Seasonal Trends of Formaldehyde and Acetaldehyde in the Megacity of São Paulo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Study Period
2.2. Formaldehyde and Acetaldehyde Measurements
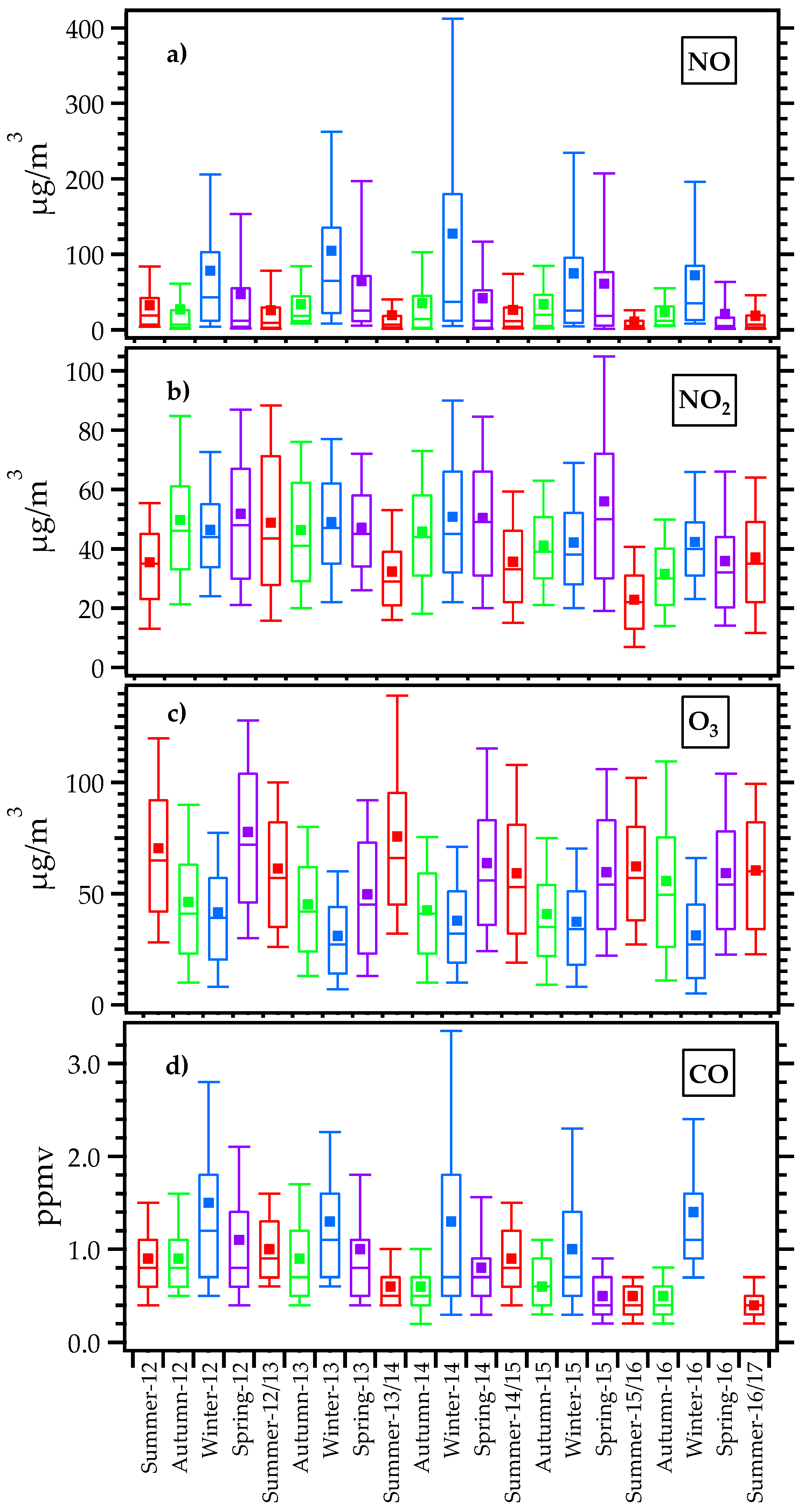
2.3. NO, NO2, CO, and Ozone Measurements
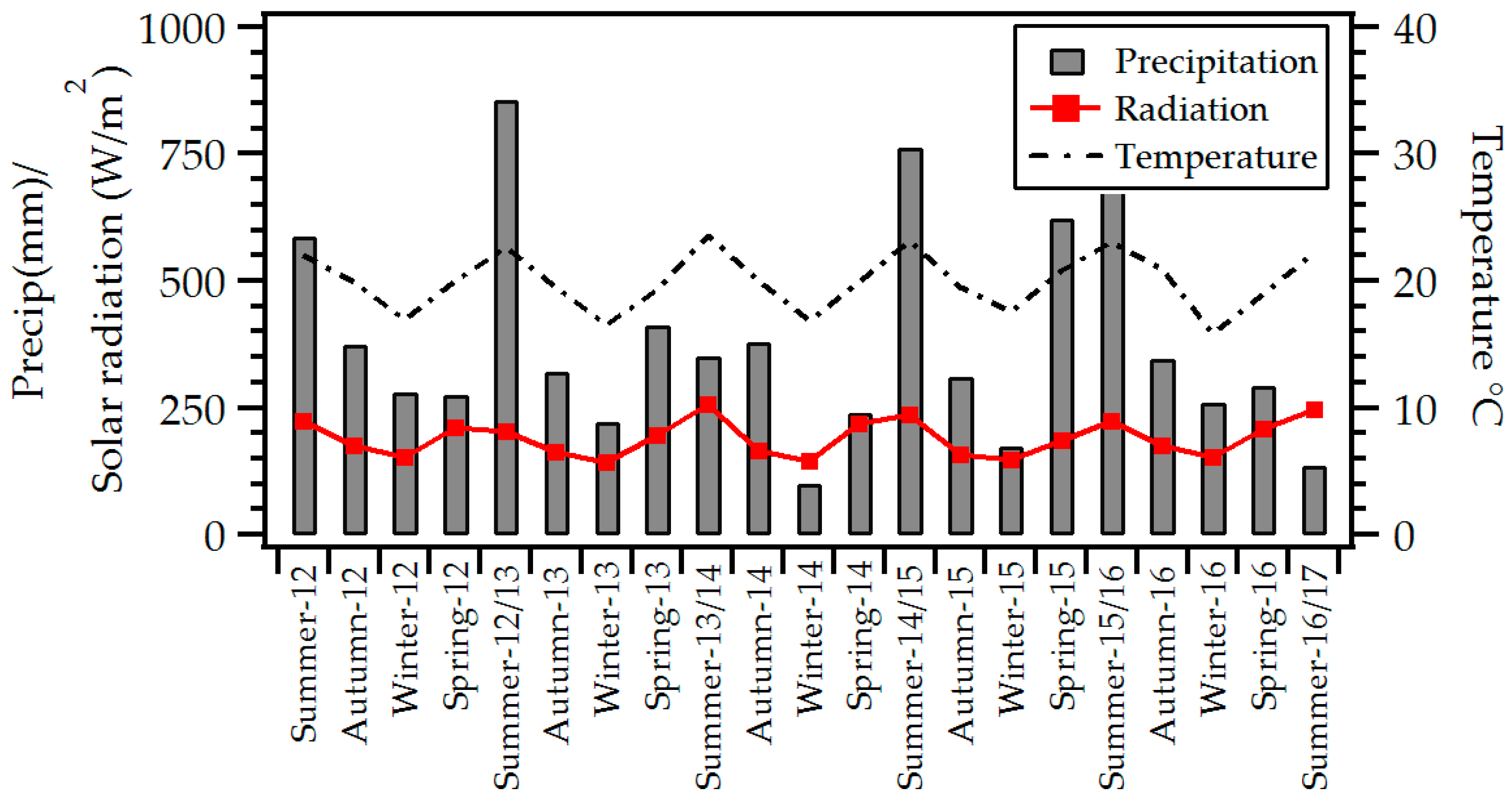
2.4. Meteorological Parameters and Ancillary Data
3. Results and Discussion
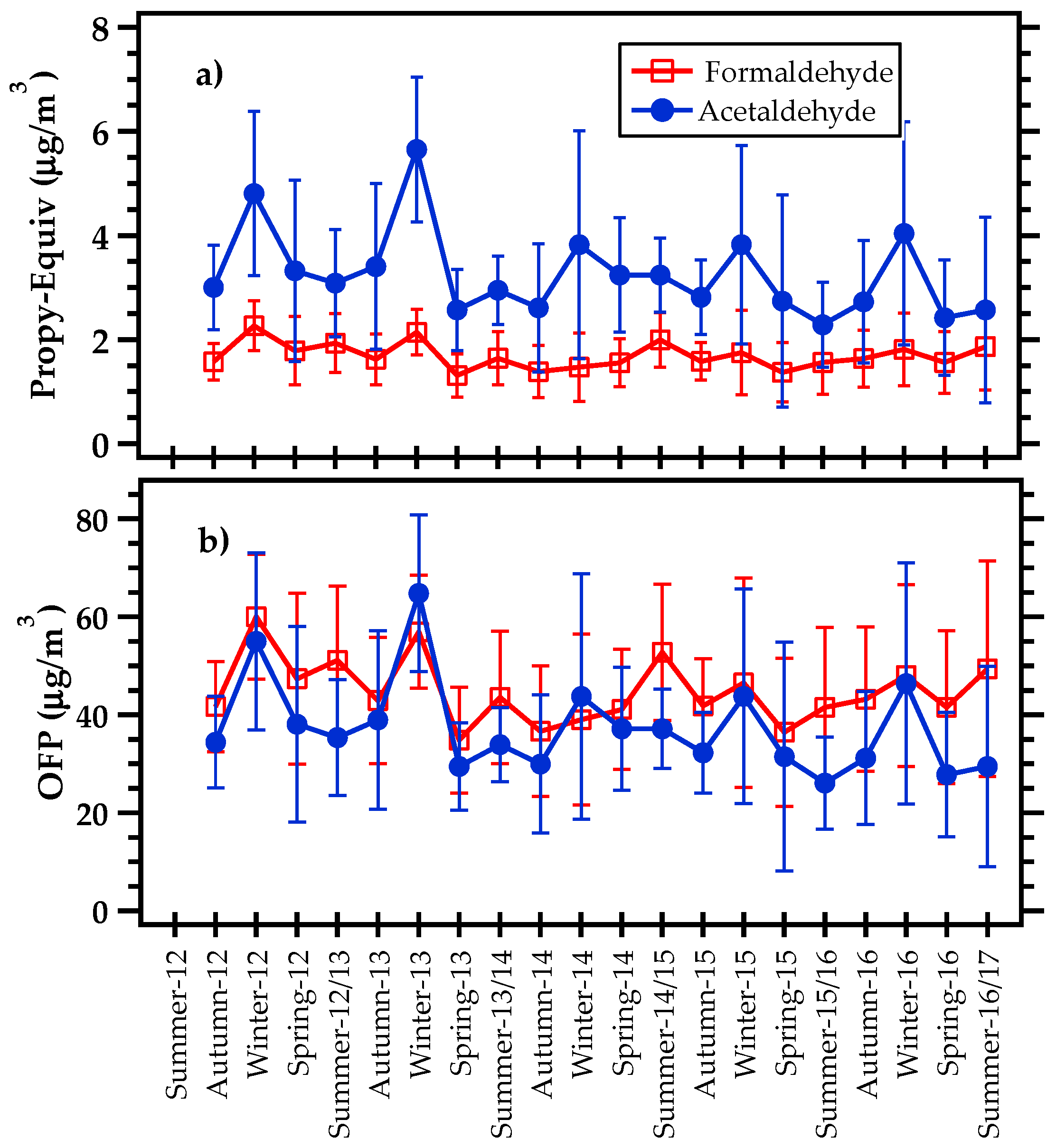
3.1. Seasonal Variations in Formaldehyde and Acetaldehyde Concentrations
3.2. Seasonal Meteorological, NO, NO2, CO, and Ozone Variations
3.3. F/A Ratio
3.4. Primary vs Secondary Sources of Aldehydes in the MASP
3.5. OH Reactivity and Ozone Formation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Instituto Brasileiro de Geografia e Estatística (IBGE); Brazilian Institute of Geography and Statistics. Available online: http://cod.ibge.gov.br/QHF (accessed on 25 May 2017).
- União da Indústria da Cana de Açúcar (UNICA). Available online: http://www.unicadata.com.br (accessed on 7 July 2017).
- Felix, J.D.; Willey, J.D.; Thomas, R.K.; Mullaugh, K.M.; Avery, G.B.; Kieber, R.J.; Mead, R.N.; Helms, J.; Giubbina, F.F.; Campos, M.L.A.M.; et al. Removal of atmospheric ethanol by wet deposition. Glob. Biogeochem. Cycles 2017, 31, 348–356. [Google Scholar] [CrossRef]
- Giubbina, F.F.; Scaramboni, C.; de Martinis, B.S.; Godoy-Silva, D.; Nogueira, R.F.P.; Campos, M.L.A.M. A simple method for simultaneous determination of acetaldehyde, acetone, methanol, and ethanol in the atmosphere and natural waters. Anal. Methods 2017, 9, 2915–2922. [Google Scholar] [CrossRef]
- De Gouw, J.A.; McKeen, S.A.; Aikin, K.C.; Brock, C.A.; Brown, S.S.; Gilman, J.B.; Graus, M.; Hanisco, T.; Holloway, J.S.; Kaiser, J.; et al. Airborne measurements of the atmospheric emissions from a fuel ethanol refinery. J. Geophys. Res. Atmos. 2015, 120, 4385–4397. [Google Scholar] [CrossRef]
- MCTI Estimativas Anuais de Emissões de Gases de Efeito Estufa no Brasil. 2016. Available online: http://sirene.mcti.gov.br/documents/1686653/1706227/LIVRO_MCTIC_EstimativaDeGases_Publica%C3%A7%C3%A3o_210x297mm_FINAL_WEB.pdf/61e78a4d-5ebe-49cd-bd16-4ebca30ad6cd (accessed on 7 July 2017).
- Instituto Ekos Brasil. Inventário de Emissões e Remoções Antrópicas de Gases de Efeito Estufa do Município de São Paulo de 2003 a 2009 com atualização para 2010 e 2011 Nos Setores Energia e Resíduos. Available online: http://www.prefeitura.sp.gov.br/cidade/secretarias/upload/meio_ambiente/arquiv (accessed on 7 July 2017).
- Kumar, P.; Andrade, M.F.; Ynoue, R.Y.; Fornaro, A.; de Freitas, E.D.; Martins, J.; Martins, L.D.; Albuquerque, T.; Zhang, Y.; Morawska, L. New directions: From biofuels to wood stoves: The modern and ancient air quality challenges in the megacity of São Paulo. Atmos. Environ. 2016, 140, 364–369. [Google Scholar] [CrossRef] [Green Version]
- CETESB Qualidade do ar no Estado de São Paulo—2016. São Paulo, 2017. Available online: http://ar.cetesb.sp.gov.br/publicacoes-relatorios/ (accessed on 2 May 2017).
- Carvalho, V.S.B.; Freitas, E.D.; Martins, L.D.; Martins, J.A.; Mazzoli, C.R.; Andrade, M.F. Air quality status and trends over the Metropolitan Area of Sao Paulo, Brazil as a result of emission control policies. Environ. Sci. Policy 2015, 47, 68–79. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.J.; Andrade, M.F.; Miranda, R.M. Traffic-related air quality trends in São Paulo, Brazil. J. Geophys. Res. Atmos. 2015, 120, 6290–6304. [Google Scholar] [CrossRef]
- Andrade, M.F.; Kumar, P.; Freitas, E.D.; Ynoue, R.Y.; Martins, J.; Martins, L.D.; Nogueira, T.; Perez-Martinez, P.; Miranda, R.M.; Albuquerque, T.; et al. Air quality in the megacity of São Paulo: Evolution over the last 30 years and future perspectives. Atmos. Environ. 2017, 159, 66–82. [Google Scholar] [CrossRef]
- Nogueira, T.; de Sales Cordeiro, D.; Muñoz, R.A.A.; Fornaro, A.; Miguel, A.H.; Andrade, M.F. Bioethanol and Biodiesel as Vehicular Fuels in Brazil—Assessment of Atmospheric Impacts from the Long Period of Biofuels Use. In Biofuels—Status Perspect; InTech: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.J.; Miranda, R.M.; Nogueira, T.; Guardani, M.L.; Fornaro, A.; Ynoue, R.; Andrade, M.F. Emission factors of air pollutants from vehicles measured inside road tunnels in São Paulo: Case study comparison. Int. J. Environ. Sci. Technol. 2014, 11, 2155–2168. [Google Scholar] [CrossRef]
- Orlando, J.P.; Alvim, D.S.; Yamazaki, A.; Corrêa, S.M.; Gatti, L.V. Ozone precursors for the São Paulo Metropolitan Area. Sci. Total Environ. 2010, 408, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ccoyllo, O.R.; Ynoue, R.Y.; Martins, L.D.; Andrade, M.F. Impacts of ozone precursor limitation and meteorological variables on ozone concentration in São Paulo, Brazil. Atmos. Environ. 2006, 40, 552–562. [Google Scholar] [CrossRef]
- ANP Agência Nacional de Petróleo, Gás Natural e Biocombustíveis. National Agency for Oil, Natural Gas, and Biofuels. Available online: http://www.anp.gov.br (accessed on 1 May 2017).
- Goldemberg, J. Ethanol for a Sustainable Energy Future. Science 2007, 315, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.D.; Andrade, M.F. Ozone Formation Potentials of Volatile Organic Compounds and Ozone Sensitivity to Their Emission in the Megacity of São Paulo, Brazil. Water Air Soil Pollut. 2008, 195, 201–213. [Google Scholar] [CrossRef]
- Salvo, A.; Geiger, F.M. Reduction in local ozone levels in urban São Paulo due to a shift from ethanol to gasoline use. Nat. Geosci. 2014, 7, 450–458. [Google Scholar] [CrossRef]
- Madronich, S. Atmospheric chemistry: Ethanol and ozone. Nat. Geosci. 2014, 7, 395–397. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Effects of ethanol (E85) versus gasoline vehicles on cancer and mortality in the United States. Environ. Sci. Technol. 2007, 41, 4150–4157. [Google Scholar] [CrossRef] [PubMed]
- Ginnebaugh, D.L.; Jacobson, M.Z. Examining the impacts of ethanol (E85) versus gasoline photochemical production of smog in a fog using near-explicit gas- and aqueous-chemistry mechanisms. Environ. Res. Lett. 2012, 7, 45901. [Google Scholar] [CrossRef]
- CETESB-QUALAR São Paulo State Environmental Agency (CETESB). Available online: http://www.cetesb.sp.gov.br (accessed on 2 May 2017).
- Environmental Protection Agency (EPA). Method TO-11A Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography. In Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, 2nd ed.; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999. [Google Scholar]
- Grosjean, D.; Miguel, A.H.; Tavares, T.M. Urban air pollution in Brazil: Acetaldehyde and other carbonyls. Atmos. Environ. B Urban Atmos. 1990, 24, 101–106. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Guarieiro, L.L.N.; Cardoso, M.P.; Souza, L.; Gisele, O.; Andrade, J.B.; Carvalho, L.S.; da Rocha, G.O.; de Andrade, J.B. Acetaldehyde and formaldehyde concentrations from sites impacted by heavy-duty diesel vehicles and their correlation with the fuel composition: Diesel and diesel/biodiesel blends. Fuel 2012, 92, 258–263. [Google Scholar] [CrossRef]
- Corrêa, S.M.; Martins, E.M.; Arbilla, G. Formaldehyde and acetaldehyde in a high traffic street of Rio de Janeiro, Brazil. Atmos. Environ. 2003, 37, 23–29. [Google Scholar] [CrossRef]
- Pinto, J.P.; Solci, M.C. Comparison of rural and urban atmospheric aldehydes in Londrina, Brazil. J. Braz. Chem. Soc. 2007, 18, 928–936. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; Tan, J. Seasonal and diurnal characteristics of atmospheric carbonyls in Nanning, China. Atmos. Res. 2016, 169, 46–53. [Google Scholar] [CrossRef]
- Cheng, Y.; Lee, S.C.; Huang, Y.; Ho, K.F.; Ho, S.S.H.; Yau, P.S.; Louie, P.K.K.; Zhang, R.J. Diurnal and seasonal trends of carbonyl compounds in roadside, urban, and suburban environment of Hong Kong. Atmos. Environ. 2014, 89, 43–51. [Google Scholar] [CrossRef]
- Lü, H.; Cai, Q.Y.; Wen, S.; Chi, Y.; Guo, S.; Sheng, G.; Fu, J. Seasonal and diurnal variations of carbonyl compounds in the urban atmosphere of Guangzhou, China. Sci. Total Environ. 2010, 408, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Grosselin, B.; Daële, V.; Mellouki, A.; Mu, Y. Seasonal, diurnal and nocturnal variations of carbonyl compounds in the semi-urban environment of Orléans, France. J. Environ. Sci. 2016, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.D.; Andrade, M.F.; Ynoue, R.Y.; Albuquerque, E.L.; Tomaz, E.; Vasconcellos, P.C. Ambiental volatile organic compounds in the megacity of Sao Paulo. Quim. Nova 2008, 31, 2009–2013. [Google Scholar] [CrossRef]
- Montero, L.; Vasconcellos, P.C.; Souza, S.R.; Pires, M.A.F.; Sánchez-Ccoyllo, O.R.; Andrade, M.F.; Carvalho, L.R.F. Measurements of Atmospheric Carboxylic Acids and Carbonyl Compounds in São Paulo City, Brazil. Environ. Sci. Technol. 2001, 35, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, P.C.; Carvalho, L.R.F.; Pool, C.S. Volatile organic compounds inside urban tunnels of São Paulo City, Brazil. J. Braz. Chem. Soc. 2005, 16, 1210–1216. [Google Scholar] [CrossRef]
- Alvim, D.S.; Gatti, L.V.; dos Santos, M.H.; Yamazaki, A. Estudos dos compostos orgânicos voláteis precursores de ozônio na cidade de São Paulo. Eng. Sanit. Ambient. 2011, 16, 189–196. [Google Scholar] [CrossRef]
- Nogueira, T.; Dominutti, P.A.; Carvalho, L.R.F.; Fornaro, A.; Andrade, M.F. Formaldehyde and acetaldehyde measurements in urban atmosphere impacted by the use of ethanol biofuel: Metropolitan Area of Sao Paulo (MASP), 2012–2013. Fuel 2014, 134, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Pires, M.; Carvalho, L.R.F. An artifact in air carbonyls sampling using C18 DNPH-coated cartridge. Anal. Chim. Acta 1998, 367, 223–231. [Google Scholar] [CrossRef]
- CETESB Concentrações de Formaldeído e Acetaldeído na Atmosfera. Estaçâo Pinheiros, São Paulo-SP (2012–2013). 2015; Volume 1. Available online: http://ar.cetesb.sp.gov.br/publicacoes-relatorios/ (accessed on 2 May 2017).
- IAG Weather Station Bulletin. Available online: http://www.estacao.iag.usp.br (accessed on 2 May 2017).
- Dominutti, P.A.; Nogueira, T.; Borbon, A.; Andrade, M.F.; Fornaro, A. One-year of NMHCs Hourly Observations in São Paulo Megacity: Meteorological and Traffic Emissions Effects in a Large Ethanol Burning Context. Atmos. Environ. 2016, 142, 371–382. [Google Scholar] [CrossRef]
- Gentner, D.R.; Harley, R.A.; Miller, A.M.; Goldstein, A.H. Diurnal and Seasonal Variability of Gasoline-Related Volatile Organic Compound Emissions in Riverside, California. Environ. Sci. Technol. 2009, 43, 4247–4252. [Google Scholar] [CrossRef] [PubMed]
- De Gouw, J.A.; Gilman, J.B.; Borbon, A.; Warneke, C.; Kuster, W.C.; Goldan, P.D.; Holloway, J.S.; Peischl, J.; Ryerson, T.B.; Parrish, D.D.; et al. Increasing atmospheric burden of ethanol in the United States. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Gentner, D.R.; Worton, D.R.; Isaacman, G.; Davis, L.C.; Dallmann, T.R.; Wood, E.C.; Herndon, S.C.; Goldstein, A.H.; Harley, R.A. Chemical composition of gas-phase organic carbon emissions from motor vehicles and implications for ozone production. Environ. Sci. Technol. 2013, 47, 11837–11848. [Google Scholar] [CrossRef] [PubMed]
- Massambani, O.; Andrade, F. Seasonal behavior of tropospheric ozone in the Sao Paulo (Brazil) metropolitan area. Atmos. Environ. 1994, 28, 3165–3169. [Google Scholar] [CrossRef]
- De Oliveira, A.P.; Machado, A.J.; Escobedo, J.F.; Soares, J. Diurnal evolution of solar radiation at the surface in the city of Sao Paulo: Seasonal variation and modeling. Theor. Appl. Climatol. 2002, 71, 231–249. [Google Scholar] [CrossRef]
- IAG-USP Boletim Climatológico Anual da Estação Meteorológica do IAG/USP. 2015, Volume 18. Available online: http://www.estacao.iag.usp.br/boletim.php (accessed on 3 May 2017).
- IAG-USP Boletim Climatológico Anual da Estação Meteorológica do IAG/USP. 2013. Available online: http://www.estacao.iag.usp.br/boletim.php (accessed on 3 May 2017).
- Rozante, J.; Rozante, V.; Souza Alvim, D.; Ocimar Manzi, A.; Barboza Chiquetto, J.; Siqueira D’Amelio, M.; Moreira, D. Variations of Carbon Monoxide Concentrations in the Megacity of São Paulo from 2000 to 2015 in Different Time Scales. Atmosphere 2017, 8, 81. [Google Scholar] [CrossRef]
- Wolfe, G.M.; Kaiser, J.; Hanisco, T.F.; Keutsch, F.N.; de Gouw, J.A.; Gilman, J.B.; Graus, M.; Hatch, C.D.; Holloway, J.; Horowitz, L.W.; et al. Formaldehyde production from isoprene oxidation across NOx regimes. Atmos. Chem. Phys. 2016, 16, 2597–2610. [Google Scholar] [CrossRef]
- Brito, J.; Wurm, F.; Yáñez-Serrano, A.M.; de Assunção, J.V.; Godoy, J.M.; Artaxo, P. Vehicular Emission Ratios of VOCs in a Megacity Impacted by Extensive Ethanol Use: Results of Ambient Measurements in Sao Paulo, Brazil. Environ. Sci. Technol. 2015, 49, 11381–11387. [Google Scholar] [CrossRef] [PubMed]
- Borbon, A.; Fontaine, H.; Veillerot, M.; Locoge, N.; Galloo, J.C.; Guillermo, R. An investigation into the traffic-related fraction of isoprene at an urban location. Atmos. Environ. 2001, 35, 3749–3760. [Google Scholar] [CrossRef]
- Von Schneidemesser, E.; Monks, P.S.; Gros, V.; Gauduin, J.; Sanchez, O. How important is biogenic isoprene in an urban environment? A study in London and Paris. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Wang, A.; Ge, Y.; Tan, J.; Fu, M.; Shah, A.N.; Ding, Y.; Zhao, H.; Liang, B. On-road pollutant emission and fuel consumption characteristics of buses in Beijing. J. Environ. Sci. 2011, 23, 419–426. [Google Scholar] [CrossRef]
- Wang, J.L.; Chew, C.; Chang, C.Y.; Liao, W.C.; Lung, S.C.C.; Chen, W.N.; Lee, P.J.; Lin, P.H.; Chang, C.C. Biogenic isoprene in subtropical urban settings and implications forair quality. Atmos. Environ. 2013, 79, 369–379. [Google Scholar] [CrossRef]
- Wagner, P.; Kuttler, W. Biogenic and anthropogenic isoprene in the near-surface urban atmosphere—A case study in Essen, Germany. Sci. Total Environ. 2014, 475, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.G.; Lanning, J.A.; Barrell, R.; Miyagishima, J.; Jones, R.H.; Wolfe, P. Sources and sinks of formaldehyde and acetaldehyde: An analysis of Denver’s ambient concentration data. Atmos. Environ. 1996, 30, 2113–2123. [Google Scholar] [CrossRef]
- Müller, K.; Haferkorn, S.; Grabmer, W.; Wisthaler, A.; Hansel, A.; Kreuzwieser, J.; Cojocariu, C.; Rennenberg, H.; Herrmann, H. Biogenic carbonyl compounds within and above a coniferous forest in Germany. Atmos. Environ. 2006, 40, 81–91. [Google Scholar] [CrossRef]
- Villanueva-Fierro, I.; Popp, C.J.; Martin, R.S. Biogenic emissions and ambient concentrations of hydrocarbons, carbonyl compounds and organic acids from ponderosa pine and cottonwood trees at rural and forested sites in Central New Mexico. Atmos. Environ. 2004, 38, 249–260. [Google Scholar] [CrossRef]
- Stockwell, W.R.; Lawson, C.V.; Saunders, E.; Goliff, W.S. A review of tropospheric atmospheric chemistry and Gas-Phase chemical mechanisms for air quality modeling. Atmosphere 2012, 3, 1–32. [Google Scholar] [CrossRef]
- Altshuller, A.P. Production of aldehydes as primary emissions and from secondary atmospheric reactions of alkenes and alkanes during the night and early morning hours. Atmos. Environ. A Gen. Top. 1993, 27, 21–32. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. CHAPTER 6—Rates and Mechanisms of Gas-Phase Reactions in Irradiated Organic—NOx—Air Mixtures. In Chemistry of the Upper and Lower Atmosphere; Finlayson-Pitts, B.J., Pitts, J.N., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 179–263. [Google Scholar]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Y.; Li, J.; Xiong, B.; Feng, J.; Wen, S.; Sheng, G.; Fu, J.; Wu, M. Characteristics of carbonyl compounds in ambient air of Shanghai, China. J. Atmos. Chem. 2008, 61, 1–20. [Google Scholar] [CrossRef]
- Pang, X.; Lee, X. Temporal variations of atmospheric carbonyls in urban ambient air and street canyons of a Mountainous city in Southwest China. Atmos. Environ. 2010, 44, 2098–2106. [Google Scholar] [CrossRef]
- Duan, J.; Guo, S.; Tan, J.; Wang, S.; Chai, F. Characteristics of atmospheric carbonyls during haze days in Beijing, China. Atmos. Res. 2012, 114, 17–27. [Google Scholar] [CrossRef]
- Nogueira, T.; Souza, K.F.; Fornaro, A.; Andrade, M.F.; Carvalho, L.R.F. On-road emissions of carbonyls from vehicles powered by biofuel blends in traffic tunnels in the Metropolitan Area of Sao Paulo, Brazil. Atmos. Environ. 2015, 108, 88–97. [Google Scholar] [CrossRef]
- Niven, R.K. Ethanol in gasoline: Environmental impacts and sustainability review article. Renew. Sustain. Energy Rev. 2005, 9, 535–555. [Google Scholar] [CrossRef]
- Corrêa, S.M.; Arbilla, G. Carbonyl emissions in diesel and biodiesel exhaust. Atmos. Environ. 2008, 42, 769–775. [Google Scholar] [CrossRef]
- McDonald, B.C.; Gentner, D.R.; Goldstein, A.H.; Harley, R.A. Long-Term Trends in Motor Vehicle Emissions in U.S. Urban Areas. Environ. Sci. Technol. 2013, 47, 10022–10031. [Google Scholar] [CrossRef] [PubMed]
- Possanzini, M.; Palo, V.D.; Cecinato, A. Sources and photodecomposition of formaldehyde and acetaldehyde in Rome ambient air. Atmos. Environ. 2002, 36, 3195–3201. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.P.L. Updated Maximum Incremental Reactivity Scale and Hydrocarbon Bin Reactivities for Regulatory Applications. 2010. Available online: https://www.arb.ca.gov/research/reactivity/mir09.pdf (accessed on 15 May 2017).
- Derwent, R.G.; Jenkin, M.E.; Pilling, M.J.; Carter, W.P.L.; Kaduwela, A. Reactivity scales as comparative tools for chemical mechanisms. J. Air Waste Manag. Assoc. 2010, 60, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.B.N.; Martins, E.M.; Corrêa, S.M. Role of carbonyls and aromatics in the formation of tropospheric ozone in Rio de Janeiro, Brazil. Environ. Monit. Assess. 2016, 188, 289. [Google Scholar] [CrossRef] [PubMed]
- Chameides, W.L.; Fehsenfeld, F.; Rodgers, M.O.; Cardelino, C.; Martinez, J.; Parrish, D.; Lonneman, W.; Lawson, D.R.; Rasmussen, R.A.; Zimmerman, P.; et al. Ozone precursor relationships in the ambient atmosphere. J. Geophys. Res. 1992, 97, 6037. [Google Scholar] [CrossRef]
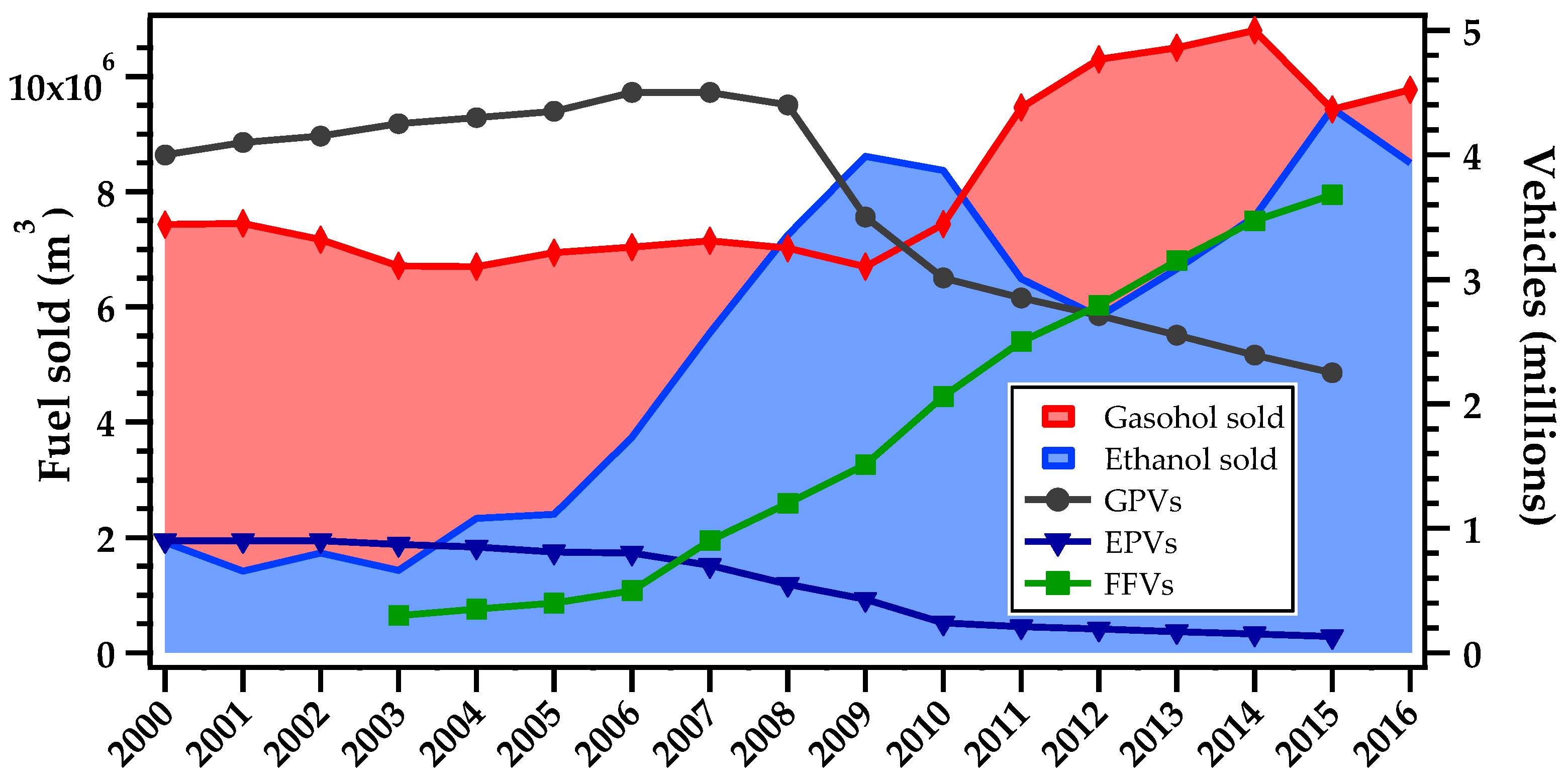
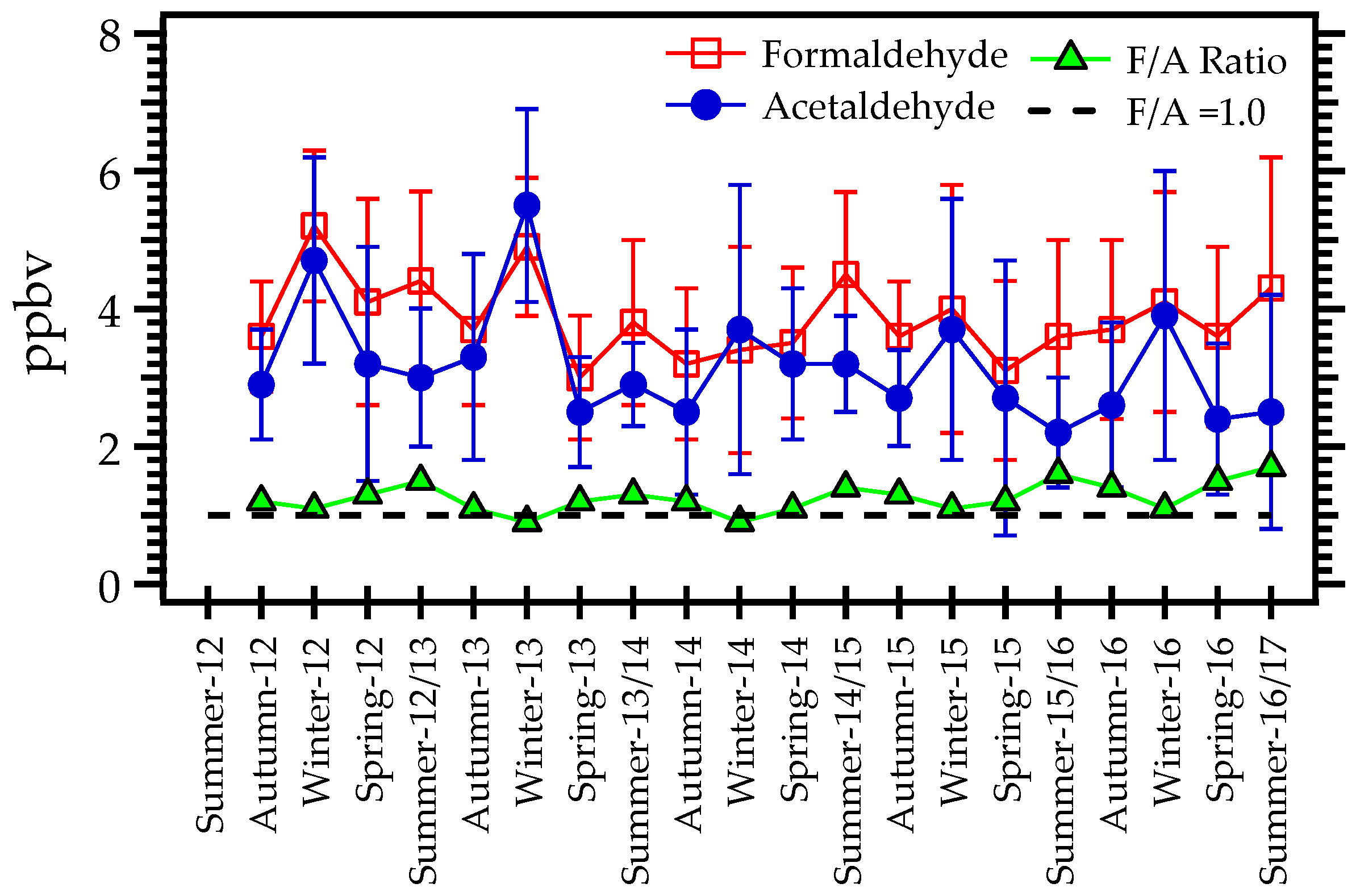
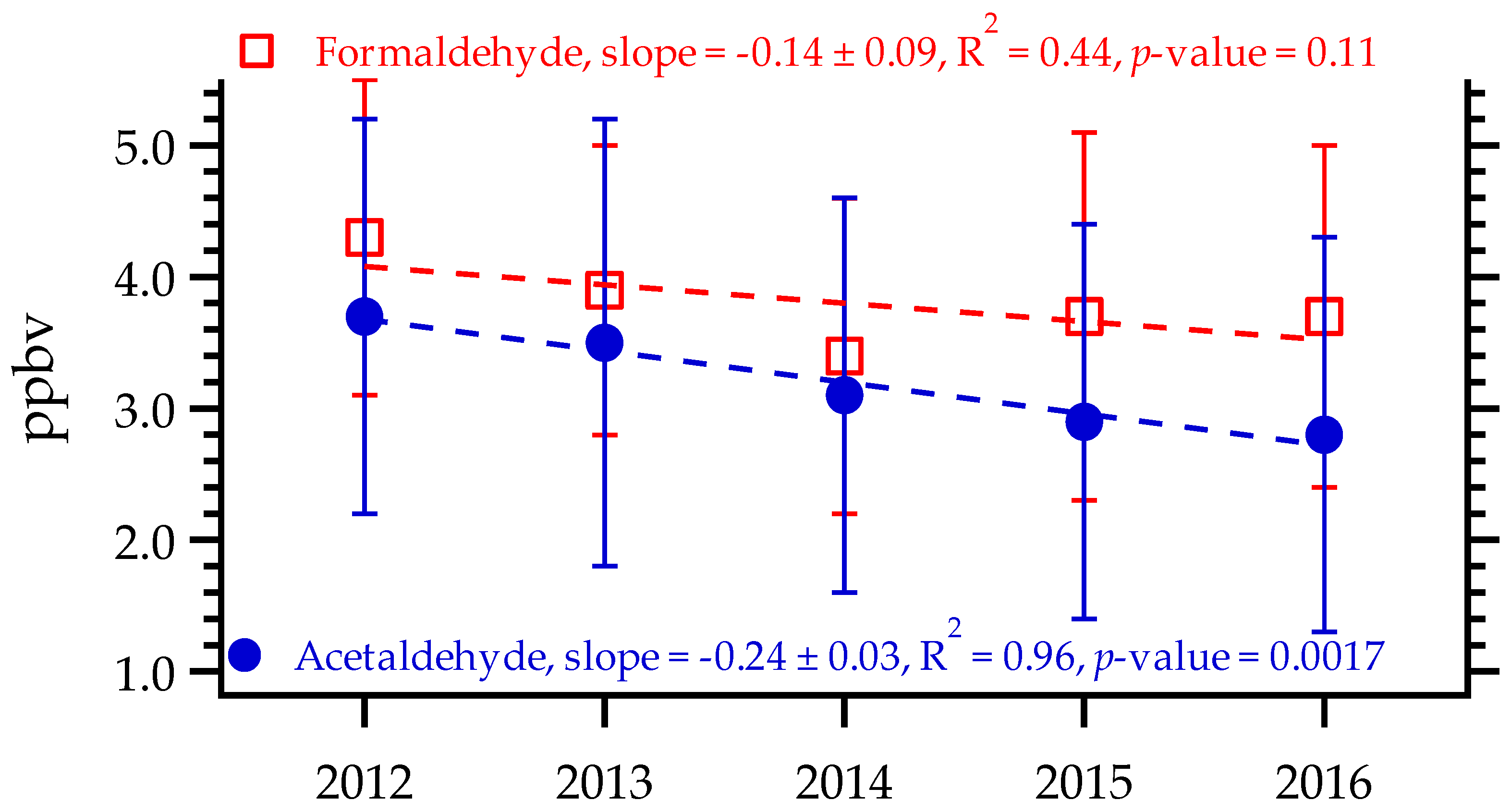
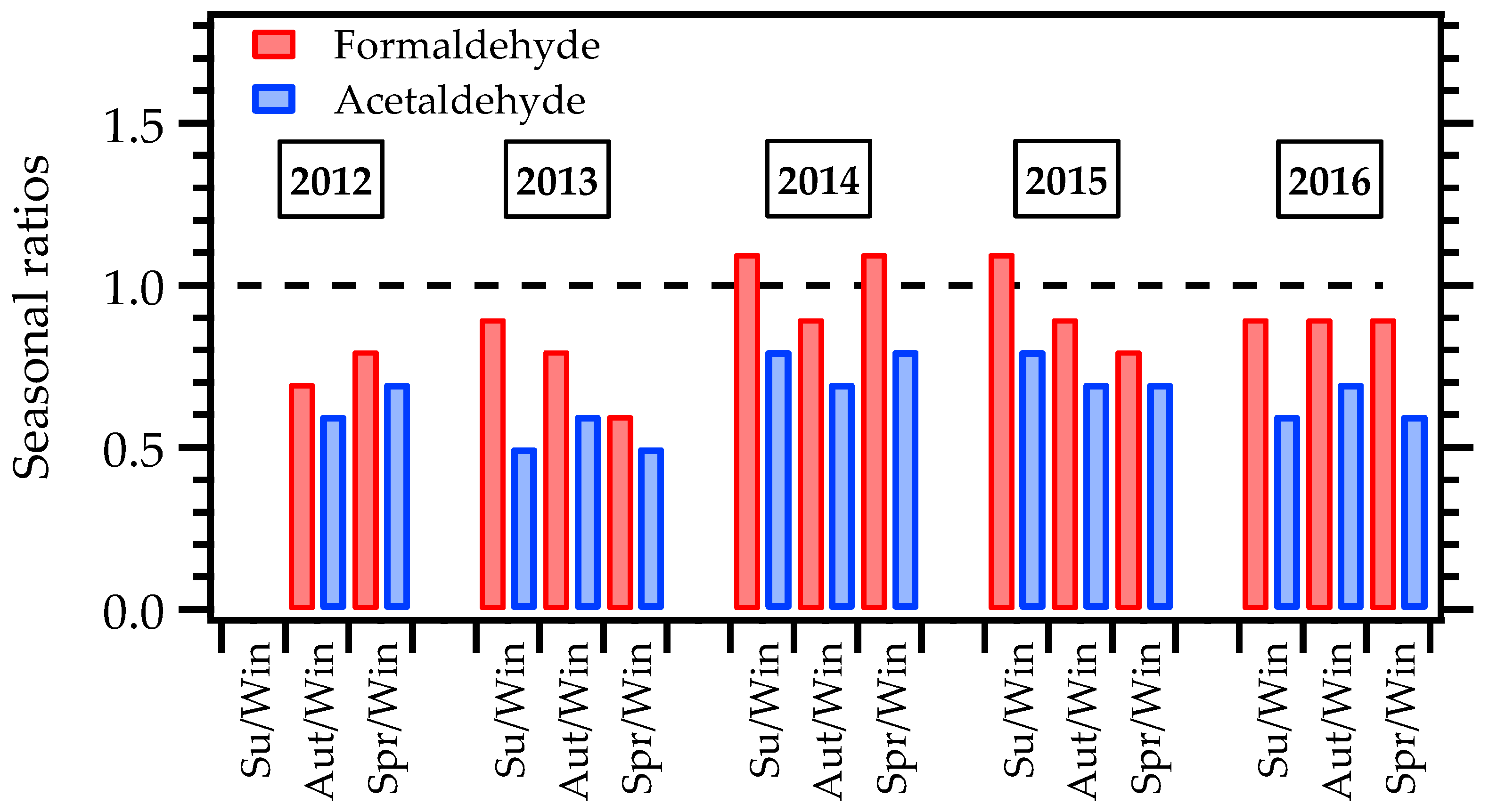
| Variable | Formaldehyde and Acetaldehyde | NOx (NO and NO2) | CO | Ozone |
|---|---|---|---|---|
| Method | DNPH method + HPLC technique | Chemiluminescence | Nondispersive infrared photometry | Ultraviolet photometry |
| Analyzer | Shimadzu LC-10A | Thermo-electron (42i) | Thermo-electron (48i) | Thermo-electron (49i) |
| Precision | <1.5% | ±0.4 ppbv | ±0.1 ppmv | ±1 ppbv |
| Detection Limit | 0.01 ppbv | 0.50 ppbv | 0.04 ppmv | 0.50 ppbv |
| Variable | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| (Min–Max) | (Min–Max) | (Min–Max) | (Min–Max) | (Min–Max) | |
| Formaldehyde | 4.3 ± 1.2 | 3.9 ± 1.1 | 3.4 ± 1.2 | 3.7 ± 1.4 | 3.7 ± 1.3 |
| (1.7–7.7) | (2.1–6.4) | (1.2–6.2) | (1.3–6.9) | (1.4–6.5) | |
| Acetaldehyde | 3.7 ± 1.5 | 3.5 ± 1.7 | 3.1 ± 1.5 | 2.9 ± 1.5 | 2.8 ± 1.5 |
| (1.4–8.9) | (1.3–8.3) | (0.9–9.1) | (0.6–7.7) | (0.9–7.1) | |
| F/A ratio | 1.2 | 1.1 | 1.1 | 1.3 | 1.3 |
| N of samples | 48 | 35 | 49 | 57 | 57 |
| Pollutant | Summer | Autumn | Winter | Spring | Su/Win | Aut/Win | Spr/Win | Reference |
|---|---|---|---|---|---|---|---|---|
| Locale | ||||||||
| Formaldehyde | ||||||||
| MASP | 4.3 ± 1.4 | 3.5 ± 1.0 | 4.3 ± 1.4 | 3.5 ± 1.2 | 1.0 | 0.8 | 0.8 | This work |
| Londrina, Brazil | 4.11 ± 0.97 | n.a. | 5.07 ± 2.09 | n.a. | 0.8 | n.a. | n.a. | Pinto and Solci. [29] |
| Nanning, China * | 8.7 | 4.1 | 2.5 | 6.4 | 3.5 | 1.7 | 2.6 | Guo et al. [30] |
| Hong Kong (Roadside) * | 5.5 ± 2.2 | 5.9 ± 1.2 | 4.6 ± 1.1 | 4.7 ± 1.0 | 1.2 | 1.3 | 1.0 | |
| Hong Kong (Urban) * | 8.7 ± 2.1 | 3.2 ± 0.8 | 2.9 ± 1.3 | 3.2 ± 1.0 | 3.0 | 1.1 | 1.1 | Cheng et al. [31] |
| Hong Kong (Background) * | 2.1 ± 2.0 | 2.1 ± 0.7 | 1.9 ± 0.5 | 1.6 ± 0.6 | 1.1 | 1.1 | 0.8 | |
| Guangzhou, China * | 11.0 | 3.8 | 4.5 | 4.7 | 2.4 | 0.9 | 1.0 | Lü et al. [32] |
| Guangzhou, China * | 8.9 | n.a. | 3.2 | 5.8 | 2.8 | n.a. | 1.8 | Lü et al. [32] |
| Orléans, France | 3.08 ± 2.21 | 2.28 ± 0.82 | 1.46 ± 0.4 | 2.16 ± 0.59 | 2.1 | 1.6 | 1.5 | Jiang et al. [33] |
| Acetaldehyde | ||||||||
| MASP | 2.8 ± 1.0 | 2.8 ± 1.1 | 4.3 ± 1.8 | 2.8 ± 1.3 | 0.7 | 0.7 | 0.7 | This work |
| Londrina, Brazil | 3.02 ± 1.10 | n.a. | 5.72 ± 2.66 | n.a. | 0.5 | n.a. | n.a. | Pinto and Solci [29] |
| Nanning, China * | 10.4 | 4.6 | 4.7 | 14.9 | 2.3 | 1.0 | 3.3 | Guo et al. [30] |
| Hong Kong (Roadside) * | 1.3 ± 0.8 | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.3 ± 0.4 | 0.8 | 1.3 | 0.8 | |
| Hong Kong (Urban) * | 0.8 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.5 | 1.0 ± 0.5 | 0.7 | 0.9 | 0.9 | Cheng et al. [31] |
| Hong Kong (Background) * | 0.5 ± 0.6 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.5 ± 0.3 | 0.6 | 0.9 | 0.6 | |
| Guangzhou, China * | 5.8 | 3.9 | 3.1 | 1.9 | 1.8 | 1.2 | 0.6 | Lü et al. [32] |
| Guangzhou, China * | 9.5 | n.a. | 2.1 | 3.4 | 4.6 | n.a. | 1.6 | Lü et al. [32] |
| Orléans, France | 1.0 ± 0.5 | 0.7 ± 0.3 | 0.7 ± 0.2 | 1.0 ± 0.3 | 1.6 | 1.0 | 1.5 | Jiang et al. [33] |
| Summer | Formaldehyde | Acetaldehyde | NO | NO2 | NOx | CO | Ozone |
| Formaldehyde | 1.00 | 0.92 | 0.38 | 0.49 | 0.43 | 0.42 | 0.65 |
| Acetaldehyde | 1.00 | 0.55 | 0.64 | 0.60 | 0.61 | 0.57 | |
| NO | 1.00 | 0.79 | 0.96 | 0.45 | 0.11 | ||
| NO2 | 1.00 | 0.91 | 0.80 | 0.03 | |||
| NOx | 1.00 | 0.76 | 0.12 | ||||
| CO | 1.00 | 0.26 | |||||
| Ozone | 1.00 | ||||||
| Winter | Formaldehyde | Acetaldehyde | NO | NO2 | NOx | CO | Ozone |
| Formaldehyde | 1.00 | 0.90 | 0.59 | 0.74 | 0.63 | 0.70 | 0.46 |
| Acetaldehyde | 1.00 | 0.79 | 0.79 | 0.82 | 0.85 | 0.33 | |
| NO | 1.00 | 0.61 | 0.99 | 0.90 | 0.12 | ||
| NO2 | 1.00 | 0.67 | 0.71 | 0.39 | |||
| NOx | 1.00 | 0.84 | 0.15 | ||||
| CO | 1.00 | 0.22 | |||||
| Ozone | 1.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, T.; Dominutti, P.A.; Fornaro, A.; Andrade, M.D.F. Seasonal Trends of Formaldehyde and Acetaldehyde in the Megacity of São Paulo. Atmosphere 2017, 8, 144. https://doi.org/10.3390/atmos8080144
Nogueira T, Dominutti PA, Fornaro A, Andrade MDF. Seasonal Trends of Formaldehyde and Acetaldehyde in the Megacity of São Paulo. Atmosphere. 2017; 8(8):144. https://doi.org/10.3390/atmos8080144
Chicago/Turabian StyleNogueira, Thiago, Pamela A. Dominutti, Adalgiza Fornaro, and Maria De Fatima Andrade. 2017. "Seasonal Trends of Formaldehyde and Acetaldehyde in the Megacity of São Paulo" Atmosphere 8, no. 8: 144. https://doi.org/10.3390/atmos8080144





