Occurrence and Potential Sources of Quinones Associated with PM2.5 in Guadalajara, Mexico
Abstract
:1. Introduction
2. Experimental
2.1. Standards and Material
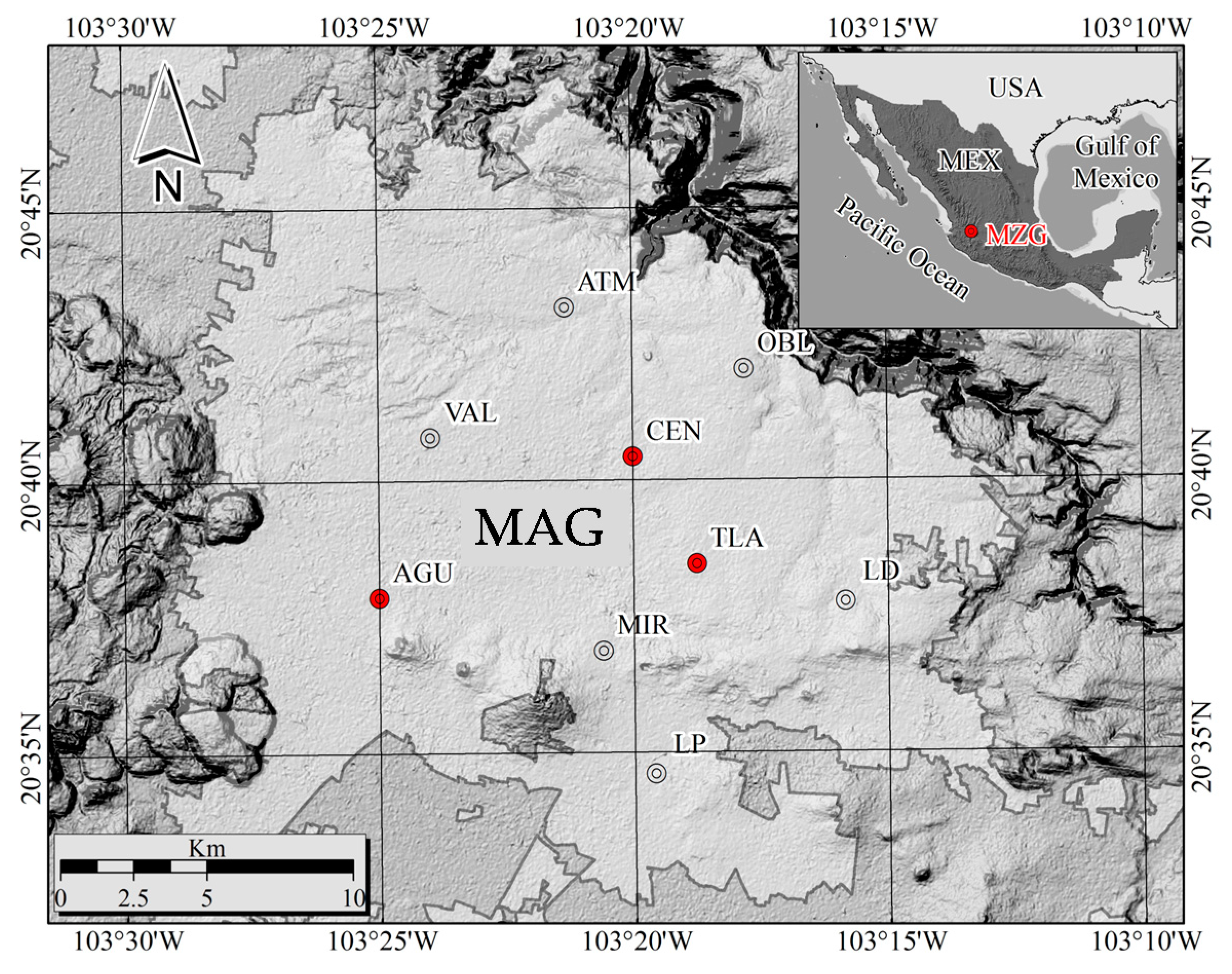
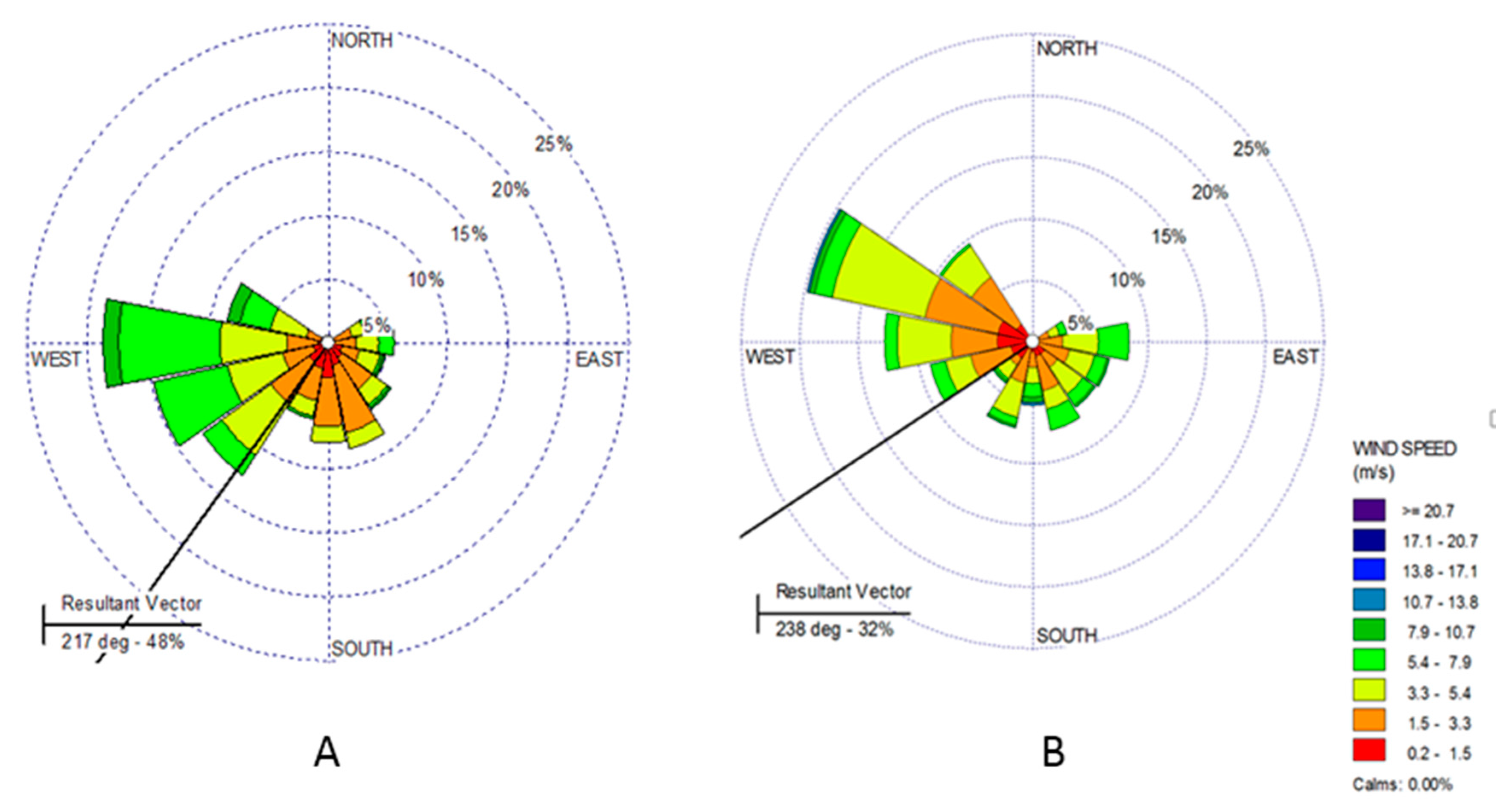
2.2. Sampling of PM2.5 and Meteorological Conditions
2.3. Analysis of Quinones in PM2.5
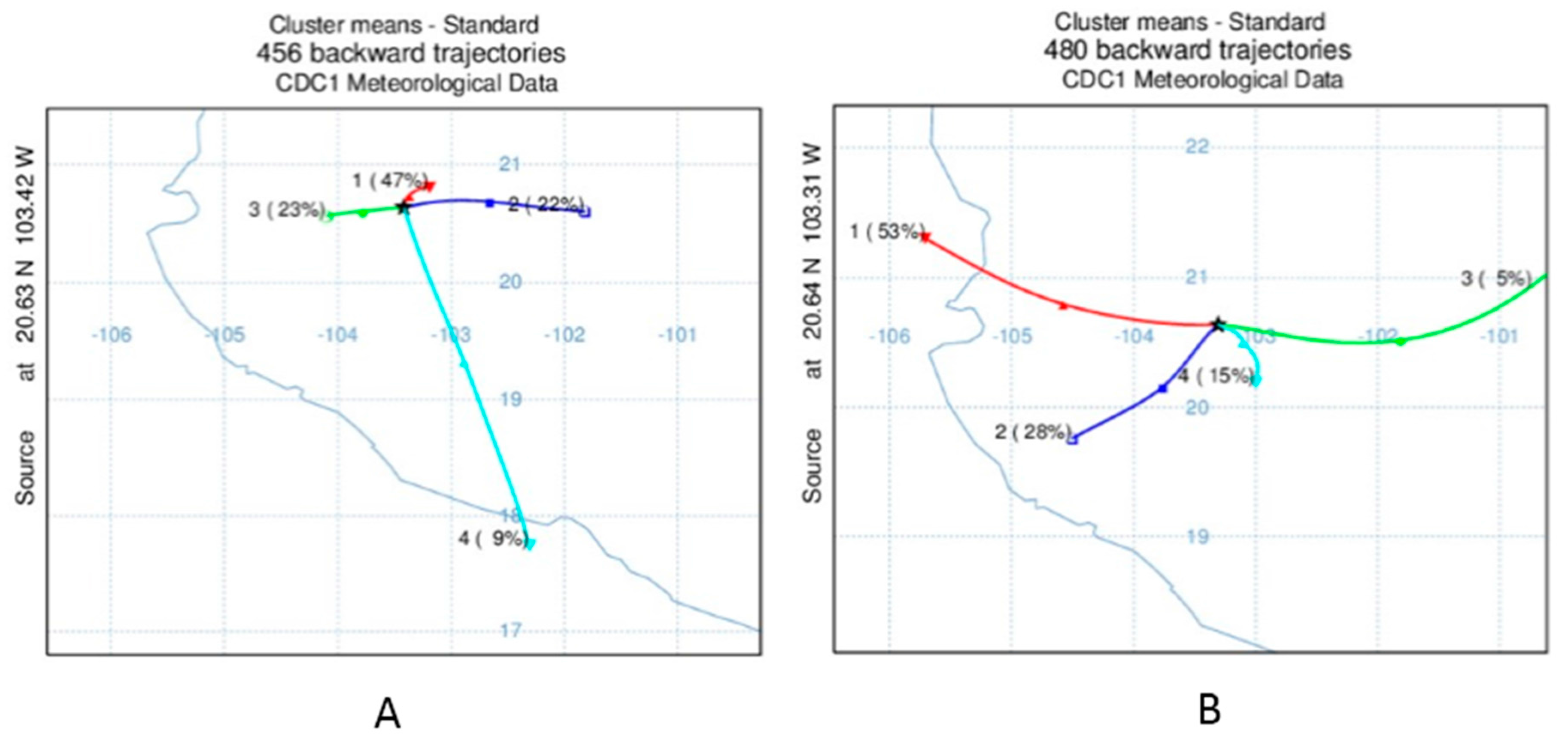
2.4. Statistical and Trajectory Analysis
3. Results and Discussion
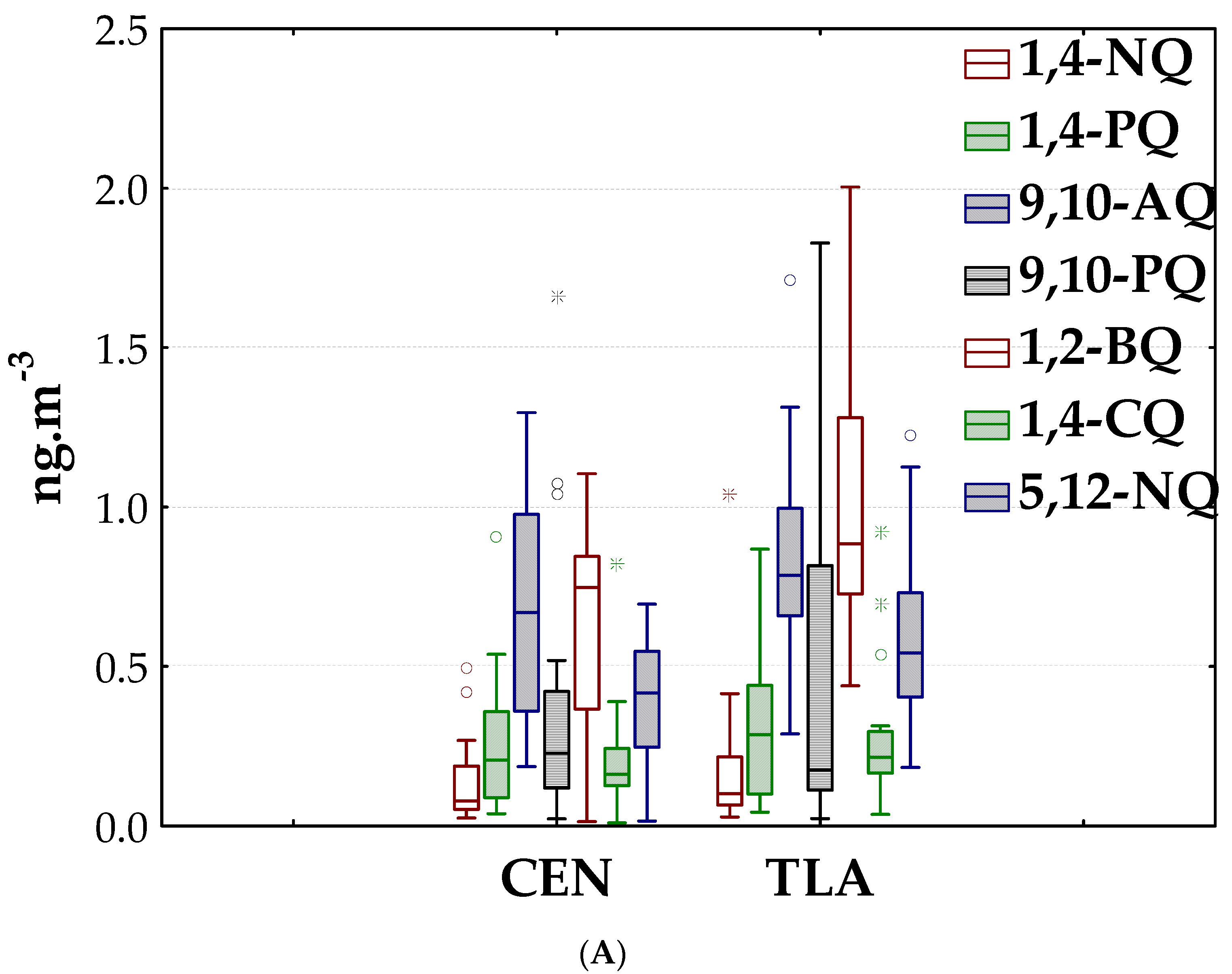
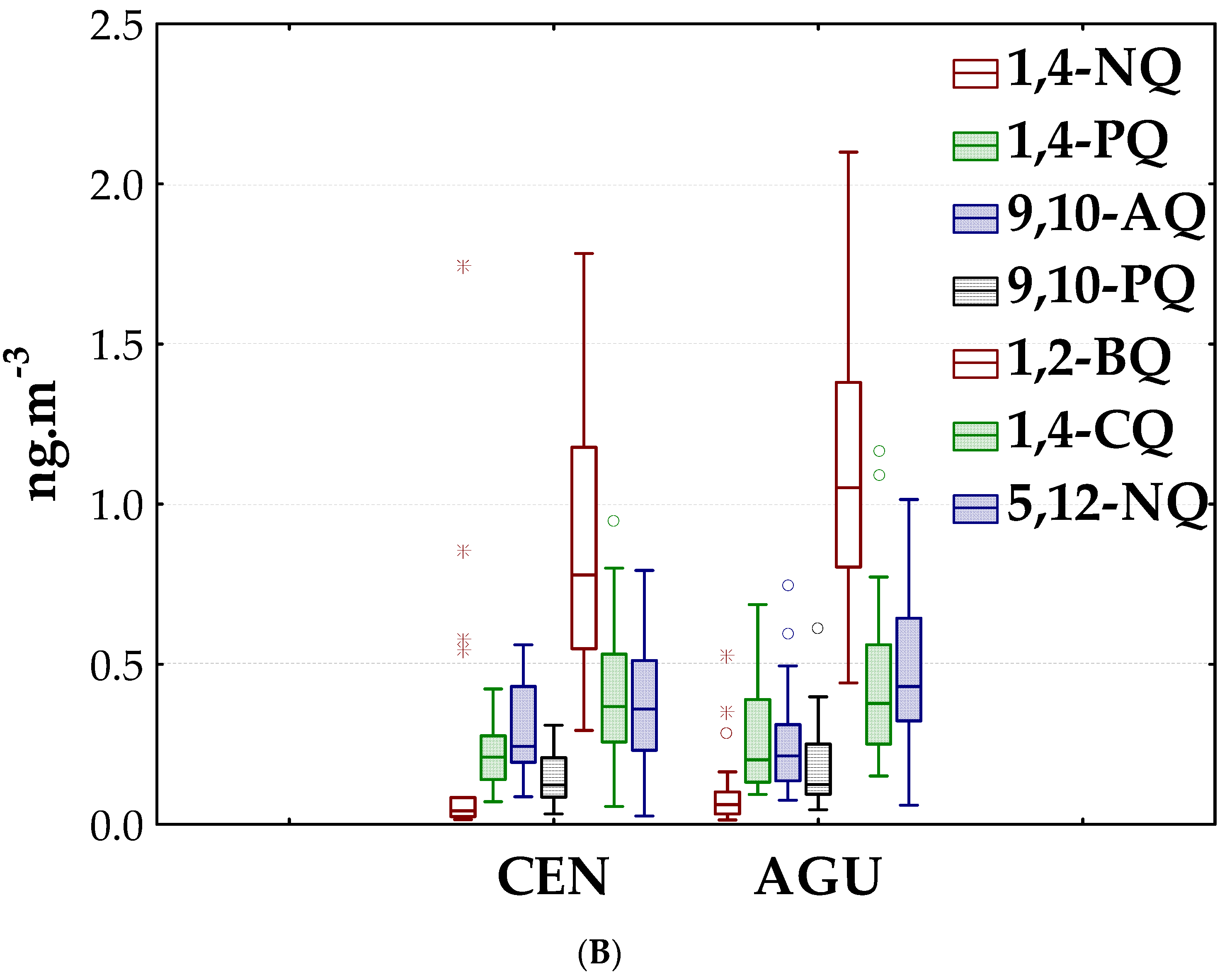
3.1. Quinones in PM2.5
3.1.1. Environmental Levels
3.1.2. Spatial and Seasonal Patterns
3.2. Potential Sources of Quinones
3.2.1. Correlation with Criteria Atmospheric Pollutants
3.2.2. Analysis of Ratios of Quinone/Precursor
3.2.3. Principal Component Analysis (PCA)
3.3. Photochemical Formation of Quinones
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kappos, A.D.; Bruckmann, P.; Eikmann, T.; Englert, N.; Heinrich, U.; Hoppe, P.; Koch, E.; Krause, G.H.; Kreyling, W.G.; Rauchfuss, K.; et al. Health effects of particles in ambient air. Int. J. Hyg. Environ. Health 2004, 207, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Englert, N. Fine particles and human health—A review of epidemiological studies. Toxicol. Lett. 2004, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- De Kok, T.M.C.M.; Driece, H.A.L.; Hogervorst, J.G.F.; Briedé, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.U.; Durant, J.L.; Penman, B.W.; Crespi, C.L.; Hemond, H.F.; Lafleur, A.L.; Cass, G.R. Human-cell mutagens in respirable airborne particles in the Northeastern United States. 1. Mutagenicity of fractionated samples. Environ. Sci. Technol. 2004, 38, 682–689. [Google Scholar] [CrossRef]
- Pedersen, D.U.; Durant, J.L.; Taghizadeh, K.; Hemond, H.F.; Lafleur, A.L.; Cass, G.R. Human cell mutagens in respirable airborne particles from the Northeastern United States. 2. Quantification of mutagens and other organic compounds. Environ. Sci. Technol. 2005, 39, 9547–9560. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of Quinones in Toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Knecht, A.L.; Goodale, B.C.; Truong, L.; Simonich, M.T.; Swanson, A.J.; Matzke, M.M.; Anderson, K.A.; Waters, K.M.; Tanguay, R.L. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharm. 2013, 271, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, K.; Talkano, H.; Inoue, K.I.; Ichinose, T.; Yanaqisawa, R.; Tomura, S.; Kumagai, Y. Effects of phenanthraquinone on allergic airway inflammation in mice. Clin. Exp. Allergy 2005, 35, 1243–1248. [Google Scholar] [PubMed]
- Zielinska, B.; Sagebiel, J.; McDonald, J.D.; Whitney, K.; Lawson, D.R. Emission rates and comparative chemical composition from selected in-use diesel and gasoline-fueled vehicles. J. Air Waste Manage 2004, 54, 1138–1150. [Google Scholar] [CrossRef]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Zimmermann, R.; van Langenhove, H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos. Environ. 2010, 44, 1831–1846. [Google Scholar]
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E.; Harrison, M.A.J.; Olariu, R.I.; Arsene, C. Photochemical reactions in the tropospheric aqueous phase and on particulate matter. Chem. Soc. Rev. 2006, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Atkinson, R.; Arey, J. Formation of 9,10-phenanthrenequinone by atmospheric gas-phase reactions of phenanthrene. Atmos. Environ. 2007, 41, 2025–2035. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Miguel, A.H.; Lu, R.; Purvis, K.; Grant, B.; Mayo, P.; Di Stefano, E.; Cho, A.K.; Froines, J. Atmospheric formation of 9,10-phenanthraquinone in the Los Angeles air basin. Atmos. Environ. 2008, 42, 2312–2319. [Google Scholar] [CrossRef]
- Murillo-Tovar, M.A. Desempeño de cromatografía de gases con espectrometría de masas (CGEM) por monitoreo selectivo de iones (SIM) para el análisis de oxi-HAP en partículas respirables finas (PM2.5). In Proceedings of the XXVIII Congreso Nacional de Química Analítica y el XVIII Simposio Estudiantil, Ixtapa-Guerrero, México, 27 June 2015. [Google Scholar]
- Valle-Hernández, B.L.; Múgica-Álvarez, V.; Salinas-Talavera, E.; Amador-Muñoz, O.; Murillo-Tovar, M.A.; Villalobos-Pietrini, R.; De Vizcaya-Ruíz, A. Temporal variation of nitro-polycyclic aromatic hydrocarbons in PM10 and PM2.5 collected in Northern Mexico City. Sci. Total Environ. 2010, 408, 5429–5438. [Google Scholar] [CrossRef] [PubMed]
- Draxler, R.R.; Rolph, G.D. HYSPLIT—Hybrid Single-Particle Lagrangian Integrated Trajectory Model. Available online: http://www.arl.noaa.gov/HYSPLIT.php (accessed on 11 April 2017).
- Alam, M.S.; Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Investigating PAH relative reactivity using congener profiles, quinone measurements and back trajectories. Atmos. Chem. Phys. 2014, 14, 2467–2477. [Google Scholar] [CrossRef]
- Flores Arriola, A. Determinación de Hidrocarburos Aromáticos Policíclicos Oxigenados en las Aeropartículas finas de la Zona Metropolitana de Guadalajara. Ph.D. Thesis, Universidad Autónoma de Guadalajara, Guadalajara, Jalisco, Mexico, April 2012. [Google Scholar]
- Murillo Tovar, M. A. Optimización de las condiciones analíticas para la determinación simultánea de n-alcanos, hidrocarburos aromáticos policíclicos y sus derivados oxigenados en las aeropartículas <2.5 μm. Ph.D. Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, March 2012. [Google Scholar]
- Chung, M.; Lazaro, R.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A. Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Saborit, J.; Alam, M.; Godri Pollit, K.; Stark, C.; Harrison, R. Analysis of atmospheric concentrations of quinones and polycyclic aromatic hydrocarbons in vapour and particulate phases. Atmos. Environ. 2013, 77, 974–982. [Google Scholar] [CrossRef]
- Alam, M.S.; Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Using atmospheric measurements of PAH and quinone compounds at roadside and urban background sites to assess sources and reactivity. Atmos. Environ. 2013, 77, 24–35. [Google Scholar] [CrossRef]
- Tsapakis, M.; Lagoudaki, E.; Stephanou, E.; Kavouras, I.; Koutrakis, P.; Oyola, P.; von Baer, D. The composition and sources of PM2.5 organic aerosol in two urban areas of Chile. Atmos. Environ. 2002, 36, 3851–3863. [Google Scholar] [CrossRef]
- Kong, S.; Li, X.; Li, L.; Yin, Y.; Chen, K.; Yuan, L.; Zhang, Y.; Shan, Y.; Ji, Y. Variation of polycyclic aromatic hydrocarbons in atmospheric PM2.5 during winter haze period around 2014 Chinese Spring Festival at Nanjing: Insights of source changes, air mass direction and firework particle injection. Sci. Total Environ. 2015, 520, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Z.; Chen, J.; Kong, S.; Fu, X.; Deng, H.; Shao, G.; Wu, G. Polycyclic aromatic hydrocarbons (PAHs) in atmospheric PM2.5 and PM10 at a coal-based industrial city: Implication for PAH control at industrial agglomeration regions, China. Atmos. Res. 2014, 149, 217–229. [Google Scholar] [CrossRef]
- Kong, L.; Ferry, J. Photochemical oxidation of chrysene at the silica gel-water interface. J. Photoch. Photobio. A 2004, 162, 415–421. [Google Scholar] [CrossRef]
- Díaz-Torres, J.J.; Hernández-Mena, L.; Murillo-Tovar, M.A.; León-Becerril, E.; López-López, A.; Suárez-Plascencia, C.; Aviña-Rodríguez, E.; Barradas-Gimate, A.; Ojeda-Castillo, V. Assessment of the modulation effect of rainfall on the solar radiation availability at the earth’s surface. Meteorol. Appl. 2017, 24, 180–190. [Google Scholar] [CrossRef]
- Almanza, R.; Lopez, S. Total solar radiation in Mexico using sunshine hours and meteorological data. Sol. Energy 1978, 21, 441–448. [Google Scholar] [CrossRef]
- Galindo, I.; Castro, S.; Valdés, M. Satellite derived solar irradiance over Mexico. Atmosfera 1991, 4, 189–200. [Google Scholar]
- Vione, D.; Barra, S.; De Gennaro, G.; De Rienzo, M.; Gilardoni, S.; Perrone, M.G.; Pozzoli, L. Polycyclic aromatic hydrocarbons in the atmosphere: monitoring sources, sinks and fate. II: Sinks and fate. Ann. Chim. 2004, 94, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the upper and lower atmosphere; Academic Press: New York, NY, USA, 2000; p. 265. [Google Scholar]
- Liang, J. Radiation in the atmosphere. In Chemical Modeling for Air Resources; Academic Press: Boston, USA, 2013; pp. 43–63. [Google Scholar]
- Fernández-Bremauntz, A.A.; Ashmore, M.R. Exposure of commuters to carbon monoxide in Mexico City—I. Measurement of in-vehicle concentrations. Atmos. Environ. 1995, 29, 525–532. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Andreae, M.O. Biomass burning in the tropics: impact on atmospheric chemistry and biogeochemical cycles. Science 1990, 250, 1669–1678. [Google Scholar] [CrossRef]
- Reisen, F.; Arey, J. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles basin. Environ. Sci. Technol. 2005, 39, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Bencs, L.; Wauters, E.; Hoog, J.; Deutsch, F.; Roekens, E.; Bleux, N.; Berghmans, P.; Grieken, R. Seasonal and site-specific variation in vapour and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities. Atmos. Environ. 2006, 40, 771–785. [Google Scholar] [CrossRef]
- Wang, W.; Jariyasopit, N.; Schrlau, J.S.; Jia, Y.; Tao, S.; Yu, T.W.; Dashwood, R.H.; Zhang, W.; Wang, X.; Massey-Simonich, S. Concentration and Photochemistry of PAHs, NPAHs and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011, 45, 6887–6895. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, N.; Nakao, M.; Ohba, Y.; Nakashima, K.; Kuroda, N. Concentration and trend of 9,10-phenanthrenequinone in airborne particulates collected in Nagasaki city, Japan. Chemosphere 2006, 64, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Chang, C.; Wu, Y.; Fu, P.; Yang, L.; Chen, M. Characterization, identification of ambient air and road dust polycyclic aromatic hydrocarbons in Central Taiwan Taichung. Sci. Total Environ. 2004, 327, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Di Stefano, E.; You, Y.; Rodriguez, C.E.; Schmitz, D.A.; Kumagai, Y.; Miguel, A.H.; Eiguren-Fernandez, A.; Kobayashi, T.; Avol, E.; et al. Determination of Four Quinones in Diesel Exhaust Particles, SRM 1649a, and Atmospheric PM2.5. Aerosol Sci. Technol. 2004, 38, 68–81. [Google Scholar] [CrossRef]
- Murillo-Tovar, M.A.; Saldarriaga-Noreña, H.; Hernández-Mena, L.; Campos-Ramos, A.; Cárdenas-González, B.; Ospina-Noreña, J.E.; Cosío-Ramírez, R.; Díaz-Torres, J.J.; Smith, W. Potential Sources of Trace Metals and Ionic Species in PM2.5 in Guadalajara, Mexico: A Case Study during Dry Season. Atmosphere 2015, 6, 1858–1870. [Google Scholar] [CrossRef]
- Li, X.; Kong, S.; Yin, Y.; Li, L.; Yuan, L.; Li, Q.; Xiao, H.; Chen, K. Polycyclic aromatic hydrocarbons (PAHs) in atmospheric PM2.5 around 2013 Asian Youth Games period in Nanjing. Atmos. Res. 2016, 174, 85–86. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J.E. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Cheng, H.X.; Xu, X.B.; Zhuang, G.M.; Zhao, C.D. A wintertime study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5–10 in Beijing: assessment of energy structure conversion. J. Hazard Mater. 2008, 157, 47–56. [Google Scholar] [CrossRef] [PubMed]
| Centro | Tlaquepaque | Águilas | ||||||
|---|---|---|---|---|---|---|---|---|
| Warm-dry season (n = 16) | Rainy season (n = 18) | Warm-dry season (n = 20) | Rainy season (n = 19) | |||||
| Quinones | Mean ± SD | Median(10–90th) | Mean ± SD | Median(10–90th) | Mean ± SD | Median(10–90th) | Mean ± SD | Median(10–90th) |
| 1,4-NQ | 0.15 ± 0.16 | 0.08(0.04–0.43) | 0.24 ± 0.46 | 0.04(0.02–0.66) | 0.17 ± 0.20 | 0.10(0.04–0.31) | 0.10 ± 0.14 | 0.05(0.02–0.26) |
| 1,4-PQ | 0.28 ± 0.25 | 0.20(0.06–0.53) | 0.21 ± 0.12 | 0.18(0.10–0.38) | 0.33 ± 0.26 | 0.29(0.04–0.68) | 0.22 ± 0.16 | 0.17(0.09–0.44) |
| 9,10-AQ | 0.70 ± 0.34 | 0.74(0.25–1.09) | 0.27 ± 0.14 | 0.20(0.15–0.48) | 0.86 ± 0.33 | 0.86(0.43–1.20) | 0.23 ± 0.16 | 0.18(0.10–0.45) |
| 9,10-PQ | 0.57 ± 0.72 | 0.29(0.04–1.48) | 0.15 ± 0.11 | 0.13(0.04–0.31) | 0.64 ± 0.75 | 0.24(0.06–1.85) | 0.16 ± 0.14 | 0.13(0.06–0.31) |
| 1,2-BQ | 0.63 ± 0.33 | 0.74(0.13–0.99) | 0.83 ± 0.36 | 0.79(0.48–1.36) | 1.04 ± 0.51 | 0.92(0.47–1.79) | 0.87 ± 0.39 | 0.85(0.46–1.32) |
| 1,4-CQ | 0.25 ± 0.22 | 0.18(0.11–0.40) | 0.41 ± 0.24 | 0.34(0.14–0.71) | 0.28 ± 0.22 | 0.21(0.07–0.59) | 0.40 ± 0.24 | 0.29(0.19–0.81) |
| 5,12-NQ | 0.42 ± 0.22 | 0.46(0.13–0.70) | 0.38 ± 0.20 | 0.34(0.16–0.64) | 0.62 ± 0.31 | 0.56(0.27–1.15) | 0.41 ± 0.23 | 0.37(0.07–0.65) |
| Σ 7 QNs | 2.98 ± 1.58 | 2.88(1.14–4.35) | 2.50 ± 1.18 | 2.08(1.46–4.18) | 3.93 ± 1.77 | 3.86(2.11–5.20) | 2.38 ± 1.09 | 2.30(1.31–3.37) |
| PAHs | ||||||||
| Nap | 0.03 ± 0.02 | 0.03(0.01–0.06) | 0.02 ± 0.02 | 0.02(0.01–0.04) | 0.02 ± 0.02 | 0.01(0.01–0.05) | 0.02 ± 0.02 | 0.02(0.01–0.05) |
| Acy | 0.13 ± 0.15 | 0.11(0.02–0.26) | 0.02 ± 0.01 | 0.02(0.01–0.04) | 0.26 ± 0.69 | 0.08(0.04–0.27) | 0.02 ± 0.01 | 0.02(0.01–0.04) |
| Acn | 0.10 ± 0.07 | 0.09(0.04–0.16) | 0.04 ± 0.01 | 0.04(0.03–0.06) | 0.15 ± 0.17 | 0.11(0.04–0.18) | 0.03 ± 0.01 | 0.03(0.02–0.05) |
| Flu | 0.03 ± 0.04 | 0.02(0.01–0.05) | 0.01 ± 0.00 | 0.01(0.01–0.02) | 0.13 ± 0.38 | 0.03(0.01–0.19) | 0.01 ± 0.00 | 0.01(0.01–0.02) |
| Phe | 0.36 ± 0.34 | 0.25(0.12–0.76) | 0.13 ± 0.06 | 0.13(0.08–0.21) | 0.70 ± 1.48 | 0.32(0.14–0.97) | 0.12 ± 0.05 | 0.11(0.08–0.17) |
| Ant | 0.11 ± 0.10 | 0.08(0.03–0.24) | 0.06 ± 0.03 | 0.06(0.03–0.09) | 0.29 ± 0.61 | 0.13(0.05–0.47) | 0.06 ± 0.02 | 0.05(0.03–0.09) |
| Flt | 0.18 ± 0.11 | 0.17(0.05–0.32) | 0.10 ± 0.03 | 0.10(0.06–0.14) | 0.24 ± 0.12 | 0.23(0.13–0.36) | 0.10 ± 0.05 | 0.11(0.06–0.13) |
| Pyr | 0.22 ± 0.14 | 0.20(0.06–0.39) | 0.13 ± 0.05 | 0.12(0.08–0.20) | 0.30 ± 0.14 | 0.28(0.16–0.44) | 0.13 ± 0.06 | 0.14(0.07–0.19) |
| BaA | 0.25 ± 0.16 | 0.21(0.09–0.40) | 0.09 ± 0.04 | 0.08(0.05–0.15) | 0.35 ± 0.18 | 0.30(0.17–0.66) | 0.10 ± 0.05 | 0.10(0.04–0.15) |
| Chry | 0.25 ± 0.14 | 0.21(0,08–0.40) | 0.25 ± 0.12 | 0.23(0.12–0.37) | 0.38 ± 0.18 | 0.36(0.19–0.64) | 0.27 ± 0.12 | 0.27(0.12–0.45) |
| BbF | 0.52 ± 0.36 | 0.50(0.13–1.04) | 0.19 ± 0.14 | 0.14(0.08–0.34) | 0.87 ± 0.50 | 0.80(0.39–1.70) | 0.19 ± 0.21 | 0.13(0.06–0.38) |
| BkF | 0.43 ± 0.30 | 0.33(0.12–0.71) | 0.40 ± 0.17 | 0.36(0.26–0.62) | 0.72 ± 0.36 | 0.70(0.39–1.09) | 0.36 ± 0.19 | 0.32(0.19–0.54) |
| BaP | 0.47 ± 0.27 | 0.42(0.14–0.83) | 0.52 ± 0.24 | 0.49(0.31–0.79) | 0.70 ± 0.33 | 0.63(0.35–1.10) | 0.48 ± 0.27 | 0.43(0.18–0.89) |
| IcdP | 0.69 ± 0.45 | 0.64(0.18–1.28) | 0.45 ± 0.21 | 0.39(0.23–0.70) | 1.12 ± 0.51 | 1.10(0.56–1.76) | 0.42 ± 0.27 | 0.36(0.14–0.75) |
| DBahA | 0.08 ± 0.04 | 0.09(0.03–0.12) | 0.11 ± 0.07 | 0.09(0.03–0.20) | 0.14 ± 0.06 | 0.13(0.66–0.22) | 0.11 ± 0.09 | 0.08(0.03–0.24) |
| BghiP | 0.82 ± 0.52 | 0.75(0.19–1.59) | 0.61 ± 0.23 | 0.59(0.37–0.90) | 1.25 ± 0.53 | 1.26(0.73–1.83) | 0.54 ± 0.32 | 0.47(0.24–0.93) |
| LMW | 0.75 ± 0.65 | 0.52(0.24–1.58) | 0.29 ± 0.10 | 0.29(0.19–0.41) | 1.56 ± 3.32 | 0.63(0.38–1.92) | 0.27 ± 0.10 | 0.25(0.17–0.38) |
| MMW | 0.90 ± 0.54 | 0.76(0.29–1.49) | 0.57 ± 0.22 | 0.54(0.32–0.86) | 1.27 ± 0.61 | 1.25(0.65–2.12) | 0.61 ± 0.26 | 0.64(0.32–0.87) |
| HMW | 3.01 ± 1.86 | 2.76(0.76–5.45) | 2.28 ± 0.96 | 2.10(1.44–3.57) | 4.79 ± 2.18 | 4.54(2.51–7.75) | 2.10 ± 1.29 | 1.84(0.85–3.72) |
| Ratio Quinone/PAH | Centro | Tlaquepaque Warm-Dry Season | Las Águilas Rainy Season | Full Database | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warm-Dry Season | Rainy Season | ||||||||||||||
| Median | Percentile | Median | Percentile | Median | Percentile | Median | Percentile | Median | Percentile | ||||||
| 10 | 90 | 10 | 90 | 10 | 90 | 10 | 90 | 10 | 90 | ||||||
| 1,4-NQ/Nap | 3.15 | 0.87 | 27.76 | 2.77 | 0.69 | 83.08 | 9.23 | 1.27 | 24.30 | 4.19 | 0.70 | 11.62 | 3.97 | 0.72 | 30.11 |
| 1,4-PQ/Phe | 0.70 | 0.12 | 3.49 | 1.35 | 0.95 | 2.85 | 1.17 | 0.04 | 3.93 | 1.49 | 0.79 | 2.77 | 1.32 | 0.18 | 3.52 |
| 9,10-AQ/Ant | 9.05 | 3.82 | 15.79 | 4.25 | 2.60 | 7.86 | 7.55 | 1.92 | 13.99 | 3.45 | 1.65 | 6.93 | 5.74 | 1.99 | 13.50 |
| 1,4-AQ/Ant | 20.16 | 8.79 | 78.69 | 49.35 | 23.79 | 104.85 | 16.83 | 2.17 | 43.09 | 49.16 | 28.56 | 99.85 | 38.62 | 10.03 | 98.61 |
| 9,10-PQ/Phe | 0.87 | 0.23 | 8.90 | 1.03 | 0.45 | 2.21 | 0.64 | 0.25 | 4.70 | 0.92 | 0.57 | 2.70 | 0.92 | 0.26 | 3.93 |
| 1,2-BQ/BaA | 2.29 | 0.85 | 5.81 | 8.74 | 6.46 | 12.75 | 2.98 | 2.00 | 5.06 | 9.03 | 6.60 | 13.88 | 5.79 | 1.97 | 10.84 |
| 1,4-CQ/Chry | 0.71 | 0.41 | 2.51 | 1.50 | 1.01 | 2.09 | 0.59 | 0.22 | 1.77 | 1.47 | 1.06 | 1.88 | 1.30 | 0.41 | 2.12 |
| PCA | CEN (n = 37) | TLA (n = 23) | AGU (n = 22) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Eigenvalues | 3.09 | 2.15 | 1.97 | 3.59 | 2.21 | 2.02 | 3.87 | 3.60 | 1.21 |
| Variance accumulated (%) | 30.89 | 52.34 | 72.04 | 35.93 | 58.01 | 78.21 | 38.74 | 74.69 | 86.79 |
| 1,4-NQ | 0.17 | 0.51 | −0.05 | −0.12 | 0.92 | −0.00 | 0.68 | 0.18 | −0.04 |
| 1.4-PQ | −0.07 | 0.14 | 0.81 | 0.05 | −0.19 | 0.76 | 0.90 | 0.14 | 0.12 |
| 9,10-AQ | 0.76 | −0.10 | 0.28 | 0.53 | −0.12 | 0.48 | 0.90 | 0.13 | 0.10 |
| 9,10-PQ | 0.08 | 0.05 | 0.99 | 0.14 | 0.57 | 0.80 | 0.09 | 0.04 | 0.99 |
| 1,2-BQ | 0.08 | 0.77 | 0.02 | 0.86 | −0.11 | 0.25 | 0.28 | 0.95 | 0.00 |
| 1,4-CQ | −0.25 | 0.77 | 0.34 | 0.35 | −0.05 | 0.53 | −0.07 | 0.95 | 0.00 |
| 5,12-NQ | 0.40 | 0.60 | 0.36 | 0.72 | −0.09 | 0.43 | 0.42 | 0.77 | 0.11 |
| LMW | 0.86 | −0.04 | −0.07 | −0.07 | 0.99 | −0.15 | 0.68 | 0.50 | 0.43 |
| MMW | 0.86 | 0.30 | −0.11 | 0.96 | 0.14 | 0.02 | 0.37 | 0.90 | 0.08 |
| HMW | 0.88 | 0.47 | −0.09 | 0.99 | −0.05 | 0.10 | 0.97 | 0.25 | 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barradas-Gimate, A.; Murillo-Tovar, M.A.; Díaz-Torres, J.D.J.; Hernández-Mena, L.; Saldarriaga-Noreña, H.; Delgado-Saborit, J.M.; López-López, A. Occurrence and Potential Sources of Quinones Associated with PM2.5 in Guadalajara, Mexico. Atmosphere 2017, 8, 140. https://doi.org/10.3390/atmos8080140
Barradas-Gimate A, Murillo-Tovar MA, Díaz-Torres JDJ, Hernández-Mena L, Saldarriaga-Noreña H, Delgado-Saborit JM, López-López A. Occurrence and Potential Sources of Quinones Associated with PM2.5 in Guadalajara, Mexico. Atmosphere. 2017; 8(8):140. https://doi.org/10.3390/atmos8080140
Chicago/Turabian StyleBarradas-Gimate, Adriana, Mario Alfonso Murillo-Tovar, José De Jesús Díaz-Torres, Leonel Hernández-Mena, Hugo Saldarriaga-Noreña, Juana Maria Delgado-Saborit, and Alberto López-López. 2017. "Occurrence and Potential Sources of Quinones Associated with PM2.5 in Guadalajara, Mexico" Atmosphere 8, no. 8: 140. https://doi.org/10.3390/atmos8080140






