Variations of Energy Fluxes and Ecosystem Evapotranspiration in a Young Secondary Dry Dipterocarp Forest in Western Thailand
Abstract
:1. Introduction
2. Materials and Methods
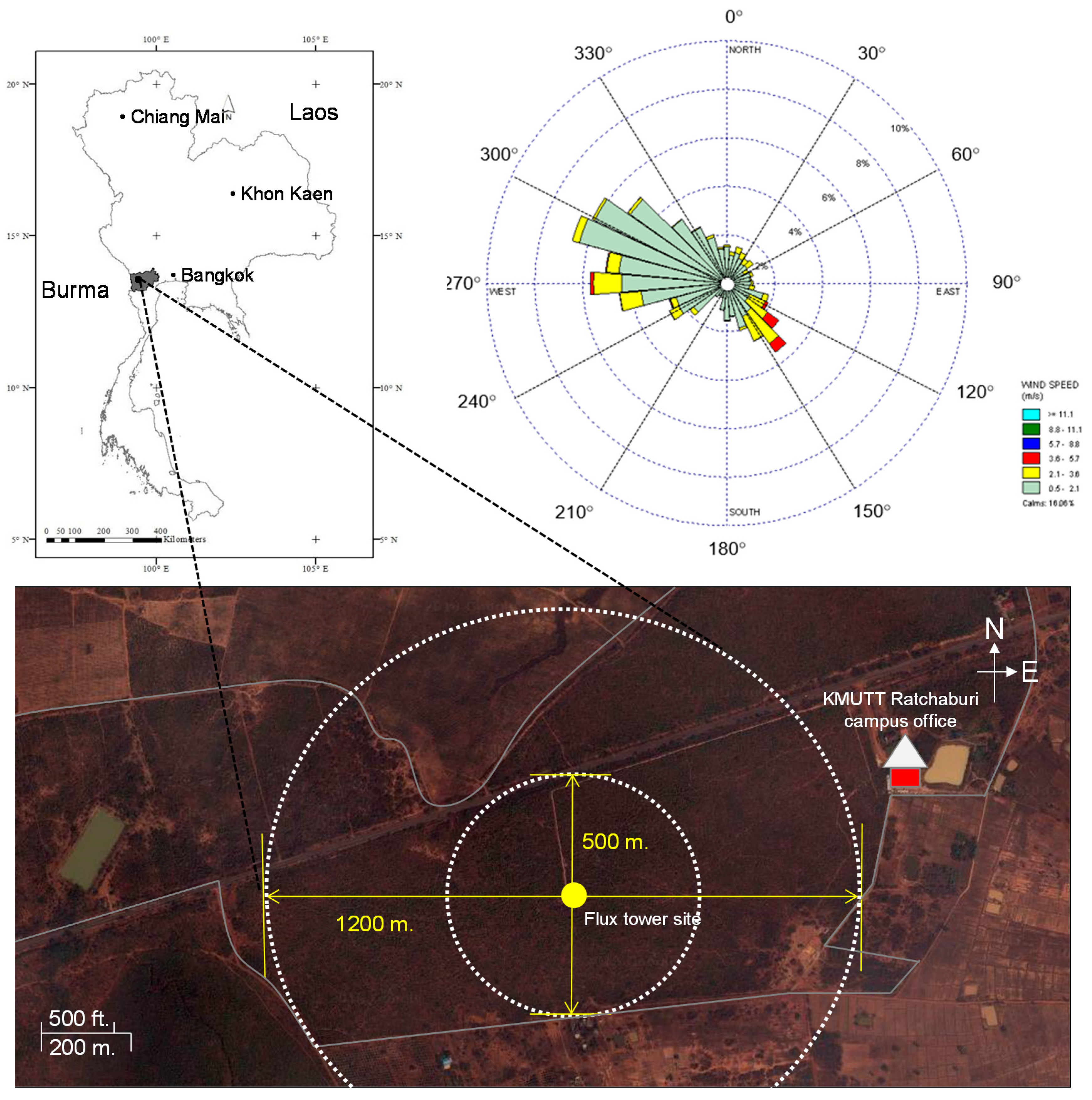
2.1. Site Description
2.2. Instrumentation and Flux Measurements
2.3. Data Processing and Correction
3. Results and Discussion
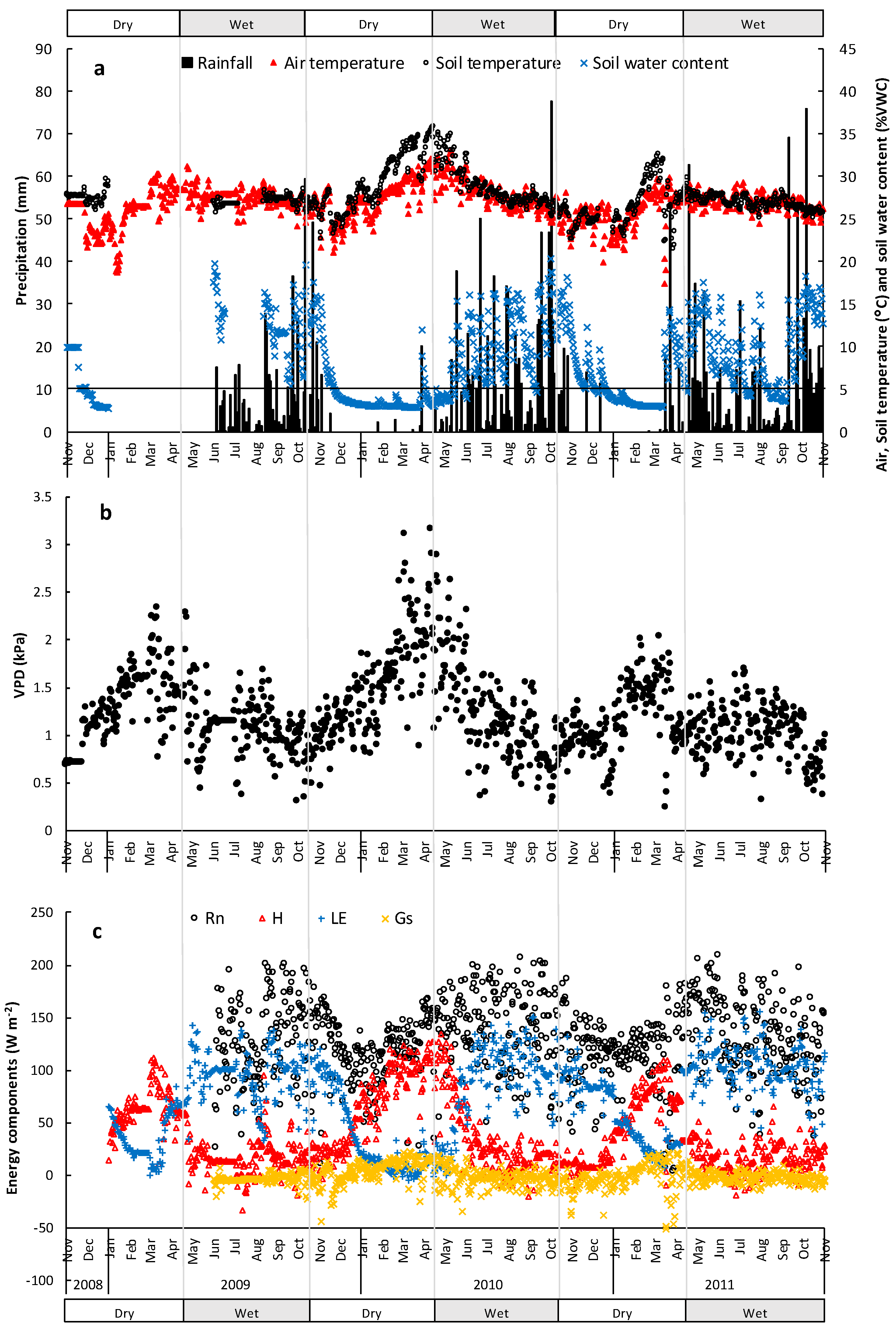
3.1. Micrometeorological Conditions
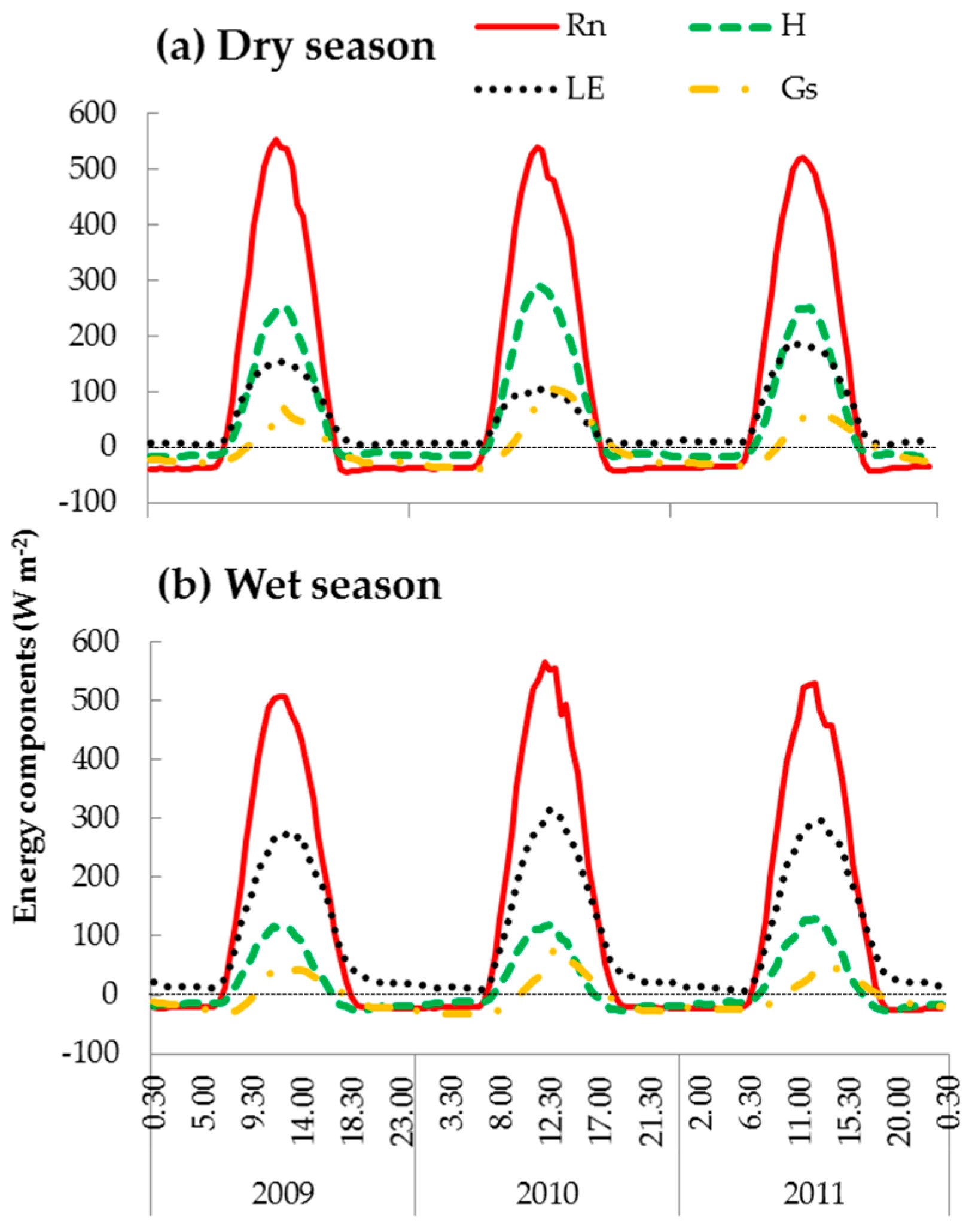
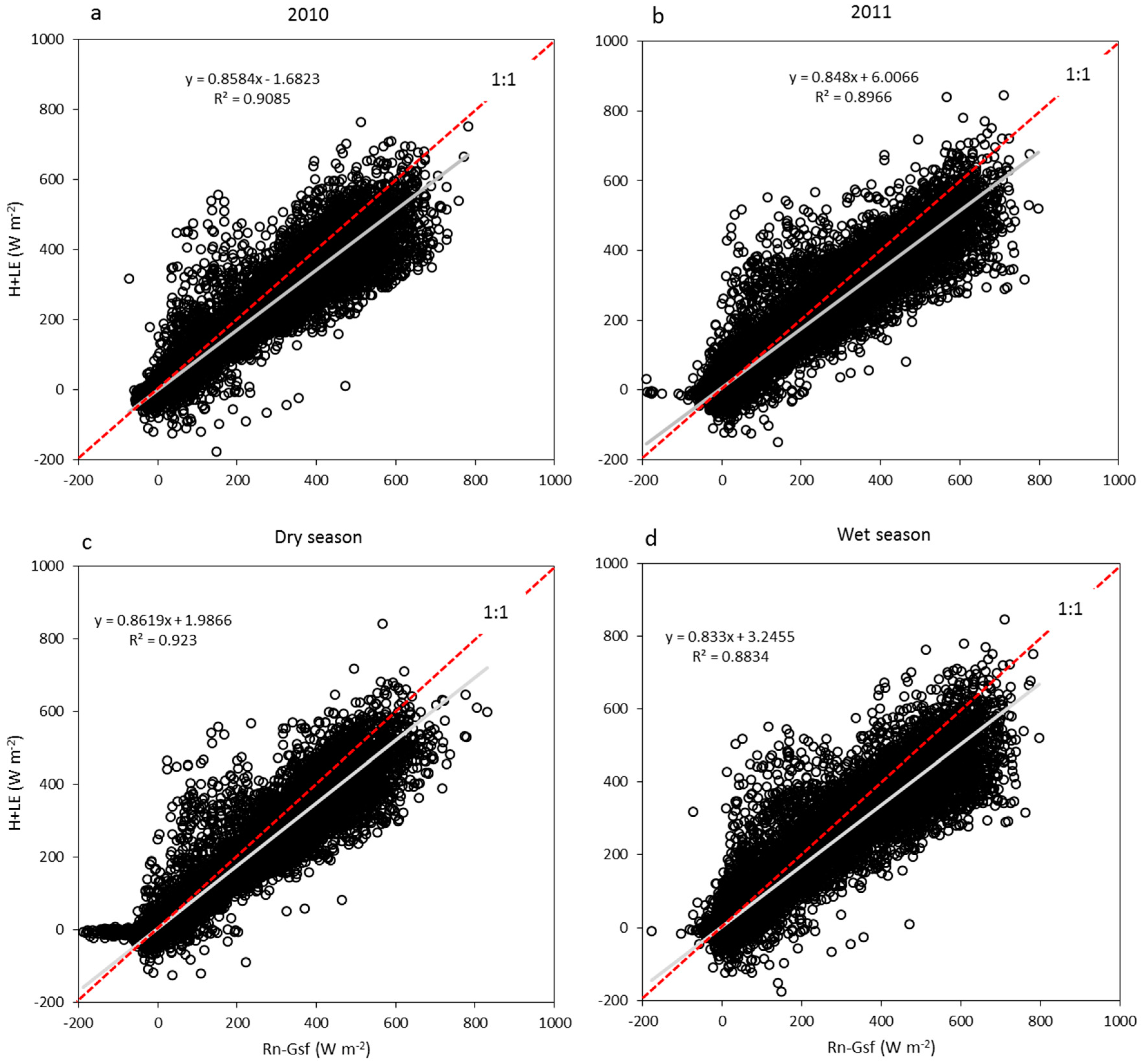
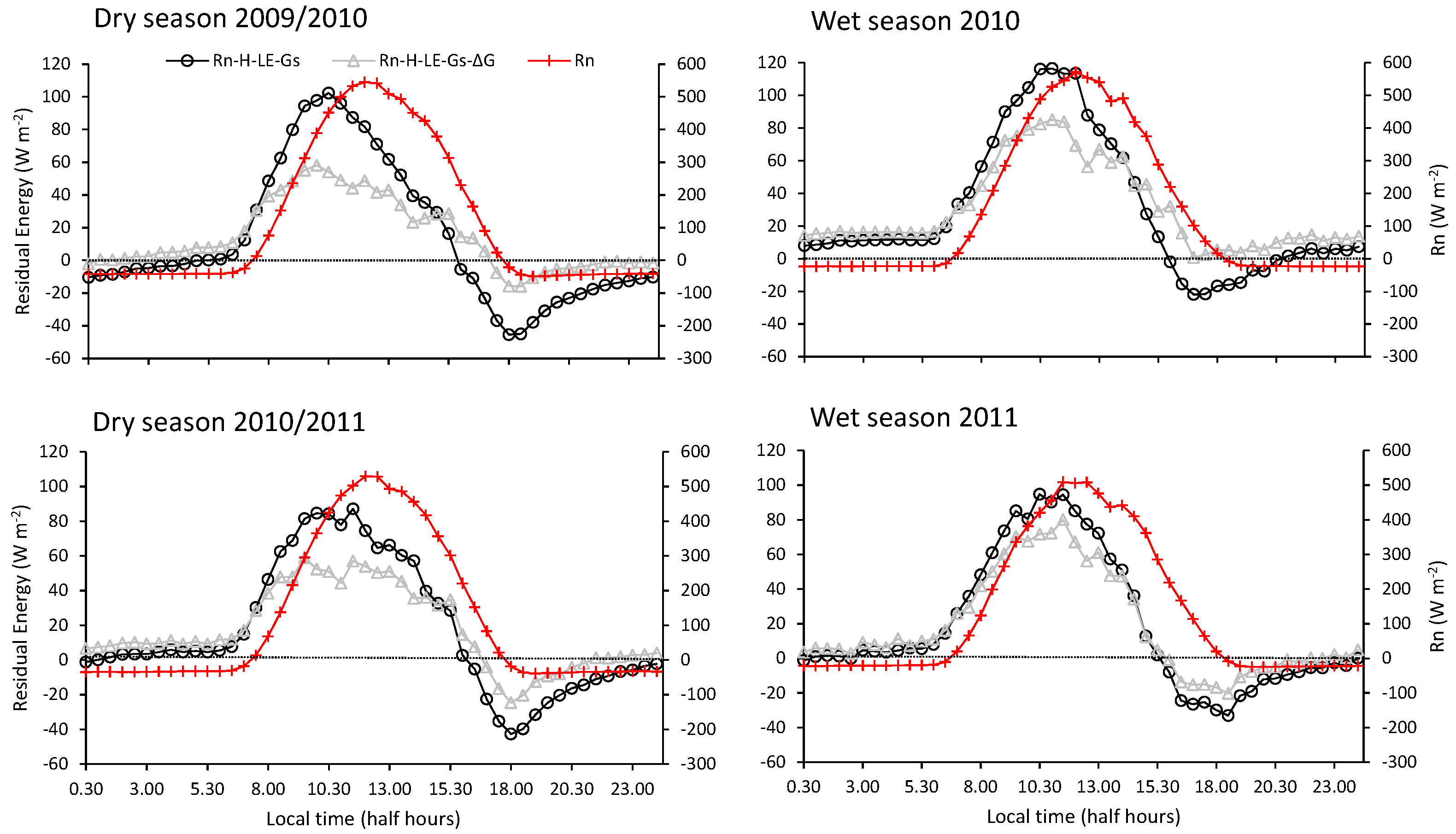
3.2. Energy Dissipation and Partitioning
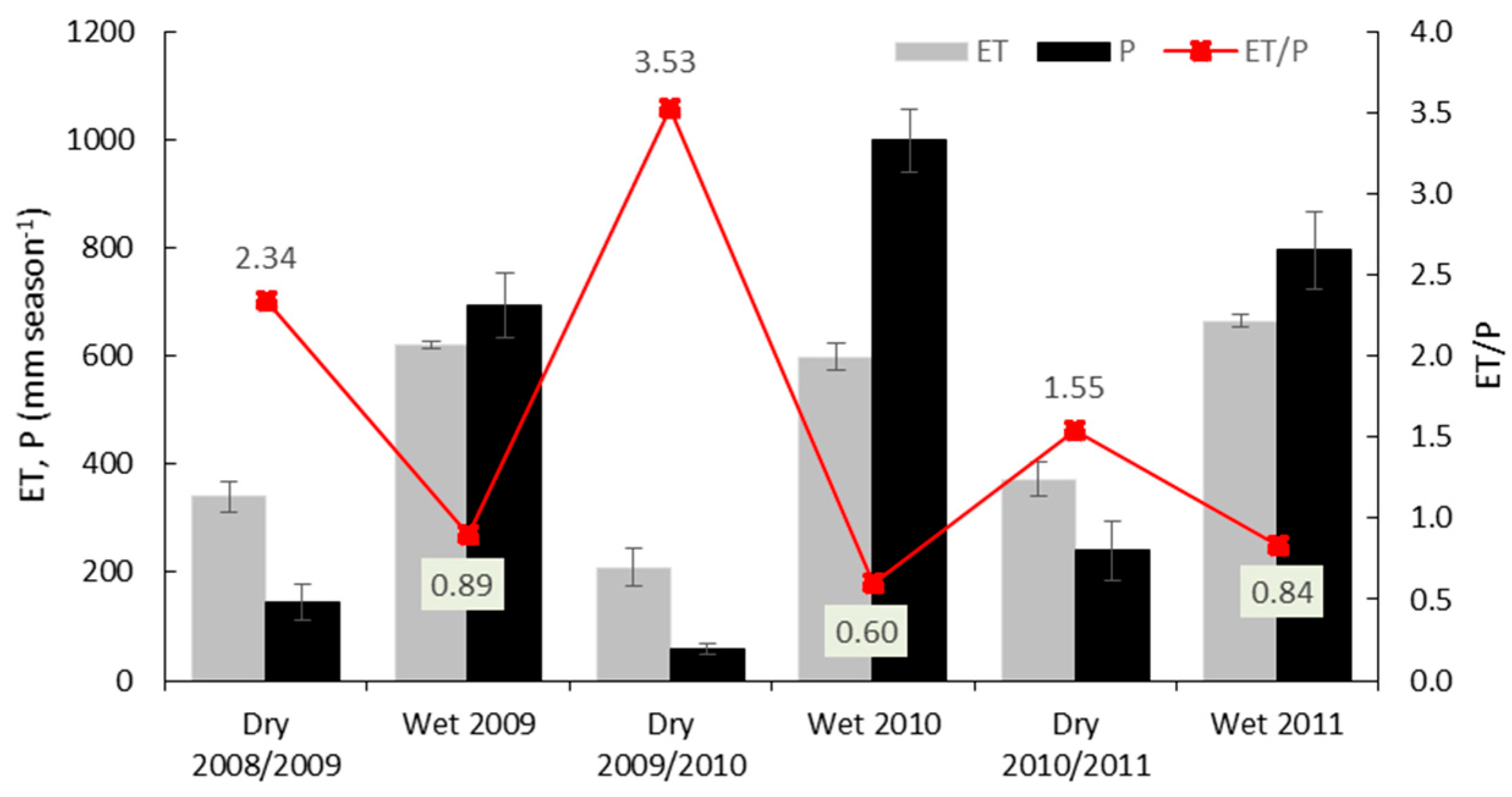
3.3. Variations in Ecosystem Evapotranspiration
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, B.A. Combining national forest type maps with annual global tree cover maps to better understand forest change over time: Case study for Thailand. Appl. Geogr. 2015, 62, 294–300. [Google Scholar] [CrossRef]
- Leinenkugel, P.; Wolters, M.L.; Oppelt, N.; Kuenzer, C. Tree cover and forest cover dynamics in the Mekong Basin from 2001 to 2011. Remote Sens. Environ. 2015, 158, 376–392. [Google Scholar] [CrossRef]
- Flint, E.P. Changes in land use in South and Southeast Asia from 1880 to 1980: A data base prepared as part of a coordinated research program on carbon fluxes in the tropics. Chemosphere 1994, 29, 1015–1062. [Google Scholar] [CrossRef]
- Wright, S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Kanzaki, M.; Hara, M.; Ohkubo, T.; Preechapanya, P.; Choocharoen, C. Secondary forest succession after the cessation of swidden cultivation in the montane forest area in Northern Thailand. For. Ecol. Manag. 2008, 255, 1994–2006. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E. Tropical secondary forests. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Bonner, M.T.L.; Schmidt, S.; Shoo, L.P. A meta-analytical global comparison of aboveground biomass accumulation between tropical secondary forests and monoculture plantations. For. Ecol. Manag. 2013, 291, 73–86. [Google Scholar] [CrossRef]
- International Tropical Timber Organization. ITTO Guidelines for the Restoration, Management and Rehabilitation of Degraded and Secondary Tropical Forests; International Tropical Timber Organization: Yokohama, Japan, 2002; p. 86. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2015, Desk Reference; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; p. 253. Available online: http://www.fao.org/3/a-i4808e.pdf (accessed on 16 July 2016).
- Sommer, R.; Abreu Sa, T.D.; Vielhauer, K.; Araújo, A.C.; Fölster, H.; Vlek, P.L.G. Transpiration and canopy conductance of secondary vegetation in the eastern Amazon. Agric. For. Meteorol. 2002, 112, 103–121. [Google Scholar] [CrossRef]
- Giambelluca, T.W.; Nullet, M.A.; Ziegler, A.D.; Tran, L. Latent and sensible energy flux over deforested land surfaces in the eastern Amazon and northern Thailand. Singap. J. Trop. Geogr. 2000, 21, 107–130. [Google Scholar] [CrossRef]
- Tanaka, N.; Kume, T.; Yoshifuji, N.; Tanaka, K.; Takizawa, H.; Shiraki, K.; Tantasirin, C.; Tangtham, N.; Suzuki, M. A review of evapotranspiration estimates from tropical forest in Thailand and adjacent regions. Agric. For. Meteorol. 2008, 148, 807–819. [Google Scholar] [CrossRef]
- Toda, M.; Nishida, K.; Ohte, N.; Tani, M.; Mushiake, K. Observations of energy fluxes and evapotranspiration over terrestrial complex land covers in the tropical monsoon environment. J. Meteorol. Soc. Jpn. 2002, 80, 465–484. [Google Scholar] [CrossRef]
- Phianchroen, M.; Duangphakdee, O.; Chanchae, P. Instruction of Plant in Dry Dipterocarp Forest at King Mongkut’s University of Technology Thonburi at Ratchaburi Campus; King Mongkut’s University of Technology Thonburi press: Bangkok, Thailand, 2008; p. 170. (In Thai) [Google Scholar]
- Arya, S.P. Introduction to Micrometeorology, 2nd ed.; Academic Press: Cambridge, MA, USA, 1988; p. 307. [Google Scholar]
- Stull, R.B. An Introduction to Boundary Layer Meteorology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; p. 670. [Google Scholar]
- Hanpattanakit, P.; Leclerc, M.Y.; Mcmillan, A.M.S.; Limtong, P.; Maeght, J.L.; Panuthai, S.; Inubushi, K.; Chidthaisong, A. Multiple timescale variations and controls of soil respiration in a tropical dry dipterocarp forest, western Thailand. Plant Soil 2015, 390, 167–181. [Google Scholar] [CrossRef]
- Kaimal, J.C.; Finnigan, J.J. Atmospheric Boundary Layer Flow: Their Structure and Measurement; Oxford University Press: New York, NY, USA, 1994; p. 289. [Google Scholar]
- Bonan, G.B. Ecological Climatology: Concepts and Applications; Cambridge University Press: Cambridge, UK, 2002; p. 678. [Google Scholar]
- Foken, T. Micrometeorology; Springer: Berlin/Heidelberg, Germany, 2008; p. 308. [Google Scholar]
- Oliphant, A.J.; Grimmond, C.S.B.; Zutter, H.N.; Schmid, H.P.; Su, H.B.; Scott, S.L.; Offer, B.; Randolph, J.C.; Ehman, J. Heat storage and energy balance fluxes for a temperate deciduous forest. Agric. For. Meteorol. 2004, 126, 185–201. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Caselles, V.; Rubio, E.M. Analysis of the energy balance closure over a FLUXNET boreal forest in Finland. Hydrol. Earth Syst. Sci. 2010, 14, 1487–1497. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Vickers, D.; Thomas, C.; Law, B.E. Random and systematic CO2 flux sampling errors for tower measurements over forests in the convective boundary layer. Agric. For. Meteorol. 2009, 149, 73–83. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapor transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Moncrieff, J.; Clement, R.; Finnigan, J.; Meyers, T. Averaging, detrending, and filtering of eddy covariance time series. In Handbook of Micrometeorology: A Guide for Surface Flux Measurements; Lee, X., Massman, W.J., Law, B.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 7–31. [Google Scholar]
- Wolf, S.; Eugster, W.; Potvin, C.; Buchmann, N. Strong seasonal variations in net ecosystem CO2 exchange of a tropical pasture and afforestation in Panama. Agric. For. Meteorol. 2011, 151, 1139–1151. [Google Scholar] [CrossRef]
- Barnard, P.L.; Allan, J.; Hansen, J.E.; Kaminsky, G.M.; Ruggiero, P.; Doria, A. The impact of the 2009–10 El Niño Modoki on U.S. West Coast beaches. Geophys. Res. Lett. 2011, 38, 13604. [Google Scholar] [CrossRef]
- Rosenberg, N.J.; Blad, B.L.; Verma, S.B. Microclimate: The Biological Environment, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1983; p. 495. [Google Scholar]
- Henry, J.G.; Heinke, G.W. Environmental Science and Engineering, 2nd ed.; Prentice-Hall International, Inc.: London, UK, 1996; p. 778. [Google Scholar]
- Hirai, K.; Tanaka, H.; Suksawang., S. Albedo characteristics of mixed deciduous forest and teak plantation in Thailand. Proceeding of the International Conference on Tropical Forest and Climate Change: Status, Issues and Challenges (TFCC’98), Makati, Philippines, 19–22 October 1998. [Google Scholar]
- Stoy, P.C.; Mauder, M.; Foken, T.; Marcolla, B.; Boegh, E.; Ibrom, A.; Arain, M.A.; Arneth, A.; Aurela, M.; Bernhofer, C.; et al. A data-driven analysis of energy balance closure across FLUXNET research site: The role of landscape scale heterogeneity. Agric. For. Meteorol. 2013, 152, 137–152. [Google Scholar] [CrossRef]
- Wilson, K.; Goldstein, A.; Falge, E.; Aubinet, M.; Baldocchi, D.; Berbigier, P.; Bernhofer, C.; Ceulemans, R.; Dolman, H.; Field, C.; et al. Energy balance closure at FLUXNET site. Agric. For. Meteorol. 2002, 133, 233–243. [Google Scholar] [CrossRef]
- Foken, T.; Wimmer, F.; Mauder, M.; Thomas, C.; Liebethal, C. Some aspects of the energy balance closure problem. Atmos. Chem. Phys. 2006, 6, 4395–4402. [Google Scholar] [CrossRef]
- Cava, A.D.; Contini, D.; Donateo, A.; Martano, P. Analysis of short-term closure of the surface energy balance above short vegetation. Agric. For. Meteorol. 2008, 148, 82–93. [Google Scholar] [CrossRef]
- Lindroth, A.; Molder, M.; Lagergren, F. Heat storage in forest biomass improves energy balance closure. Biogeosciences 2010, 7, 301–313. [Google Scholar] [CrossRef]
- Da Rocha, H.R.; Goulden, M.L.; Miller, S.D.; Menton, M.C.; Pinto, L.; Freitas, H.C.; Figueira, A.M.S. Seasonality of water and heat fluxes over a tropical forest in eastern Amazonia. Ecol. Appl. 2004, 14, 22–32. [Google Scholar] [CrossRef]
- Kim, W.; Komori, D.; Cho, J.; Kanae, S.; Oki, T. Long-term analysis of evapotranspiration over a diverse land use area in northern Thailand. Hydrol. Res. Lett. 2014, 8, 45–50. [Google Scholar] [CrossRef]
- Tanaka, K.; Kosugi, Y.; Nakamura, A. Impact of leaf physiological characteristics on seasonal variation in CO2, latent and sensible heat exchanges over a tree plantation. Agric. For. Meteorol. 2002, 114, 103–122. [Google Scholar] [CrossRef]
| Seasonality | Monthly | Rn | H | LE | Gsf | Albedo | Rn | H | LE | Gsf |
|---|---|---|---|---|---|---|---|---|---|---|
| (W m−2) | (%) | |||||||||
| Wet season 2009 | May | * | * | * | * | * | * | * | * | * |
| Jun | 122.71 | 13.52 | 100.49 | −5.30 | 0.17 | 100 | 11 | 82 | −4 | |
| Jul | 110.66 | 4.01 | 96.55 | −4.48 | 0.17 | 100 | 4 | 87 | −4 | |
| Aug | 132.47 | 30.72 | 83.42 | −3.75 | 0.17 | 100 | 23 | 63 | −3 | |
| Sep | 142.77 | 15.80 | 99.82 | −2.33 | 0.16 | 100 | 11 | 70 | −2 | |
| Oct | 140.12 | 19.15 | 94.76 | −3.53 | 0.15 | 100 | 14 | 68 | −3 | |
| average | 129.75 | 16.64 | 95.01 | −3.88 | 0.16 | 100 | 13 | 74 | −3 | |
| SE | 13.21 | 9.67 | 6.89 | 1.11 | 0.01 | |||||
| Dry season 2009/2010 | Nov | 131.62 | 19.67 | 93.55 | −6.27 | 0.15 | 100 | 15 | 71 | −5 |
| Dec | 106.50 | 41.12 | 48.11 | 2.01 | 0.16 | 100 | 39 | 45 | 2 | |
| Jan | 96.33 | 67.69 | 14.50 | 3.66 | 0.17 | 100 | 70 | 15 | 4 | |
| Feb | 120.42 | 94.56 | 7.04 | 11.32 | 0.17 | 100 | 79 | 6 | 9 | |
| Mar | 118.35 | 96.41 | 6.05 | 10.91 | 0.18 | 100 | 81 | 5 | 9 | |
| Apr | 144.20 | 106.97 | 15.47 | 8.15 | 0.17 | 100 | 74 | 11 | 6 | |
| average | 119.57 | 71.07 | 30.79 | 4.96 | 0.17 | 100 | 60 | 25 | 4 | |
| SE | 17.12 | 34.74 | 34.39 | 6.67 | 0.01 | |||||
| Wet season 2010 | May | 142.73 | 74.98 | 46.23 | −0.18 | 0.16 | 100 | 53 | 32 | 0 |
| Jun | 150.98 | 31.04 | 102.35 | −2.37 | 0.17 | 100 | 21 | 68 | −2 | |
| Jul | 143.83 | 15.30 | 108.01 | −3.82 | 0.17 | 100 | 11 | 75 | −3 | |
| Aug | 131.65 | 9.88 | 104.97 | −4.86 | 0.17 | 100 | 8 | 80 | −4 | |
| Sep | 156.80 | 17.64 | 102.58 | −2.16 | 0.16 | 100 | 11 | 65 | −1 | |
| Oct | 121.08 | 11.10 | 92.48 | −9.18 | 0.15 | 100 | 9 | 76 | −8 | |
| average | 141.18 | 26.66 | 92.77 | −3.76 | 0.16 | 100 | 19 | 66 | −3 | |
| SE | 13.00 | 24.85 | 23.39 | 3.09 | 0.01 | |||||
| Dry season 2010/2011 | Nov | 115.86 | 6.29 | 87.77 | −7.55 | 0.15 | 100 | 5 | 76 | −7 |
| Dec | 113.10 | 15.74 | 81.93 | −6.48 | 0.15 | 100 | 14 | 72 | −6 | |
| Jan | 114.45 | 50.36 | 77.76 | −2.32 | 0.16 | 100 | 44 | 68 | −2 | |
| Feb | 119.32 | 78.96 | 27.02 | 9.13 | 0.16 | 100 | 66 | 23 | 8 | |
| Mar | 93.91 | 75.62 | 22.66 | −2.16 | 0.16 | 100 | 81 | 24 | −2 | |
| Apr | 150.65 | 41.91 | 84.35 | 4.24 | 0.15 | 100 | 28 | 56 | 3 | |
| average | 117.88 | 44.81 | 63.58 | −0.86 | 0.15 | 100 | 40 | 53 | −1 | |
| SE | 18.38 | 29.94 | 30.22 | 6.42 | 0.01 | |||||
| Wet season 2011 | May | 163.37 | 20.41 | 114.58 | −1.26 | 0.15 | 100 | 12 | 70 | −1 |
| Jun | 125.02 | 3.24 | 101.79 | −2.63 | 0.16 | 100 | 3 | 81 | −2 | |
| Jul | 128.42 | 12.33 | 113.48 | −3.01 | 0.16 | 100 | 10 | 88 | −2 | |
| Aug | 132.23 | 23.78 | 98.69 | −0.86 | 0.16 | 100 | 18 | 75 | −1 | |
| Sep | 121.43 | 12.81 | 106.40 | −2.04 | 0.15 | 100 | 11 | 88 | −2 | |
| Oct | 112.20 | 18.71 | 84.85 | −5.12 | 0.15 | 100 | 17 | 76 | −5 | |
| average | 130.45 | 15.21 | 103.30 | −2.49 | 0.16 | 100 | 12 | 80 | −2 | |
| SE | 17.52 | 7.35 | 10.99 | 1.52 | 0.01 | |||||
| Total dry | 118.73 | 57.94 | 47.18 | 2.05 | 0.16 | 100 | 50 | 39 | 2 | |
| Total wet | 144.76 | 21.24 | 100.79 | −2.97 | 0.16 | 100 | 14 | 73 | −3 | |
| Total all season | 131.75 | 39.59 | 73.99 | −0.46 | 0.16 | 100 | 32 | 56 | −1 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanwangsri, M.; Hanpattanakit, P.; Chidthaisong, A. Variations of Energy Fluxes and Ecosystem Evapotranspiration in a Young Secondary Dry Dipterocarp Forest in Western Thailand. Atmosphere 2017, 8, 152. https://doi.org/10.3390/atmos8080152
Sanwangsri M, Hanpattanakit P, Chidthaisong A. Variations of Energy Fluxes and Ecosystem Evapotranspiration in a Young Secondary Dry Dipterocarp Forest in Western Thailand. Atmosphere. 2017; 8(8):152. https://doi.org/10.3390/atmos8080152
Chicago/Turabian StyleSanwangsri, Montri, Phongthep Hanpattanakit, and Amnat Chidthaisong. 2017. "Variations of Energy Fluxes and Ecosystem Evapotranspiration in a Young Secondary Dry Dipterocarp Forest in Western Thailand" Atmosphere 8, no. 8: 152. https://doi.org/10.3390/atmos8080152




