bioavailability and bioaccumulation of iron in the sea. In the Biogeochemistry of Iron in Seawater; Turner, D.R., Hunter, K., Eds.; John Wiley: New York, NY, USA, 2001; pp. 41–84. [Google Scholar]
- Duce, R.A.; Liss, P.S.; Merrill, J.T.; Atlas, E.L.; Buat-Menard, P.; Hicks, B.B.; Miller, J.M.; Prospero, J.M.; Arimoto, R.; Church, T.M. The atmospheric input of trace species to the world ocean. Global Biogeochem. Cycles 1991, 5, 193–259. [Google Scholar] [CrossRef]
- Gao, Y.; Kaufman, Y.J.; Tanré, D.; Kolber, D.; Falkowski, P.G. Seasonal distributions of aeolian iron fluxes to the global ocean. Geophys. Res. Lett. 2001, 28, 29–32. [Google Scholar] [CrossRef]
- Prospero, J.M.; Ginoux, P.; Torres, O.; Nicholson, S.E.; Gill, T.E. Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev. Geophys. 2002, 40. [Google Scholar] [CrossRef]
- Li, F.; Ginoux, P.; Ramaswamy, V. Distribution, transport, and deposition of mineral dust in the Southern Ocean and Antarctica: Contribution of major sources. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Guieu, C.; Bonnet, S.; Wagener, T.; Loÿe-Pilot, M.D. Biomass burning as a source of dissolved iron to the open ocean? Geophys. Res. Lett. 2005, 321, 205–213. [Google Scholar] [CrossRef]
- Fu, H.B.; Shang, G.F.; Lin, J.; Hu, Y.J.; Hu, Q.Q.; Guo, L.; Zhang, Y.C.; Chen, J.M. Fractional iron solubility of aerosol particles enhanced by biomass burning and ship emission in Shanghai, East China. Sci. Total Environ. 2014, 481, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Lin, J.; Shang, G.; Dong, W.; Grassian, V.H.; Carmichael, G.R.; Li, Y.; Chen, J. Solubility of iron from combustion source particles in acidic media linked to iron speciation. Environ. Sci. Technol. Lett. 2012, 46, 11119–11127. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.; Duvall, R.; Shafer, M.; Schauer, J. The origin of water soluble particulate iron in the Asian atmospheric outflow. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Sedwick, P.N.; Sholkovitz, E.R.; Church, T.M. Impact of anthropogenic combustion emissions on the fractional solubility of aerosol iron: Evidence from the Sargasso Sea. Geochem. Geophys. Geosyst. 2007, 8. [Google Scholar] [CrossRef]
- Luo, C.; Mahowald, N.; Bond, T.; Chuang, P.; Artaxo, P.; Siefert, R.; Chen, Y.; Schauer, J. Combustion iron distribution and deposition. Global Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Jickells, T.D.; Spokes, L.J. Atmospheric iron inputs to the oceans. In The Biogeochemistry of Iron in Seawater; Turner, D.R., Hunter, K., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 85–121. [Google Scholar]
- Johansen, A.M.; Siefert, R.L.; Hoffmann, M.R. Chemical composition of aerosols collected over the tropical North Atlantic Ocean. J. Geophys. Res. Atmos. 2000, 105, 15277–15312. [Google Scholar] [CrossRef]
- Baker, A.; Jickells, T. Mineral particle size as a control on aerosol iron solubility. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Schroth, A.W.; Crusius, J.; Sholkovitz, E.R.; Bostick, B.C. Iron solubility driven by speciation in dust sources to the ocean. Nat. Geosci. 2009, 2, 337–340. [Google Scholar] [CrossRef]
- Dentener, F.J.; Carmichael, G.R.; Zhang, Y.; Lelieveld, J.; Crutzen, P.J. Role of mineral aerosol as a reactive surface in the global troposphere. J. Geophys. Res. Atmos. 1996, 101, 22869–22889. [Google Scholar] [CrossRef]
- Underwood, G.; Song, C.; Phadnis, M.; Carmichael, G.; Grassian, V. Heterogeneous reactions of NO2 and HNO3 on oxides and mineral dust: A combined laboratory and modeling study. J. Geophys. Res. Atmos. 2001, 106, 18055–18066. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Liu, X.; Zhang, J.; Lin, Y.; Yao, X.; Gao, H.; Zhang, D.; Chen, J.; Wang, W.; et al. Air pollution-aerosol interactions produce more bioavailable iron for ocean ecosystems. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Faust, B.C.; Zepp, R.G. Photochemistry of Aqueous Iron (III)-Polycarboxylate Complexes: Roles in the Chemistry of Atmospheric And Surface Waters; Environmental Protection Agency: Athens, GA, USA, January 1993.
- Zhu, X.; Prospero, J.; Millero, F. Diel variability of soluble Fe (II) and soluble total Fe in North African dust in the trade winds at Barbados. J. Geophys. Res. Biogeosci. 1997, 102, 21297–221305. [Google Scholar] [CrossRef]
- Kieber, R.J.; Hardison, D.; Whitehead, R.F.; Willey, J.D. Photochemical production of Fe (II) in rainwater. Environ. Sci. Technol. Lett. 2003, 37, 4610–4616. [Google Scholar] [CrossRef]
- Meskhidze, N.; Chameides, W.; Nenes, A.; Chen, G. Iron mobilization in mineral dust: Can anthropogenic SO2 emissions affect ocean productivity? Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Fan, S.M.; Moxim, W.J.; Levy, H. Aeolian input of bioavailable iron to the ocean. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y. Air-to-sea flux of soluble iron: Is it driven more by HNO3 or SO2?—An examination in the light of dust aging. Atmos. Chem. Phys. Discuss. 2007, 7, 10043–10063. [Google Scholar] [CrossRef]
- Xu, N.; Gao, Y. Characterization of hematite dissolution affected by oxalate coating, kinetics and pH. Appl. Geochem. 2008, 23, 783–793. [Google Scholar] [CrossRef]
- Luo, C.; Gao, Y. Aeolian iron mobilisation by dust-acid interactions and their implications for soluble iron deposition to the ocean: A test involving potential anthropogenic organic acidic species. Environ. Chem. 2010, 7, 153–161. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikushima, K. Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere. Environ. Sci. Technol. 1993, 27, 2227–2235. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y. Mass size distributions of water-soluble inorganic and organic ions in size-segregated aerosols over metropolitan Newark in the US east coast. Atmos. Environ. 2008, 42, 4063–4078. [Google Scholar] [CrossRef]
- Prabhakar, G.; Ervens, B.; Wang, Z.; Maudlin, L.C.; Coggon, M.M.; Jonsson, H.H.; Seinfeld, J.H.; Sorooshian, A. Sources of nitrate in stratocumulus cloud water: Airborne measurements during the 2011 E-PEACE and 2013 NICE studies. Atmos. Environ. 2014, 97, 166–173. [Google Scholar] [CrossRef]
- Sorooshian, A.; Murphy, S.M.; Hersey, S.; Gates, H. Comprehensive airborne characterization of aerosol from a major bovine source. Atmos. Chem. Phys. 2008, 8, 5489–5520. [Google Scholar] [CrossRef]
- Sorooshian, A.; Murphy, S.M.; Hersey, S.; Bahreini, R.; Jonsson, H.; Flagan, R.C.; Seinfeld, J.H. Constraining the contribution of organic acids and AMS m/z 44 to the organic aerosol budget: On the importance of meteorology, aerosol hygroscopicity, and region. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Kawamura, K.; Narukawa, M.; Li, S.M.; Barrie, L.A. Size distributions of dicarboxylic acids and inorganic ions in atmospheric aerosols collected during polar sunrise in the Canadian high arctic. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Whitby, K.T. The physical characteristics of sulfur aerosols. Atmos. Environ. 1978, 12, 135–159. [Google Scholar] [CrossRef]
- Mochida, M.; Kawabata, A.; Kawamura, K.; Hatsushika, H.; Yamazaki, K. Seasonal variation and origins of dicarboxylic acids in the marine atmosphere over the western North Pacific. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Cabada, J.C.; Pandis, S.N.; Subramanian, R.; Robinson, A.L.; Polidori, A.; Turpin, B. Estimating the secondary organic aerosol contribution to PM2. 5 using the EC tracer method special issue of aerosol science and technology on findings from the fine particulate matter supersites program. Aerosol Sci. Technol. 2004, 38, 140–155. [Google Scholar] [CrossRef]
- Maudlin, L.C.; Wang, Z.; Jonsson, H.H.; Sorooshian, A. Impact of wildfires on size-resolved aerosol composition at a coastal California site. Atmos. Environ. 2015, 119, 59–68. [Google Scholar] [CrossRef]
- Meng, Z.; Seinfeld, J.H. On the source of the submicrometer droplet mode of urban and regional aerosols. Aerosol Sci. Technol. 1994, 20, 253–265. [Google Scholar] [CrossRef]
- Kerminen, V.M.; Wexler, A.S. Growth laws for atmospheric aerosol particles: An examination of the bimodality of the accumulation mode. Atmos. Environ. 1995, 29, 3263–3275. [Google Scholar] [CrossRef]
- Kerminen, V.-M.; Ojanen, C.; Pakkanen, T.; Hillamo, R.; Aurela, M.; Meriläinen, J. Low-molecular-weight dicarboxylic acids in an urban and rural atmosphere. J. Aerosol Sci. 2000, 31, 349–362. [Google Scholar] [CrossRef]
- Wang, Z.; Sorooshian, A.; Prabhakar, G.; Coggon, M.M.; Jonsson, H.H. Impact of emissions from shipping, land, and the ocean on stratocumulus cloud water elemental composition during the 2011 E-PEACE field campaign. Atmos. Environ. 2014, 89, 570–580. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, G.; Zhan, J.; Zhang, J.; Li, W.; Lin, Q.; Chen, L.; Lin, H. Spatial and particle size distributions of atmospheric dissolvable iron in aerosols and its input to the Southern Ocean and coastal East Antarctica. J. Geophys. Res. Atmos. 2013, 118. [Google Scholar] [CrossRef]
- Chen, Y.; Siefert, R.L. Seasonal and spatial distributions and dry deposition fluxes of atmospheric total and labile iron over the tropical and subtropical North Atlantic Ocean. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Buck, C.S.; Landing, W.M.; Resing, J.A.; Lebon, G.T. Aerosol iron and aluminum solubility in the northwest Pacific Ocean: Results from the 2002 IOC cruise. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef]
- Xia, L.; Gao, Y. Chemical composition and size distributions of coastal aerosols observed on the us east coast. Mar. Chem. 2010, 119, 77–90. [Google Scholar] [CrossRef]
- The Weather Company. Available online: https://www.wunderground.com/ (accessed on 7 March 2017).
- Air Resources Laboratory. Available online: http://www.arl.noaa.gov/HYSPLIT_info.php (accessed on 7 March 2017).
- Siefert, R.L.; Johansen, A.M.; Hoffmann, M.R. Chemical characterization of ambient aerosol collected during the southwest monsoon and intermonsoon seasons over the Arabian Sea: Labile-Fe (II) and other trace metals. J. Geophys. Res. Atmos. 1999, 104, 3511–3526. [Google Scholar] [CrossRef]
- Johansen, A.M.; Hoffmann, M.R. Chemical characterization of ambient aerosol collected during the northeast monsoon season over the Arabian Sea: Labile-Fe (II) and other trace metals. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Baker, A.; Kelly, S.; Biswas, K.; Witt, M.; Jickells, T. Atmospheric deposition of nutrients to the Atlantic Ocean. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Kramer, J.; Laan, P.; Sarthou, G.; Timmermans, K.; De Baar, H. Distribution of dissolved aluminium in the high atmospheric input region of the subtropical waters of the North Atlantic Ocean. Mar. Chem. 2004, 88, 85–101. [Google Scholar] [CrossRef]
- Sedwick, P.N.; Church, T.M.; Bowie, A.R.; Marsay, C.M.; Ussher, S.J.; Achilles, K.; Lethaby, P.; Johnson, R.J.; Sarin, M.; McGillicuddy, D.J. Iron in the Sargasso Sea (Bermuda Atlantic Time-series Study region) during summer: Eolian imprint, spatiotemporal variability, and ecological implications. Global Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Measures, C.; Cutter, G.; Landing, W.; Powell, R. Hydrographic observations during the 2002 IOC contaminant baseline survey in the western Pacific Ocean. Geochem. Geophys. Geosystems. 2006, 7. [Google Scholar] [CrossRef]
- Hsu, S.C.; Liu, S.C.; Arimoto, R.; Liu, T.H.; Huang, Y.T.; Tsai, F.; Lin, F.J.; Kao, S.J. Dust deposition to the East China Sea and its biogeochemical implications. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Zhang, L.; Vet, R.; Wiebe, A.; Mihele, C.; Sukloff, B.; Chan, E.; Moran, M.; Iqbal, S. Characterization of the size-segregated water-soluble inorganic ions at eight Canadian rural sites. Atmos. Chem. Phys. 2008, 8, 7133–7151. [Google Scholar] [CrossRef]
- Anlauf, K.; Li, S.-M.; Leaitch, R.; Brook, J.; Hayden, K.; Toom-Sauntry, D.; Wiebe, A. Ionic composition and size characteristics of particles in the lower fraser valley: Pacific 2001 field study. Atmos. Environ. 2006, 40, 2662–2675. [Google Scholar] [CrossRef]
- Yu, J.; Flagan, R.C.; Seinfeld, J.H. Identification of products containing −COOH, −OH, and −CO in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 1998, 32, 2357–2370. [Google Scholar] [CrossRef]
- Kasper-Giebl, A.; Bauer, H.; Giebl, H.; Hitzenberger, R.; Löflund, M.; Puxbaum, H. Transport and Chemical Transformation in the Troposphere; Springer: Berlin, Germany, 2002. [Google Scholar]
- Solmon, F.; Chuang, P.; Meskhidze, N.; Chen, Y. Acidic processing of mineral dust iron by anthropogenic compounds over the north Pacific Ocean. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Sorooshian, A.; Wang, Z.; Coggon, M.M.; Jonsson, H.H.; Ervens, B. Observations of sharp oxalate reductions in stratocumulus clouds at variable altitudes: Organic acid and metal measurements during the 2011 E-PEACE campaign. Environ. Sci. Technol. 2013, 47, 7747–7756. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Wong, G.T.; Gong, G.C.; Shiah, F.K.; Huang, Y.T.; Kao, S.J.; Tsai, F.; Lung, S.C.C.; Lin, F.J.; Lin, I. Sources, solubility, and dry deposition of aerosol trace elements over the East China Sea. Mar. Chem. 2010, 120, 116–127. [Google Scholar] [CrossRef]
- Dall’Osto, M.; Beddows, D.C.; Harrison, R.M.; Onat, B. Fine iron aerosols are internally mixed with nitrate in the urban European atmosphere. Environ. Sci. Technol. 2016, 50, 4212–4220. [Google Scholar] [CrossRef] [PubMed]
- Pehkonen, S.O.; Siefert, R.; Erel, Y.; Webb, S.; Hoffmann, M.R. Photoreduction of iron oxyhydroxides in the presence of important atmospheric organic compounds. Environ. Sci. Technol. 1993, 27, 2056–2062. [Google Scholar] [CrossRef]
- Siefert, R.L.; Pehkonen, S.O.; Erel, Y.; Hoffmann, M.R. Iron photochemistry of aqueous suspensions of ambient aerosol with added organic acids. Geochim. Cosmochim. Acta. 1994, 58, 3271–3279. [Google Scholar] [CrossRef]
- Moffet, R.C.; Furutani, H.; Rödel, T.C.; Henn, T.R.; Sprau, P.O.; Laskin, A.; Uematsu, M.; Gilles, M.K. Iron speciation and mixing in single aerosol particles from the Asian continental outflow. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Luo, C.; Mahowald, N.; Meskhidze, N.; Chen, Y.; Siefert, R.; Baker, A.; Johansen, A. Estimation of iron solubility from observations and a global aerosol model. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef]
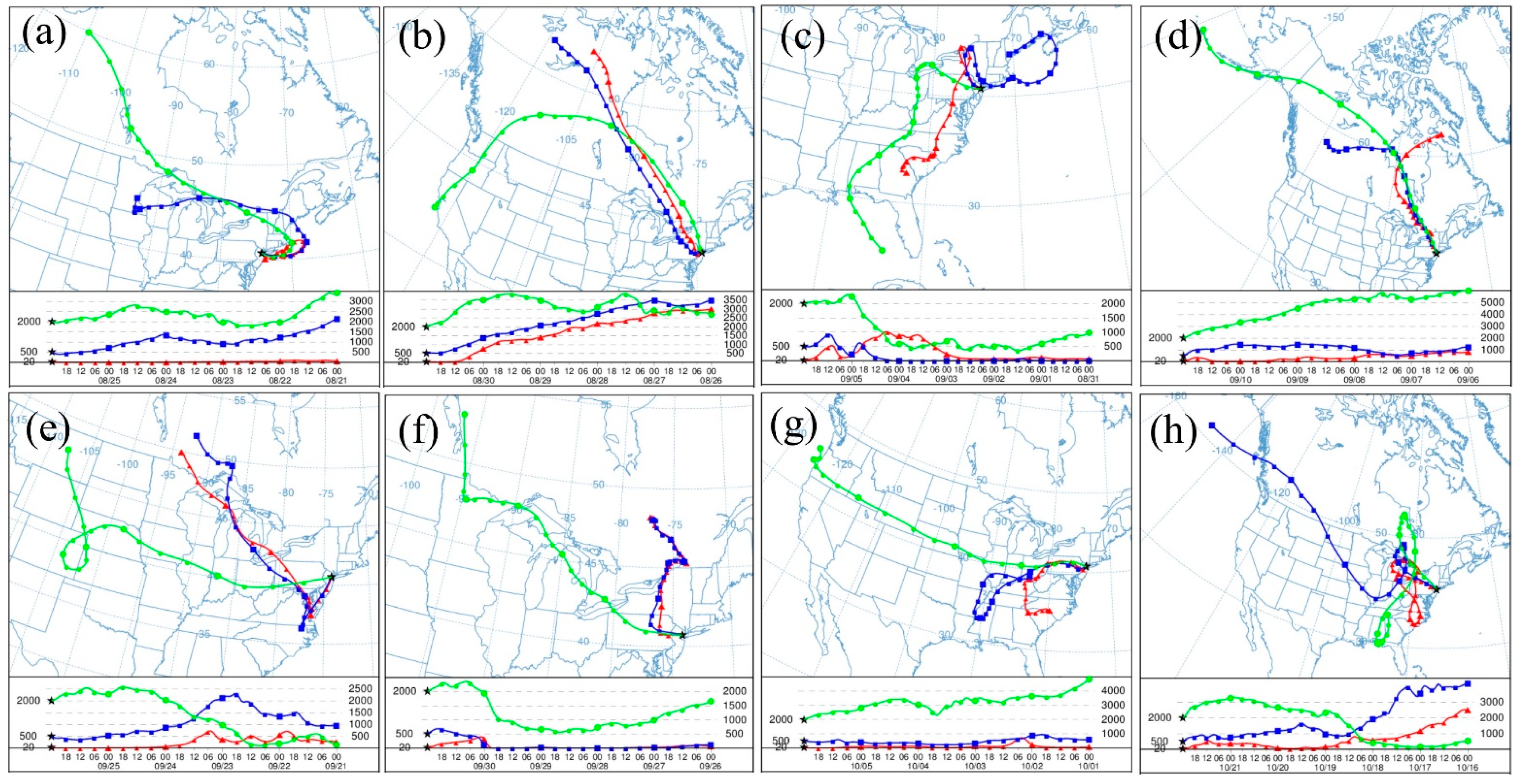


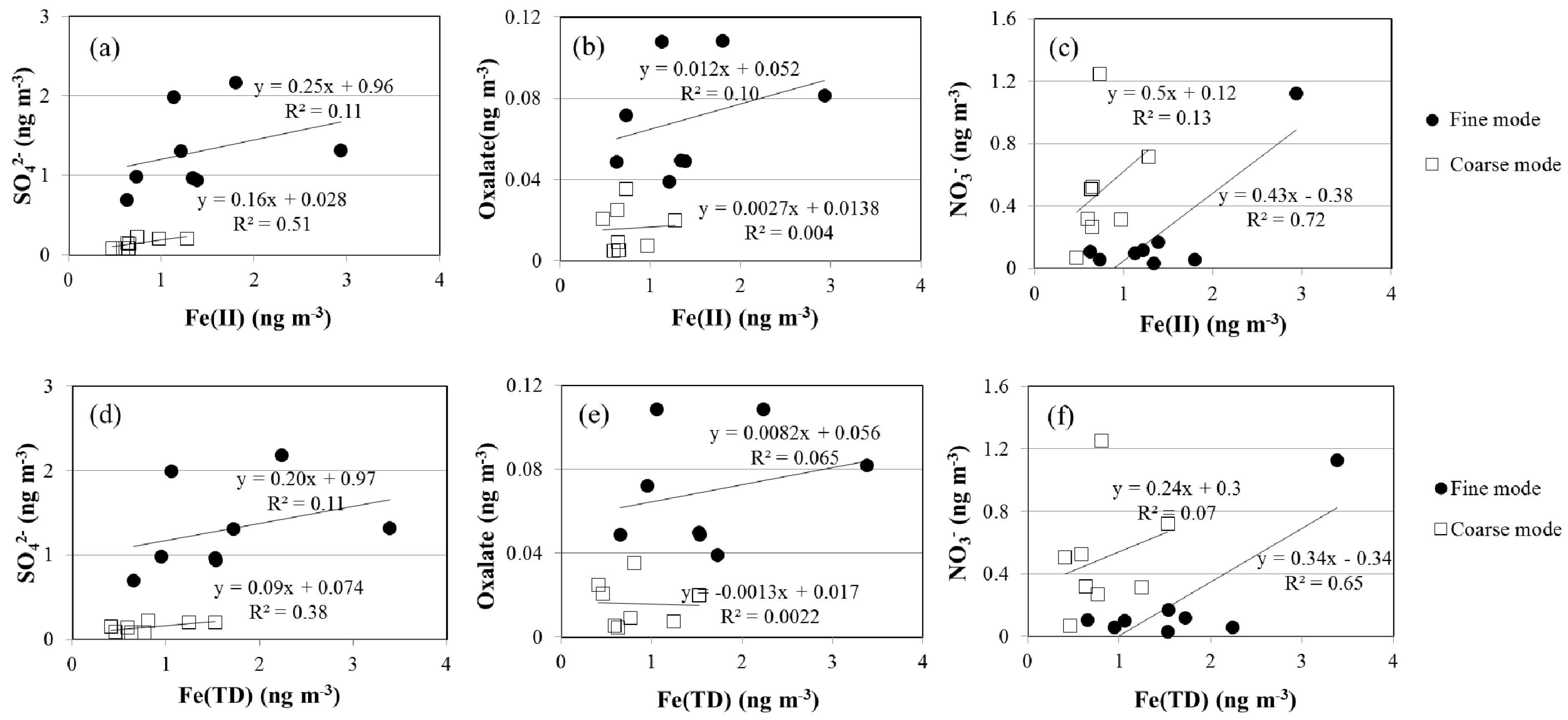
| Set | Sampling Date | Sampling Volume (m3) | Wind Speed (m/s) | Air Temperature (°C) | Relative Humidity (%) | Air Pressure (hPa) |
|---|---|---|---|---|---|---|
| No.1 | 08/21/2012–08/26/2012 | 214.2 | 2.5 | 25 | 63 | 1021 |
| No.2 | 08/26/2012–08/31/2012 | 217.8 | 3.9 | 25 | 61 | 1017 |
| No.3 | 08/31/2012–09/06/2012 | 236.7 | 3.3 | 25 | 71 | 1015 |
| No.4 | 09/06/2012–09/11/2012 | 216 | 3.3 | 23 | 66 | 1013 |
| No.5 | 09/21/2012–09/26/2012 | 215.1 | 3.6 | 19 | 65 | 1018 |
| No.6 | 09/26/2012–10/01/2012 | 214.7 | 3.3 | 19 | 73 | 1015 |
| No.7 | 10/01/2012–10/06/2012 | 215.1 | 2.8 | 20 | 78 | 1015 |
| No.8 | 10/16/2012–10/22/2012 | 190.3 | 3.6 | 15 | 65 | 1012 |
| Locations | Latitude/Longitude | Fe(II) (ng m−3) | Fe(TD) (ng m−3) | Citations |
|---|---|---|---|---|
| Northwest Pacific | 50° N–24° N, 170°E | 0.05–5.3 | 0.28–86 | Buck et al. (2006) [43] |
| East China Sea | 25° N–30° N, 120° E–127° E | 1.7–120 | Hsu et al. (2009) [53] | |
| North Atlantic | 13.17° N, 59.43° W | 2.5 ± 1.8 (0.63–8.2) | 12 ± 8.4 (2.8–33) | Zhu et al. (1997) [20] |
| North Atlantic | 10° N–30° N, 30° W–60° W | 0.19–1.2 | 0.35–20 | Chen and Siefert (2004) [42] |
| North Atlantic | ~30° N–31.5° N, ~63.5° W–65.5° W | 4.5–12 | Sedwick et al. (2007) [10] | |
| Coastal East Antarctic | 64° S–69° S 75° E–110° E | 0.53 ± 0.38 (0.18–1.3) | 1.2 ± 1.1 (0.23–3.3) | Gao et al. (2013) [41] |
| US Northeast Coast Newark | 40°74ʹ N 74°18ʹ W | 2.1 (1.2–4.2) | 2.4 (1.3–4.9) | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Gao, Y. Characterization of Atmospheric Iron Speciation and Acid Processing at Metropolitan Newark on the US East Coast. Atmosphere 2017, 8, 66. https://doi.org/10.3390/atmos8040066
Xu G, Gao Y. Characterization of Atmospheric Iron Speciation and Acid Processing at Metropolitan Newark on the US East Coast. Atmosphere. 2017; 8(4):66. https://doi.org/10.3390/atmos8040066
Chicago/Turabian StyleXu, Guojie, and Yuan Gao. 2017. "Characterization of Atmospheric Iron Speciation and Acid Processing at Metropolitan Newark on the US East Coast" Atmosphere 8, no. 4: 66. https://doi.org/10.3390/atmos8040066





