Assessment of Bacterial Aerosol in a Preschool, Primary School and High School in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling and Analysis Methods
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Total Concentration of Bacterial Aerosol
3.2. Indoor/Outdoor Ratio
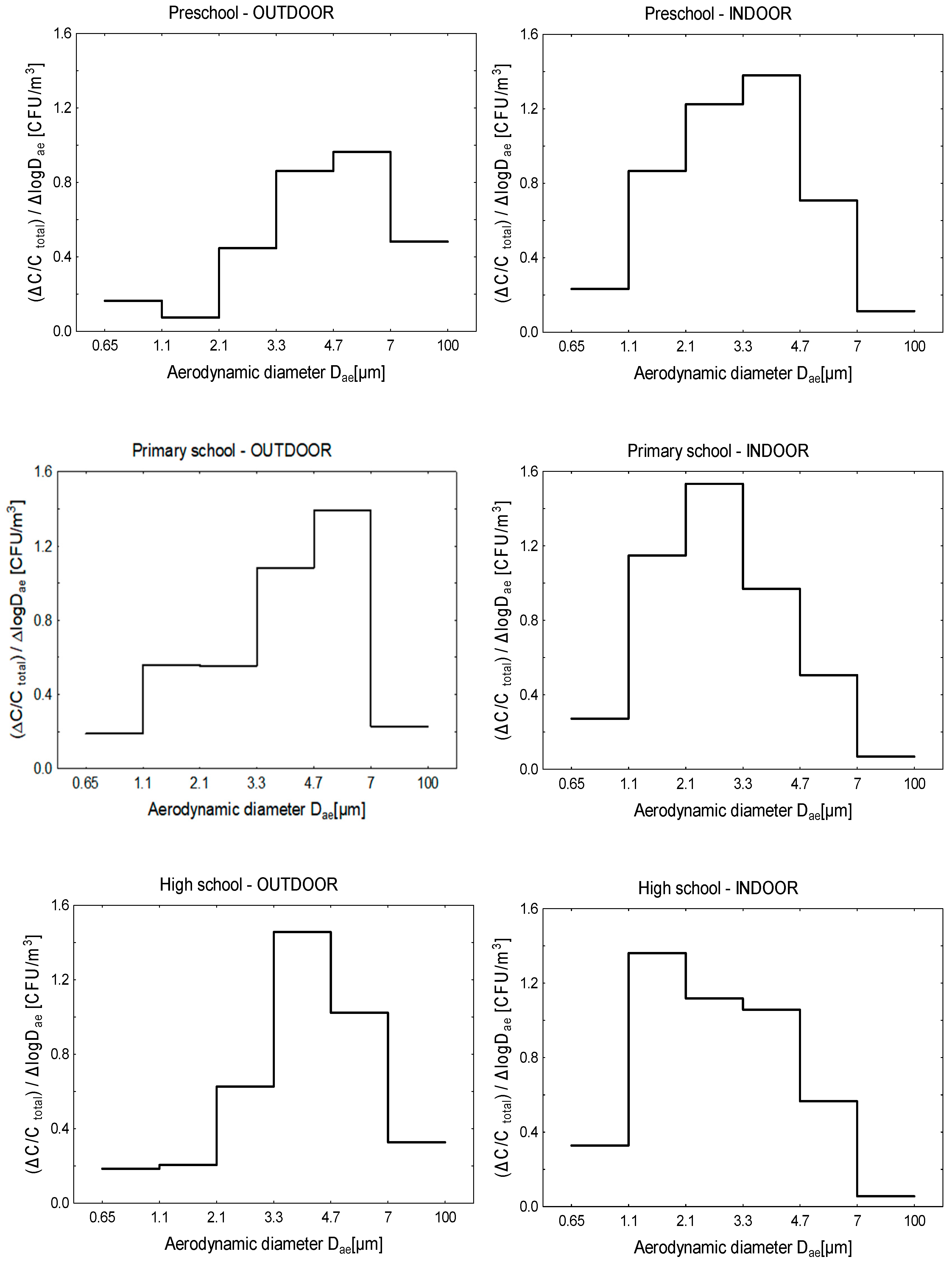
3.3. Size Distribution of Bacterial Aerosol
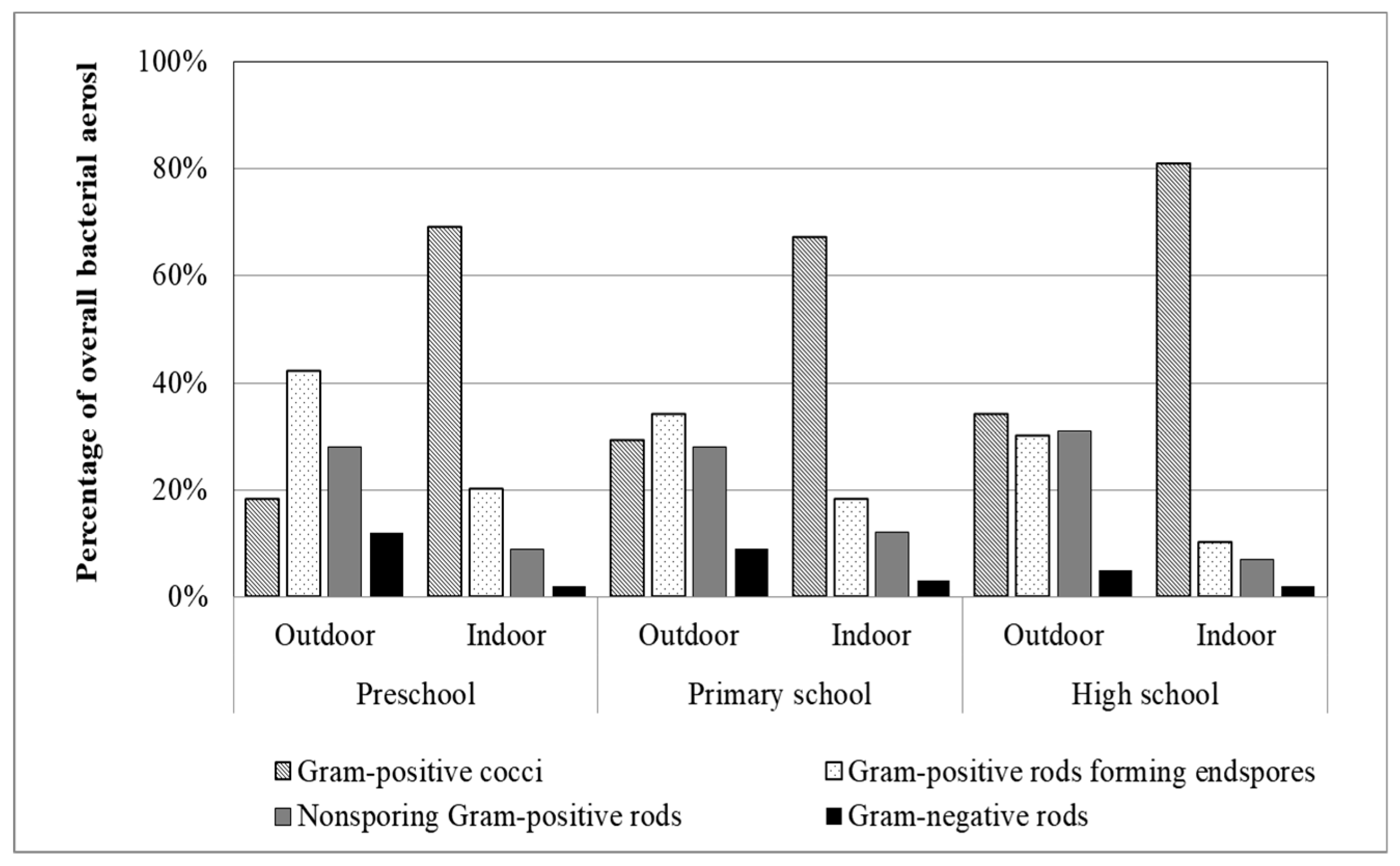
3.4. Identification of Bacterial Aerosol
3.5. Exposure Dose (ED)
- ED is the exposure dose for the indoor environment, CFU/kg
- C is the bacterial aerosol concentration, CFU/m³
- IEF is the indoor exposure fraction: hours spent over a day in each educational building, with diverse activity patterns (seven hours)
- IR is the inhalation rate coefficient characteristic for selected activity levels, m³/h [70]
- BW is the mean body weight, kg.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reynolds, S.J.; Black, D.W.; Borin, S.S.; Breuer, G.; Burmeister, L.F.; Fuortes, L.J.; Smith, T.F.; Stein, M.A.; Subramanian, P.; Thorne, P.S.; et al. Indoor environmental quality in six commercial office buildings in the midwest United States. Appl. Occup. Environ. Hyg. 2001, 16, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, M.R.; Dimitroulopoulou, C. Personal exposure of children to air pollution. Atmos. Environ. 2009, 43, 128–141. [Google Scholar] [CrossRef]
- Wichmann, J.; Lind, T.; Nilsson, M.A.M.; Bellander, T. PM2.5, soot and NO2 indoor-outdoor relationships at homes, pre-schools and schools in Stockholm, Sweden. Atmos. Environ. 2010, 44, 4536–4544. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, C.H.; Hsu, K.L. Size and seasonal distributions of airborne bioaerosols in commuting trains. Atmos. Environ. 2010, 44, 4331–4338. [Google Scholar] [CrossRef]
- Nasir, Z.A.; Colbeck, I.; Sultan, S.; Ahmed, S. Bioaerosols in residential micro-environments in low income countries: A case study from Pakistan. Environ. Pollut. 2012, 168, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canha, N.; Almeida, S.M.; do Carmo Freitas, C.; Wolterbeek, H.T. Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch. Environ. Prot. 2015, 41, 11–22. [Google Scholar] [CrossRef]
- Selgrade, M.K.; Plopper, C.G.; Gilmour, M.I.; Conolly, R.B.; Foos, B.S.P. Assessing the health effects and risks associated with children’s inhalation exposures—Asthma and allergy. J. Toxicol. Environ. Health Part A Curr. Issues 2008, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.T.B.S.; Nunes, R.A.O.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Ferraz, C.; Vaz, L.G.; Sousa, S.I.V. Asthma prevalence in Portuguese preschool children: The latest scientific evidence. Rev. Port. Pneumol. 2016, 22, 293–295. [Google Scholar] [CrossRef] [PubMed]
- CSO Central Statistical Office Education in 2015/2016 School Year. 2016. Available online: http://www.stat.gov.pl (accessed on 1 July 2017).
- Aydogdu, H.; Asan, A.; Tatman Otkun, M. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environ. Monit. Assess. 2010, 164, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, K.M.; Gerba, C.P.; Maxwell, S.L.; Kelley, S.T. Office space bacterial abundance and diversity in three metropolitan areas. PLoS ONE 2012, 7, e37849. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, P.; Sudharsanam, S.; Steinberg, R. Bio-aerosols in indoor environment: Composition, health effects and analysis. Indian J. Med. Microbiol. 2008, 26, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, Y.; Ma, X.; Wu, F.; Ma, X.; An, L.; Feng, H. Diversity and seasonal dynamics of airborne bacteria in the Mogao Grottoes, Dunhuang, China. Aerobiologia (Bologna) 2012, 28, 27–38. [Google Scholar] [CrossRef]
- Faridi, S.; Hassanvand, M.S.; Naddafi, K.; Yunesian, M.; Nabizadeh, R.; Sowlat, M.H.; Kashani, H.; Gholampour, A.; Niazi, S.; Zare, A.; et al. Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ. Sci. Pollut. Res. 2015, 22, 8190–8200. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Cellini, L. Indoor air quality in university environments. Environ. Monit. Assess. 2010, 170, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Bhangar, S.; Pasut, W.; Arens, E.A.; Taylor, J.W.; Lindow, S.E.; Nazaroff, W.W.; Bruns, T.D. Chamber bioaerosol study: Outdoor air and human occupants as sources of indoor airborne microbes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Nazaroff, W.W. Indoor bioaerosol dynamics. Indoor Air 2016, 26, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Mentese, S.; Arisoy, M.; Rad, A.Y.; Güllü, G. Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey. Clean Soil. Air. Water 2009, 37, 487–493. [Google Scholar] [CrossRef]
- Mentese, S.; Rad, A.Y.; Arisoy, M.; Gullu, G. Seasonal and spatial variations of bioaerosols in indoor urban environments, Ankara, Turkey. Indoor Built Environ. 2012, 21, 797–810. [Google Scholar] [CrossRef]
- Goyer, N.; Lavoie, J.; Lazure, L.; Marchand, G. Bioaerosols in the Workplace: Evaluations, Control and Prevention Guide. Available online: http://www.irsst.qc.ca/en/publications-tools/publication/i/818/n/bioaerosols-in-the-workplace-evaluation-control-and-prevention-guide-t-24 (accessed on 1 July 2017).
- Salleh, N.M.; Kamaruzzaman, S.N.; Sulaiman, R.; Mahbob, N.S. Indoor Air Quality at School: Ventilation Rates and It Impacts Towards Children—A review. In Proceedings of the 2nd International Conference Environmental Science and Technology, Singapore, 26–28 February 2011. [Google Scholar]
- Dumała, S.M.; Dudzińska, M.R. Microbiological indoor air quality in Polish schools. Annu. Set Environ. Prot. 2013, 15, 231–244. [Google Scholar]
- Almeida, S.M.; Canha, N.; Silva, A.; Do Carmo Freitas, M.; Pegas, P.; Alves, C.; Evtyugina, M.; Pio, C.A. Children exposure to atmospheric particles in indoor of Lisbon primary schools. Atmos. Environ. 2011, 45, 7594–7599. [Google Scholar] [CrossRef]
- Watson, A.Y.; Bates, R.R.; Kennedy, D. Assessment of Human Exposure to Air Pollution: Methods, Measurements, and Models; 1988; ISBN 0309568269. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218147/ (accessed on 1 July 2017).
- Zanobetti, A.; Schwartz, J. The effect of particulate air pollution on emergency admissions for myocardial infarction: A multicity case-crossover analysis. Environ. Health Perspect. 2005, 113, 978–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcy, N.; Canales, M.; Spratt, D.A. Healthy schools: Standardisation of culturing methods for seeking airborne pathogens in bioaerosols emitted from human sources. Aerobiologia (Bologna) 2012, 28, 413–422. [Google Scholar] [CrossRef]
- Nasir, Z.A.; Colbeck, I. Assessment of bacterial and fungal aerosol in different residential settings. Water Air. Soil Pollut. 2010, 211, 367–377. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Górny, R.L. Bacterial and fungal aerosols in air-conditioned office buildings in Warsaw, Poland—The winter season. Int. J. Occup. Saf. Ergon. 2010, 16, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Daisey, J.M.; Angell, W.J.; Apte, M.G.; Air, I.; Munksgaard, B.; Issn, I.A.I.R. Indoor air quality, ventilation and health symptoms in schools: An analysis of existing information. Indoor Air 2003, 13, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Denda, M.; Chang, S.; Elias, P.M.; Feingold, K.R. Abrupt decreases in environmental humidity induce abnormalities in permeability barrier homeostasis. J. Investig. Dermatol. 2002, 119, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.B.; Padmalal, D.; Kumaran, K.P.N. Cyanobacteria and testate amoeba as potential proxies for Holocene hydrological changes and climate variability: Evidence from tropical coastal lowlands of SW India. Quat. Int. 2017, 443, 99–114. [Google Scholar] [CrossRef]
- Mainka, A.; Zajusz-Zubek, E.; Kozielska, B.; Bragoszewska, E. Investigation of Air Pollutants in Rural Nursery School—A Case Study. In E3S Web of Conferences; 2018; Volume 28, pp. 1–8. Available online: https://www.researchgate.net/profile/Ewa_Bragoszewska/publication/322360151_Investigation_of_air_pollutants_in_rural_nursery_school_-_a_case_study/links/5a56791045851547b1bf1d81/Investigation-of-air-pollutants-in-rural-nursery-school-a-case-study.pdf (accessed on 10 January 2018).
- Mainka, A.; Zajusz-Zubek, E. Indoor air quality in urban and rural preschools in upper Silesia, Poland: Particulate Matter and Carbon Dioxide. Int. J. Environ. Res. Public Health 2015, 12, 7697–7711. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, R.; Li, W.; Xie, Z.; Song, Y. Concentrations and size distributions of viable bioaerosols under various weather conditions in a typical semi-arid city of Northwest China. J. Aerosol Sci. 2017, 106, 83–92. [Google Scholar] [CrossRef]
- Brągoszewska, E. Bacterial Aerosol Occurring in the Atmospheric Air in Gliwice and Its Share of the Total Human Exposure to the Bacteria Absorbed by Inhalation. Ph.D. Thesis, Silesian University of Technology, Gliwice, Poland, 2014. [Google Scholar]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J. Bacterial and fungal aerosols in rural nursery schools in Southern Poland. Atmosphere (Basel) 2016, 7, 142. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J. Concentration and size distribution of culturable bacteria in ambient air during spring and winter in Gliwice: A typical urban area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef]
- Nevalainen, A.; Pastuszka, J.; Liebhaber, F.; Willeke, K. Performance of bioaerosol samplers: Collection characteristics and sampler design considerations. Atmos. Environ. Part A Gen. Top. 1992, 26, 531–540. [Google Scholar] [CrossRef]
- PN-EN 12322 In Vitro Diagnostic Medical Devices. Culture Media for Microbiology. Performance Criteria for Culture Media. 2005. Available online: https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/iv-diagnostic-medical-devices_en (accessed on 1 June 2017).
- ISO 11133 Microbiology of Food, Animal Feed and Water—Preparation, Production, Storage and Performance Testing of Culture Media. 2014. Available online: https://www.iso.org/standard/53610.html (accessed on 1 June 2017).
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.S. Bacterial aerosols in an urban nursery school in Gliwice, Poland: A case study. Aerobiologia (Bologna) 2016, 32, 469–480. [Google Scholar] [CrossRef]
- Pegas, P.N.; Evtyugina, M.G.; Alves, C.A.; Nunes, T.; Cerqueira, M.; Franchi, M.; Pio, C.; Almeida, S.M.; Freitas, M.C. Outdoor/Indoor air quality in primary schools in Lisbon: A Preliminary Study. Quim. Nova 2010, 33, 1145–1149. [Google Scholar] [CrossRef]
- Yang, W.; Sohn, J.; Kim, J.; Son, B.; Park, J. Indoor air quality investigation according to age of the school buildings in Korea. J. Environ. Manag. 2009, 90, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hussin, N.H.M.; Sann, L.M.; Shamsudin, M.N.; Hashim, Z. Characterization of bacteria and fungi bioaerosol in the indoor air of selected primary schools in Malaysia. Indoor Built Environ. 2011, 20, 607–617. [Google Scholar] [CrossRef]
- Al Mijalli, S.H. Bacterial contamination of indoor air in schools of Riyadh, Saudi Arabia. Air Water Borne Dis. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Sheik, G.B.; Ismail, A.; Abd, A.; Rheam, A.; Saad, Z.; Shehri, A.; Bin, O.; Al, M. Assessment of Bacteria and Fungi in air from College of Applied Medical Sciences (Male) at AD-Dawadmi, Saudi Arabia. Int. J. Sci. Technol. Res. 2015, 4, 48–53. [Google Scholar]
- Soto, T.; Garcia Murcia, R.M.; Franco, A.; Vicente-Soler, J.; Cansado, J.; Gacto, M. Indoor airborne microbial load in a Spanish university (University of Murcia, Spain). Anales de Biología 2009, 31, 109–115. [Google Scholar]
- Dutkiewicz, J.; Górny, R.L. Biologic factors hazardous to health: Classification and criteria of exposure assessment. Med. Pr. 2002, 53, 29–39. [Google Scholar] [PubMed]
- Jo, W.-K.; Seo, Y.-J. Indoor and outdoor bioaerosol levels at recreation facilities, elementary schools, and homes. Chemosphere 2005, 61, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kim, C.N. Airborne microbiological characteristics in public buildings of Korea. Build. Environ. 2007, 42, 2188–2196. [Google Scholar] [CrossRef]
- Pastuszka, J.S.; Kyaw Tha Paw, U.; Lis, D.O.; Wlazło, A.; Ulfig, K. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atmos. Environ. 2000, 34, 3833–3842. [Google Scholar] [CrossRef]
- Stryjakowska-Sekulska, M.; Piotraszewska-Pajak, A.; Szyszka, A.; Nowicki, M.; Filipiak, M. Microbiological quality of indoor air in university rooms. Pol. J. Environ. Stud. 2007, 16, 623–632. [Google Scholar] [CrossRef]
- Burkowska, A.; Kalwasińska, A.; Walczak, M. Airborne mesophilic bacteria at the ciechocinek health resort. Pol. J. Environ. Stud. 2012, 21, 307–312. [Google Scholar]
- Bartlett, K.H.; Kennedy, S.M.; Brauer, M.; van Netten, C.; Dill, B. Evaluation and determinants of airborne bacterial concentrations in school classrooms. J. Occup. Environ. Hyg. 2004, 1, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chai, Y.; Lin, H.; So, W.W.M.; Ho, K.W.K.; Tsui, A.K.Y.; Wong, R.K.S. Distribution of bacteria in inhalable particles and its implications for health risks in kindergarten children in Hong Kong. Atmos. Environ. 2016, 128, 268–275. [Google Scholar] [CrossRef]
- Bavykin, S.G.; Mikhailovich, V.M.; Zakharyev, V.M.; Lysov, Y.P.; Kelly, J.J.; Alferov, O.S.; Gavin, I.M.; Kukhtin, A.V.; Jackman, J.; Stahl, D.A.; et al. Discrimination of Bacillus anthracis and closely related microorganisms by analysis of 16S and 23S rRNA with oligonucleotide microarray. Chem. Biol. Interact. 2008, 171, 212–235. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Brigmon, R.L.; Knox, A.; Seaman, J.; Smith, G. Effects of microbial and phosphate amendments on the bioavailability of lead (Pb) in shooting range soil. Bull. Environ. Contam. Toxicol. 2006, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Koneman, E.W.; Allen, S.D.; Janda, W.M.; Schreckenberger, P.C. Color Atlas and Textbook of Diagnostic Microbiology; 1997; ISBN 0781730147. Available online: europepmc.org/articles/pmc1900441/pdf/amjpathol00176-0005.pdf (accessed on 1 June 2017).
- Pakarinen, J.; Hyvärinen, A.; Salkinoja-Salonen, M.; Laitinen, S.; Nevalainen, A.; Mäkelä, M.J.; Haahtela, T.; Von Hertzen, L. Predominance of Gram-positive bacteria in house dust in the low-allergy risk Russian Karelia. Environ. Microbiol. 2008, 10, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Han, J.H.; Joung, Y.; Cho, S.H.; Kim, S.A.; Kim, S.B. Bacterial diversity in the indoor air of pharmaceutical environment. J. Appl. Microbiol. 2014, 116, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus Microbiome Diversity Depletion and Corynebacterium tuberculostearicum Enrichment Mediates Rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124. [Google Scholar] [CrossRef] [PubMed]
- Ege, M.J.; Mayer, M.; Schwaiger, K.; Mattes, J.; Pershagen, G.; Van Hage, M.; Scheynius, A.; Bauer, J.; Von Mutius, E. Environmental bacteria and childhood asthma. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Schram-Bijkerk, D.; Doekes, G.; Douwes, J.; Boeve, M.; Riedler, J.; Üblagger, E.; Von Mutius, E.; Benz, M.R.; Pershagen, G.; Van Hage, M.; et al. Bacterial and fungal agents in house dust and wheeze in children: The PARSIFAL study. Clin. Exp. Allergy 2005, 35, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Gehring, U.; Bolte, G.; Borte, M.; Bischof, W.; Fahlbusch, B.; Wichmann, E.E.; Heinrich, J. Exposure to endotoxin decreases the risk of atopic eczema in infancy: A cohort study. J. Allergy Clin. Immunol. 2001, 108, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.S.; Cohn, R.D.; Mav, D.; Arbes, S.J.; Zeldin, D.C. Predictors of endotoxin levels in U.S. housing. Environ. Health Perspect. 2009, 117, 763–771. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Child-Specific Exposure Factors Handbook; EPA, Environmental Protection Agency: Washington, DC, USA, 2002.
- Johnson-Restrepo, B.; Kannan, K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009, 76, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Ott, W.R.; Steinemann, A.C.; Wallace, L.A. Exposure Analysis; CRC Press: London, UK, 2006; ISBN 978-1-56670-663-6. [Google Scholar]
- U.S. EPA. Exposure Factors Handbook; EPA, Environmental Protection Agency: Washington, DC, USA, 2011.
- Górny, R.; Gołofit-Szymczak, M.; Stobnicka, A. Biological agents in kindergartens [in Polish Szkodliwe czynniki biologiczne w przedszkolach]. Bezpieczeństwo Pracy Nauka i Praktyka 2014, 2, 16–20. [Google Scholar]
| Preschool (P) | Primary school (S) | High school (H) | |
|---|---|---|---|
| School localization | away from the city center, near a quiet street (low traffic), | away from the city center, near a quiet street (low traffic) | in the city center, near a busy street (heavy vehicular traffic) |
| Building built in | 1970s | 1930s | 1930s |
| Classroom localization | on the ground floor | on the ground floor | on the ground floor |
| Equipment | writing desk, tables and chairs toys | 12 double desks, writing desk, blackboard | 20 double desks, writing desk, blackboard |
| Ventilation system | natural | natural | natural |
| Volume, m3 | 210 | 130 | 170 |
| Number of children | 25 | 24 | 16 |
| Age of children | 4–6 | 9–11 | 16–18 |
| Flor covered with | PVC and carpet | PVC | tiles |
| Indoor temperature, °C | 24.1 | 22.1 | 23 |
| Indoor relative humidity (RH), % | 26 | 29 | 31 |
| Outdoor temperature, °C | 20.2 | 18.4 | 19.1 |
| Outdoor relative humidity (RH), % | 32.4 | 31.7 | 38.6 |
| Location | Average Concentration, CFU/m−3 | SD | Min | Max | I/O Ratio |
|---|---|---|---|---|---|
| OUT | 551 | 119 | 393 | 725 | - |
| IN (P) | 1408 | 406 | 456 | 1984 | 2.55 |
| OUT | 246 | 185 | 54 | 423 | - |
| IN (S) | 2205 | 1468 | 611 | 4388 | 8.96 |
| OUT | 213 | 147 | 52 | 269 | - |
| IN (H) | 391 | 57 | 336 | 453 | 1.83 |
| Parameter | Preschool (P) | Primary School (S) | High School (H) | |||
|---|---|---|---|---|---|---|
| Short-Term Inhalation Rates by Activity Level/Hours | ||||||
| Activity levels | m3/min | hour | m3/min | hour | m3/min | hour |
| Sedentary/Passive | 0.0045 | 2 | 0.0048 | 2 | 0.0053 | 5 |
| Light Intensity | 0.011 | 2 | 0.011 | 3 | 0.012 | 1 |
| Moderate Intensity | 0.021 | 2 | 0.022 | 2 | 0.026 | 1 |
| High Intensity | 0.037 | 1 | 0.042 | 0 | 0.049 | 0 |
| BW, kg | 19 | 34 | 62 | |||
| ED, CFU/kg | 489 | 337 | 24 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brągoszewska, E.; Mainka, A.; Pastuszka, J.S.; Lizończyk, K.; Desta, Y.G. Assessment of Bacterial Aerosol in a Preschool, Primary School and High School in Poland. Atmosphere 2018, 9, 87. https://doi.org/10.3390/atmos9030087
Brągoszewska E, Mainka A, Pastuszka JS, Lizończyk K, Desta YG. Assessment of Bacterial Aerosol in a Preschool, Primary School and High School in Poland. Atmosphere. 2018; 9(3):87. https://doi.org/10.3390/atmos9030087
Chicago/Turabian StyleBrągoszewska, Ewa, Anna Mainka, Józef S. Pastuszka, Katarzyna Lizończyk, and Yitages Getachew Desta. 2018. "Assessment of Bacterial Aerosol in a Preschool, Primary School and High School in Poland" Atmosphere 9, no. 3: 87. https://doi.org/10.3390/atmos9030087






