Refinement of Modeled Aqueous-Phase Sulfate Production via the Fe- and Mn-Catalyzed Oxidation Pathway
Abstract
:1. Introduction
2. Overview of Modeled SO42− Production
3. Methodology
4. Results and Discussion
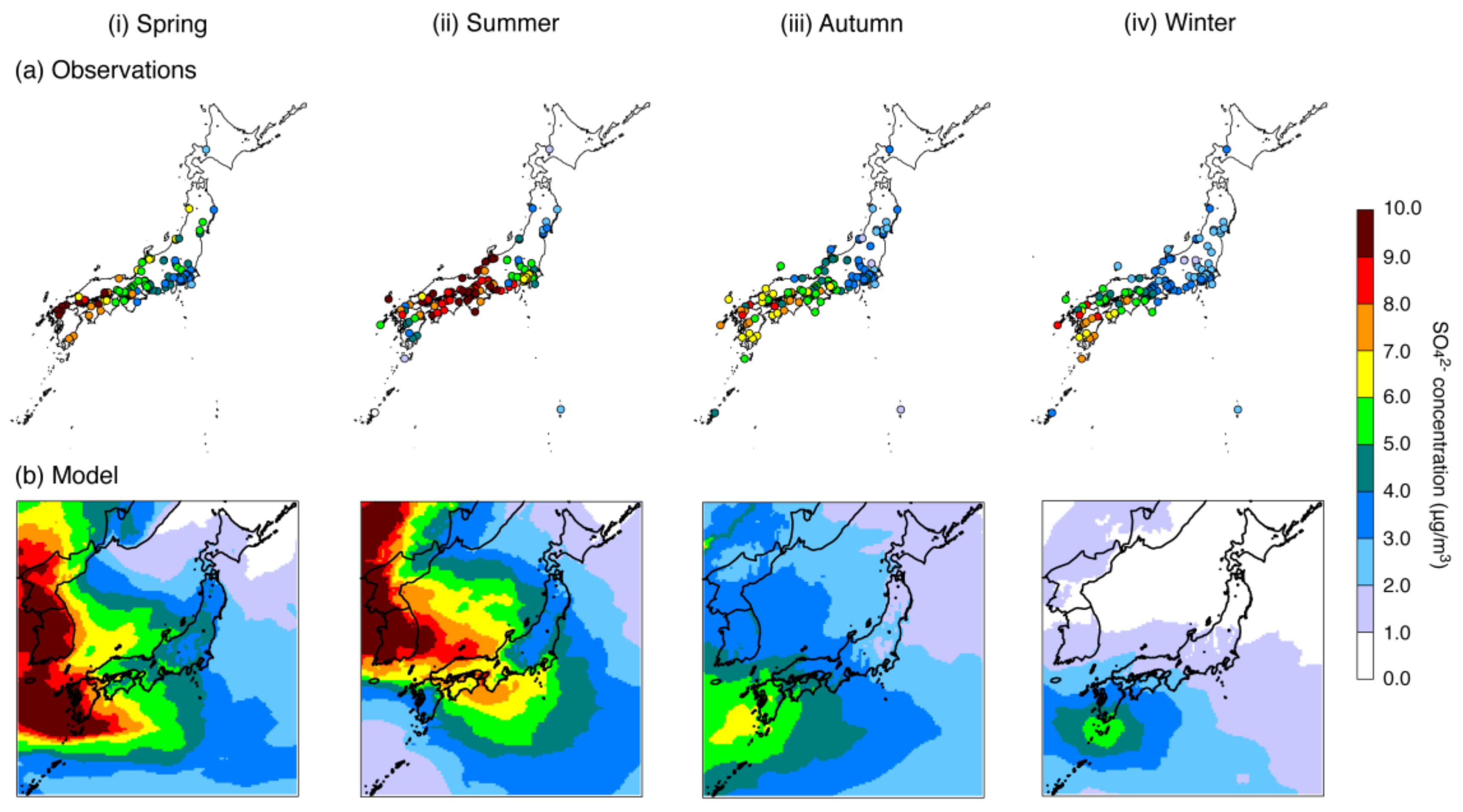
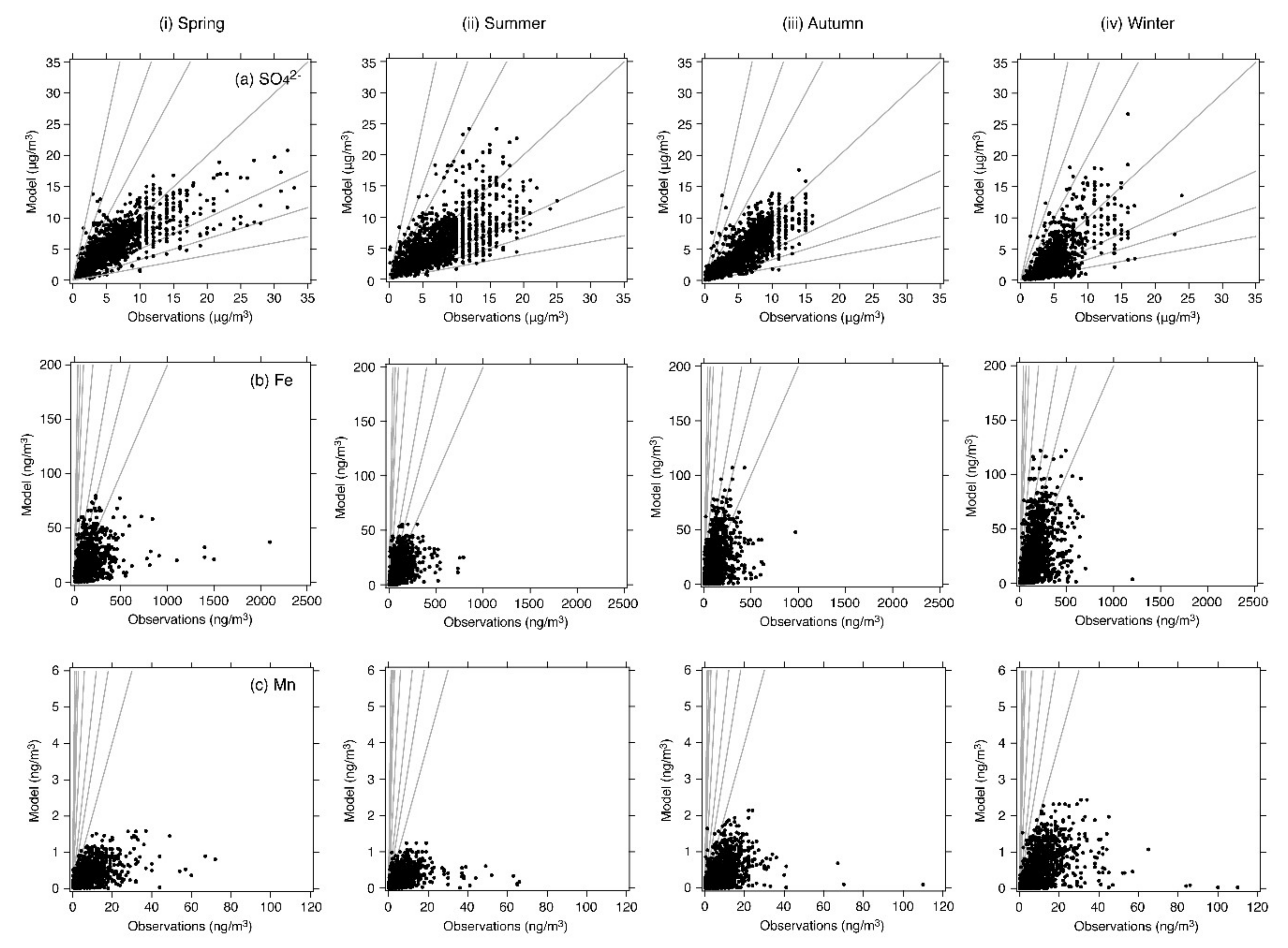
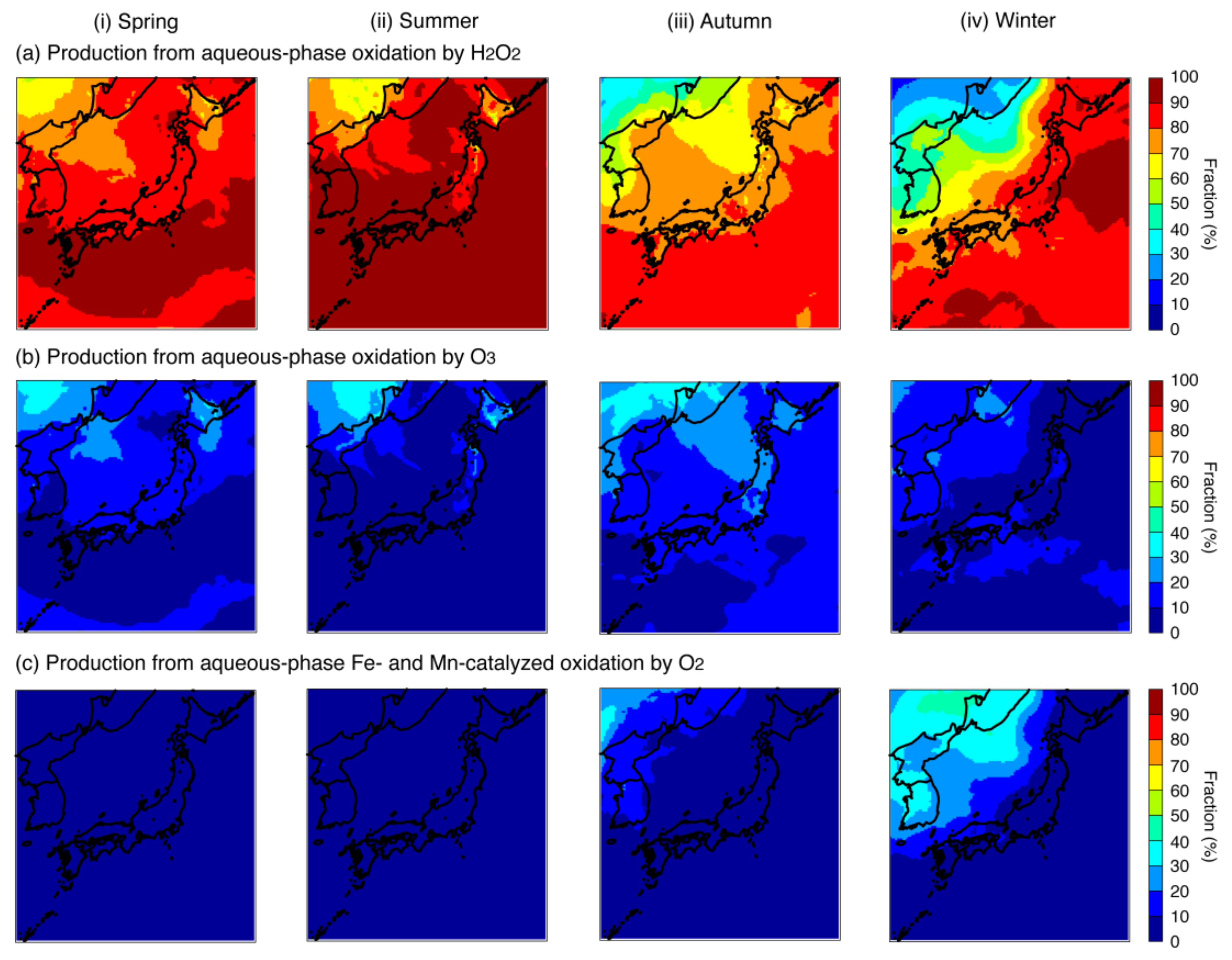
4.1. Base-Case Simulation
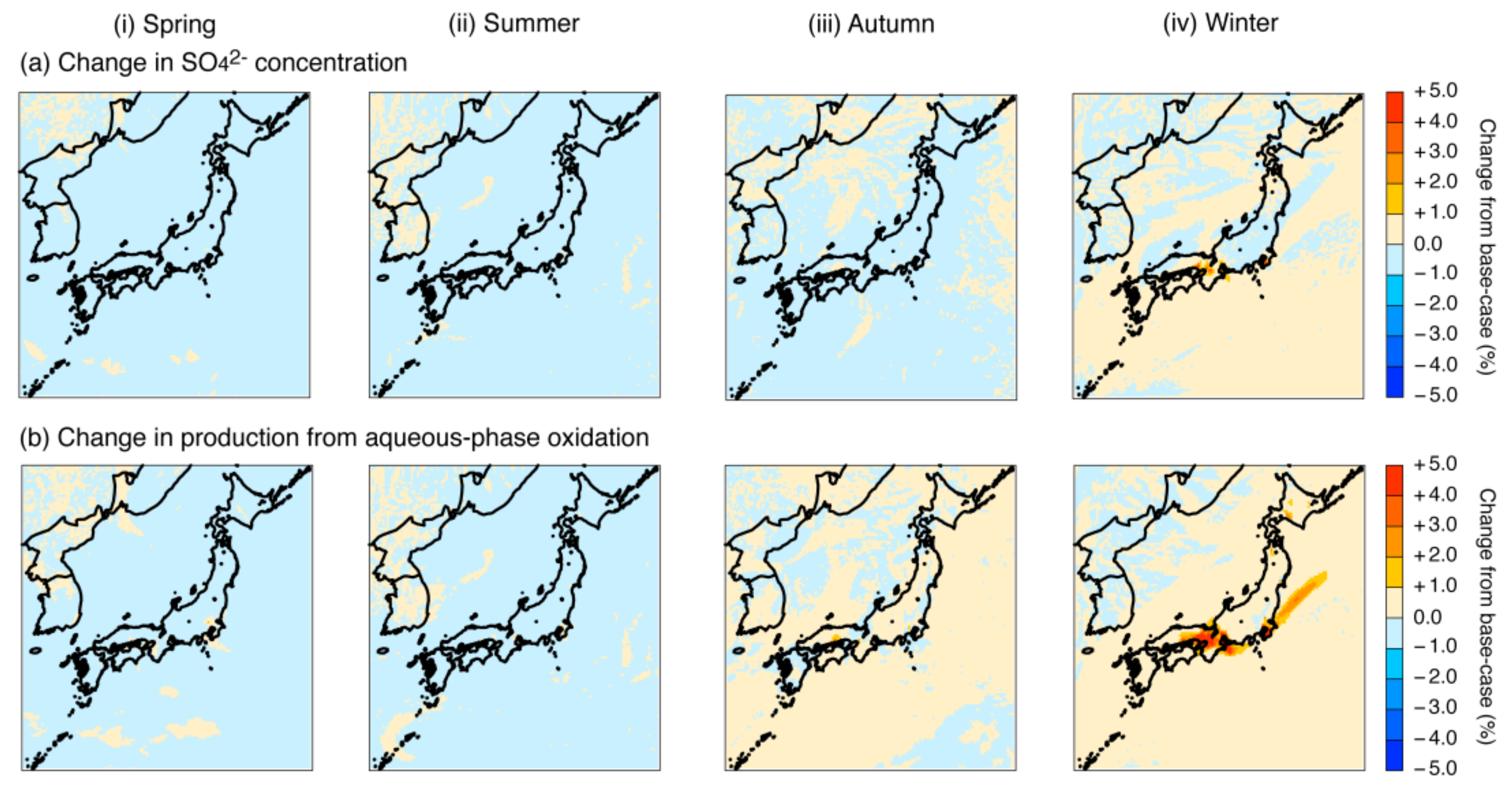
4.2. Sensitivity Simulation of the Fe and Mn Treatment
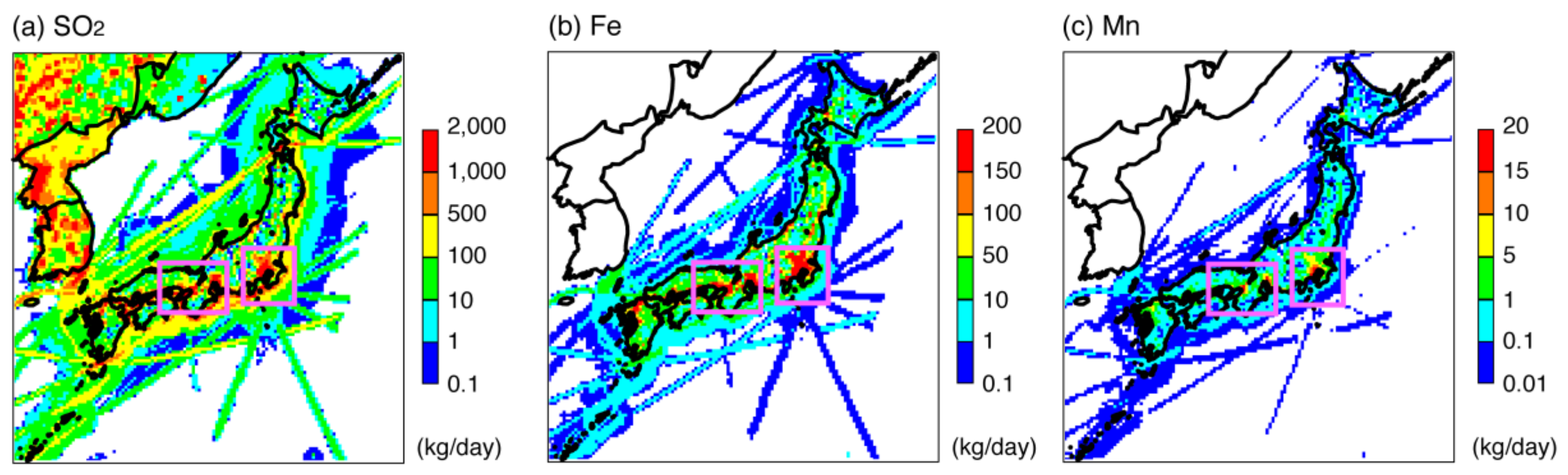
4.2.1. Adjustment of Fe and Mn Concentrations
4.2.2. Increase in the Fe and Mn Solubilities
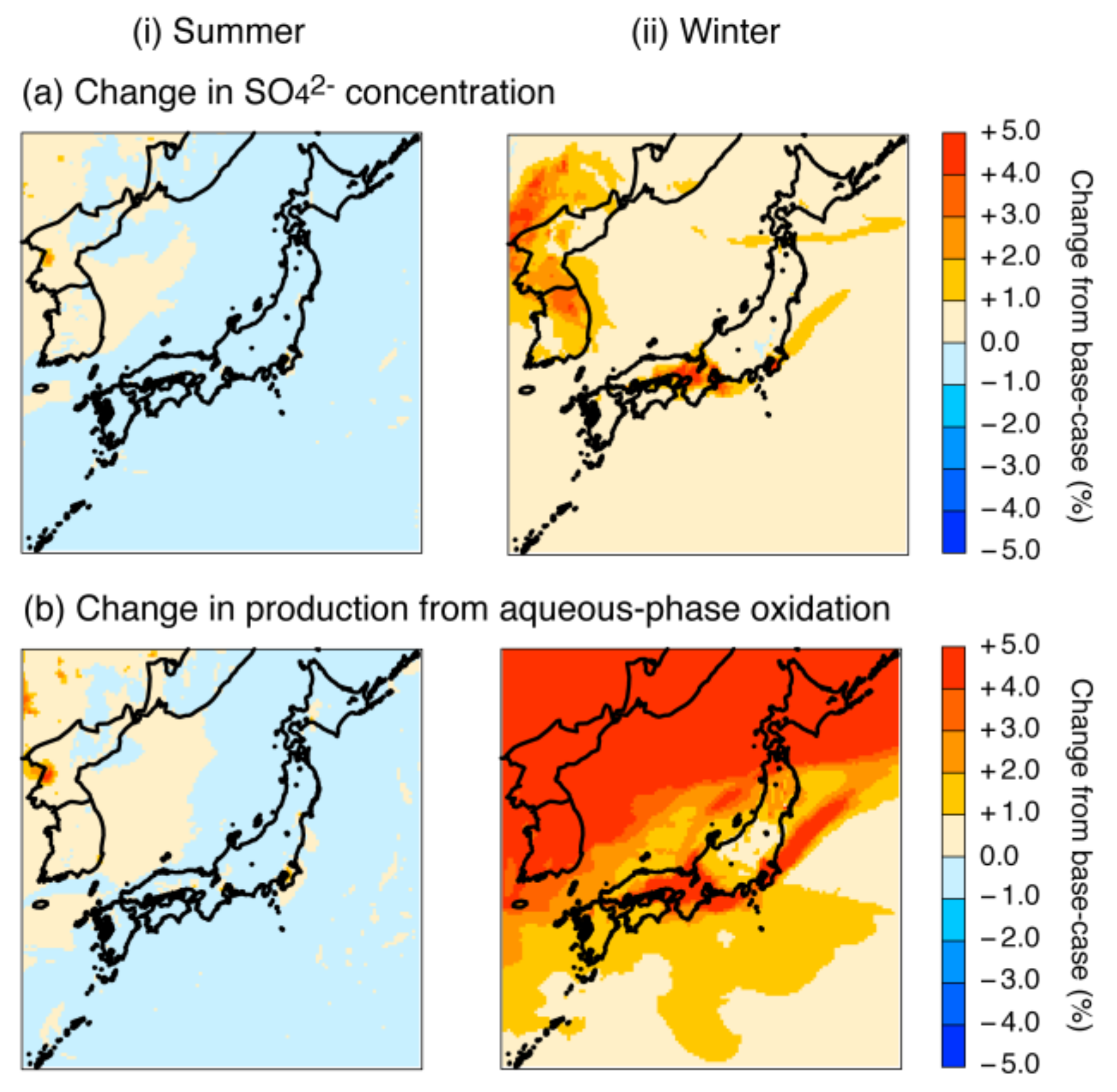
4.2.3. Revision of the Fe and Mn Rate Constant
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chatani, S.; Yamaji, K.; Sakurai, T.; Itahashi, S.; Shimadera, H.; Kitayama, K.; Hayami, H. Overview of model inter-comparison in Japan’s study for reference air quality modeling (J-STREAM). Atmosphere 2018, 9, 19. [Google Scholar] [CrossRef]
- Yamaji, K.; Kobe University, Kobe, Hyogo Prefecture, Japan. Personal communication, 2018.
- Tesche, T.W.; Morris, R.; Tonnesen, G.; McNally, D.; Boylan, J.; Brewer, P. CMAQ/CAMx annual 2002 performance evaluation over the eastern US. Atmos. Environ. 2006, 40, 4906–4919. [Google Scholar] [CrossRef]
- Zhang, Y.; Olsen, K.M.; Wang, K. Fine-scale modeling of agricultural air quality over the Southeastern United States using two air quality models. Part I. Application and Evaluation. Aerosol Air Qual. Res. 2013, 13, 1231–1252. [Google Scholar] [CrossRef]
- CMAQ v5.0 Sulfur Chemistry. Available online: https://www.airqualitymodeling.org/index.php/CMAQv5.0_Sulfur_Chemistry (accessed on 20 January 2018).
- Sander, S.P. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies Evaluation Number 15; JPL Publication: Pasadena, CA, USA, 2006. [Google Scholar]
- Whitten, G.Z.; Heo, G.; Kimura, Y.; McDonald-Buller, E.; Allen, D.T.; Carter, W.P.L.; Yarwood, G. A new condensed toluene mechanism for carbon bond CB05-TU. Atmos. Environ. 2010, 44, 5346–5355. [Google Scholar] [CrossRef]
- Carter, W.P.L. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Jacobson, M. Development and application of a new air pollution modeling system-II. Aerosol module structure and design. Atmos. Environ. 1997, 31, 131–144. [Google Scholar] [CrossRef]
- Martin, R.L.; Good, T.W. Catalyzed oxidation of sulfur dioxide in solution: The iron-manganese synergism. Atmos. Environ. 1991, 25, 2395–2399. [Google Scholar] [CrossRef]
- Alexander, B.; Park, R.J.; Jacob, D.J.; Gong, S. Transition metal-catalyzed oxidation of atmospheric sulfur: Global implications for the sulfur budget. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-NH4+-Na+-SO42−-NO3--Cl−-H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Reff, A.H.; Bhave, P.; Simon, H.; Pace, T.; Pouliot, G.; Mobley, D.; Houyoux, M. Emissions inventory of PM2.5 trace elements across the United States. Environ. Sci. Technol. 2009, 43, 5790–5796. [Google Scholar] [CrossRef]
- Appel, K.W.; Pouliot, G.A.; Simon, H.; Sarwar, G.; Pye, H.O.T.; Napelenok, S.L.; Akhtar, F.; Roselle, S.J. Evaluation of dust and trace metal estimates from the Community Multiscale Air Quality (CMAQ) model version 5.0. Geosci. Model Dev. 2013, 6, 883–899. [Google Scholar] [CrossRef]
- Simon, H.; Beck, L.; Bhave, P.V.; Divita, F.; Hsu, Y.; Luecken, D.; Mobley, J.D.; Pouliot, G.A.; Reff, A.; Sarwar, G.; et al. The development and uses of EPA’s SPECIATE database. Atmos. Pollut. Res. 2010, 1, 196–206. [Google Scholar] [CrossRef]
- Upadhyay, N.; Clements, A.L.; Fraser, M.P.; Sundblom, M.; Solomon, P.; Herckes, P. Size-differentiated chemical composition of re-suspended soil dust from the desert southwest United States. Aerosol Air Qual. Res. 2015, 15, 387–398. [Google Scholar] [CrossRef]
- Crippa, M.; Janssens-Maenhout, G.M.; Dentener, F.; Guizzardi, D.; Sindelarova, K.; Muntean, M.; Van Dingenen, R.; Granier, C. Forty years of improvements in European air quality; regional policy-industry interactions with global impacts. Atmos. Chem. Phys. 2016, 16, 3825–3841. [Google Scholar] [CrossRef] [Green Version]
- Janssens-Maenhout, G.; Crippa, M.; Guizzardi, F.; Dentener, F.; Muntean, M.; Pouliot, G.; Keating, T.; Zhang, Q.; Kurokawa, J.; Wankmuller, R.; et al. HTAP_v2.2: A mosaic of regional and global emission grid maps for 2008 and 2010 to study hemispheric transport of air pollution. Atmos. Chem. Phys. 2015, 15, 11411–11432. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, J.; Ohara, T.; Morikawa, T.; Hanayama, S.; Janssens-Maenhout, G.; Fukui, T.; Kawashima, K.; Akimoto, H. Emissions of air pollutants and greenhouse gases over Asian regions during 2000–2008: Regional Emission inventory in ASia (REAS) version 2. Atmos. Chem. Phys. 2013, 13, 11019–11058. [Google Scholar] [CrossRef]
- Chatani, S.; Morikawa, T.; Nakatsuka, S.; Matsunaga, S.; Minoura, H. Development of a framework for a high-resolution, three-dimensional regional air quality simulation and its application to predicting future air quality over Japan. Atmos. Environ. 2011, 45, 1383–1393. [Google Scholar] [CrossRef]
- Chatani, S.; Morino, Y.; Shimadera, H.; Hayami, H.; Mori, Y.; Sasaki, K.; Kajino, M.; Yokoi, T.; Morikawa, T.; Ohara, T. Multi-model analyses of dominant factors influencing elemental carbon in Tokyo metropolitan area of Japan. Aerosol Air Qual. Res. 2014, 14, 396–405. [Google Scholar] [CrossRef]
- Shimadera, H.; Hayami, H.; Chatani, S.; Morikawa, T.; Morino, Y.; Mori, Y.; Yamaji, K.; Nakatsuka, S.; Ohara, T. Urban air quality model inter-comparison study in Japan (UMICS) for improvement of PM2.5 simulation. Asian J. Atmos. Environ. 2017, in press. [Google Scholar]
- CMAQ v5.0.2 Sulfur Tracking. Available online: https://www.airqualitymodeling.org/index.php/ (accessed on 20 January 2018).
- Mathur, R.; Roselle, S.; Pouliot, G.; Sarwar, G. Diagnostic analysis of the three-dimensional sulfur distributions over the Eastern United States using the CMAQ model and measurements from the ICARTT field experiment. In Air pollution Modeling and Its Application XIX; NATO Science for Peace and Security Series; Springer: Dordrecht, The Netherlands, 2008; Volume 5, pp. 496–504. [Google Scholar] [CrossRef]
- Stein, A.F.; Saylor, R.D. Sensitivities of sulfate aerosol formation and oxidation pathways on the chemical mechanism employed in simulations. Atmos. Chem. Phys. 2012, 12, 8567–8574. [Google Scholar] [CrossRef]
- Itahashi, S.; Uno, I.; Yumimoto, K.; Irie, H.; Osada, K.; Ogata, K.; Fukushima, H.; Wang, Z.; Ohara, T. Interannual variation in the fine-mode MODIS aerosol optical depth and its relationship to the changes in sulfur dioxide emissions in China between 2000 and 2010. Atmos. Chem. Phys. 2012, 12, 2631–2640. [Google Scholar] [CrossRef] [Green Version]
- Itahashi, S.; Uno, I.; Kim, S.-T. Source contributions of sulfate aerosol over East Asia estimated by CMAQ-DDM. Environ. Sci. Technol. 2012, 46, 6733–6741. [Google Scholar] [CrossRef] [PubMed]
- Itahashi, S.; Hayami, H.; Yumimoto, K.; Uno, I. Chinese province-scale source apportionments for sulfate aerosol in 2005 evaluated by the tagged tracer method. Environ. Pollut. 2017, 220, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Itahashi, S.; Hatakeyama, S.; Shimada, K.; Tatsuta, S.; Taniguchi, Y.; Chan, C.K.; Kim, Y.-P.; Lin, N.-H.; Takami, A. Model estimation of sulfate aerosol source collected at Cape Hedo during an intensive campaign in October–November, 2015. Aerosol Air Qual. Res. 2017, 17, 3079–3090. [Google Scholar] [CrossRef]
- Itahashi, S. Toward synchronous evaluation of source apportionments for atmospheric concentration and deposition of sulfate aerosol over East Asia. J. Geophys. Res. 2018, 123. [Google Scholar] [CrossRef]
- Boylan, J.W.; Russell, A.G. PM and light extinction model performance metrics, goals, and criteria for three-dimensional air quality models. Atmos. Environ. 2006, 40, 4946–4959. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhang, B.; Shen, Z.B. Spatial and temporal variability of trace metals in aerosol from the desert region of China ant the Yellow Sea. J. Geophys. Res. 2002, 107. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, Z.; Yang, Z.; Fang, M.; Feng, J. Seasonal variations and sources of various elements in the atmospheric aerosols in Qingdao, China. Atmos. Res. 2007, 85, 27–37. [Google Scholar] [CrossRef]
- Itahashi, S.; Uno, I.; Osada, K.; Kamiguchi, Y.; Yamamoto, S.; Tamura, K.; Wang, Z.; Kurosaki, Y.; Kanaya, Y. Nitrate transboundary heavy pollution over East Asia in winter. Atmos. Chem. Phys. 2017, 17, 3823–3843. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics—From Air Pollution to Climate Change, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Walcek, C.J.; Taylor, G.R. A theoretical method for computing vertical distributions of acidity and sulfate production within cumulus clouds. J. Atmos. Sci. 1986, 43, 339–355. [Google Scholar] [CrossRef]
- Siefert, R.L.; Johansen, A.M.; Hoffman, M.R.; Pehkonen, S.O. Measurements of trace metal (Fe, Cu, Mn, Cr) oxidation states in fog and stratus clouds. J. Air Waste Manag. Assoc. 1998, 48, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Desboeufs, K.V.; Sofikitis, A.; Losno, R.; Colin, J.L.; Ausset, P. Dissolution and solubility of trace metals from natural and anthropogenic aerosol particulate matter. Chemosphere 2005, 58, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ibusuki, T.; Takeuchi, K. Sulfur dioxide oxidation by oxygen catalyzed by mixtures of manganese(II) and iron(III) in aqueous solutions at environmental reaction conditions. Atmos. Environ. 1987, 21, 1555–1560. [Google Scholar] [CrossRef]
- CAMx Overview. Available online: http://www.camx.com/about/default.aspx (accessed on 30 January 2018).
- Fahey, K.M.; Carlton, A.G.; Pye, H.O.T.; Baek, J.; Hutzell, W.T.; Stainier, C.O.; Baker, K.R.; Appel, K.W.; Jaoui, M.; Offenberg, J.H. A framework for expanding aqueous chemistry in the Community Multiscale Air Quality (CMAQ) model version 5.1. Geosci. Model Dev. 2017, 10, 1587–1605. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Ma, Q.; Ma, J.; Chu, B.; Ji, D.; Tang, G.; Liu, C.; Zhang, H.; Hao, J. Pathways of sulfate enhancement by natural and anthropogenic mineral aerosols in China. Sci. Rep. 2014, 4, 4172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Itahashi, S.; Uno, I.; Pan, X.L.; Osada, K.; Yamamoto, S.; Nishizawa, T.; Tamura, K.; Wang, Z. Modeling the long-range transport of particulate matters for January in East Asia using NAQPMS and CMAQ. Aerosol Air Qual. Res. 2017, 17, 3065–3078. [Google Scholar] [CrossRef]
- Fu, X.; Wang, S.; Chang, X.; Cai, S.; Xing, J.; Hao, J. Modeling analysis of secondary inorganic aerosols over China: Pollution characteristics, and meteorological and dust impacts. Sci. Rep. 2016, 6, 35992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhang, Q.; Zhang, Y.; He, K.B.; Wang, K.; Zheng, G.J.; Duan, F.K.; Ma, Y.L.; Kimoto, T. Heterogeneous chemistry: A mechanism missing in current models to explain secondary inorganic aerosol formation during January 2013 haze episode in North China. Atmos. Chem. Phys. 2015, 15, 2031–2049. [Google Scholar] [CrossRef]
- Li, G.; Bei, N.; Cao, J.; Huang, R.; Wu, J.; Feng, T.; Wang, Y.; Liu, S.; Zhang, Q.; Tie, X.; et al. A possible pathway for rapid growth of sulfate during haze days in China. Atmos. Chem. Phys. 2017, 17, 3301–3316. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, R.; Gomez, M.E.; Yang, L.; Levy Zamora, M.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zheng, G.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.; Gao, M.; Zhang, Q.; He, K.; Carmichael, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, e1601530. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Kilmont, Z.; Zhang, Q.; Martin, R.V.; Zheng, B.; Heyes, C.; Cofala, J.; Zhang, Y.; He, K. Comparison and evaluation of anthropogenic emissions of SO2 and NOx over China. Atmos. Chem. Phys. 2018, 18, 3433–3456. [Google Scholar]
- Lei, Y.; Zhang, Q.; He, K.B.; Streets, D.G. Primary anthropogenic aerosol emission trends for China, 1990–2005. Atmos. Chem. Phys. 2011, 11, 931–954. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Zhao, D.; Cheng, K.; Lu, L.; He, M.; Hao, J. Anthropogenic atmospheric emissions of antimony and its spatial distribution characteristics in China. Environ. Sci. Technol. 2012, 46, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Cheng, H.; Li, Q.; Lin, C. Anthropogenic atmospheric emissions of cadmium in China. Atmos. Environ. 2013, 79, 155–160. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, T.; Li, Q.; Lu, L.; Lin, C. Anthropogenic Chromium Emissions in China from 1990 to 2009. PLoS ONE 2014, 9, e87753. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Wang, Y.; Tian, H.; Gao, X.; Zhang, Y.; Wu, X.; Zhu, C.; Gao, J. Atmospheric Emission Characteristics and Control Policies of Five Precedent-Controlled Toxic Heavy Metals from Anthropogenic Sources in China. Environ. Sci. Technol. 2015, 49, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Hao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 2015, 15, 10127–10147. [Google Scholar] [CrossRef]
- Moteki, N.; Adachi, K.; Ohata, S.; Yoshida, A.; Harigaya, T.; Koike, M.; Kondo, Y. Anthropogenic iron oxide aerosols enhance atmospheric heating. Nat. Commun. 2017, 8, 15329. [Google Scholar] [CrossRef] [PubMed]


| Spring | Summer | Autumn | Winter | ||
|---|---|---|---|---|---|
| SO42− | N | 1583 | 1824 | 1910 | 1923 |
| Mean (observations) (μg/m3) | 5.78 | 7.18 | 4.89 | 4.00 | |
| Mean (model) (μg/m3) | 5.27 | 5.61 | 4.03 | 2.44 | |
| R | 0.78 (p < 0.001) | 0.69 (p < 0.001) | 0.84 (p < 0.001) | 0.73 (p < 0.001) | |
| MFB (%) | −4.9 | −22.3 | −19.0 | −72.2 | |
| MFE (%) | +33.0 | +40.2 | +39.7 | +79.3 | |
| % within a factor of 2 | 88.3 | 81.4 | 81.8 | 40.6 | |
| % within a factor of 3 | 96.8 | 95.4 | 93.6 | 63.1 | |
| % within a factor of 5 | 99.3 | 99.5 | 97.6 | 86.9 | |
| Fe | N | 1412 | 1669 | 1755 | 1763 |
| Mean (observation) (ng/m3) | 131.01 | 88.20 | 101.77 | 132.68 | |
| Mean (model) (ng/m3) | 17.35 | 12.00 | 16.48 | 21.14 | |
| R | 0.36 (p < 0.001) | 0.47 (p < 0.001) | 0.45 (p < 0.001) | 0.49 (p < 0.001) | |
| MFB (%) | –142.3 | –145.1 | –137.4 | –143.0 | |
| MFE (%) | +142.8 | +145.9 | +139.8 | +143.8 | |
| % within a factor of 2 | 5.5 | 4.4 | 8.4 | 6.3 | |
| % within a factor of 3 | 13.2 | 11.9 | 17.8 | 15.1 | |
| % within a factor of 5 | 33.4 | 30.3 | 37.1 | 33.6 | |
| Mn | N | 1324 | 1574 | 1657 | 1643 |
| Mean (observation) (ng/m3) | 7.76 | 5.93 | 7.23 | 8.88 | |
| Mean (model) (ng/m3) | 0.37 | 0.25 | 0.36 | 0.44 | |
| R | 0.48 (p < 0.001) | 0.37 (p < 0.001) | 0.42 (p < 0.001) | 0.45 (p < 0.001) | |
| MFB (%) | −174.0 | −178.1 | −175.6 | −178.0 | |
| MFE (%) | +174.2 | +178.2 | +175.9 | +178.1 | |
| % within a factor of 2 | 1.1 | 0.8 | 1.0 | 0.6 | |
| % within a factor of 3 | 2.4 | 1.4 | 2.4 | 1.3 | |
| % within a factor of 5 | 6.0 | 2.9 | 5.6 | 3.5 |
| Spring | Summer | Autumn | Winter | ||
|---|---|---|---|---|---|
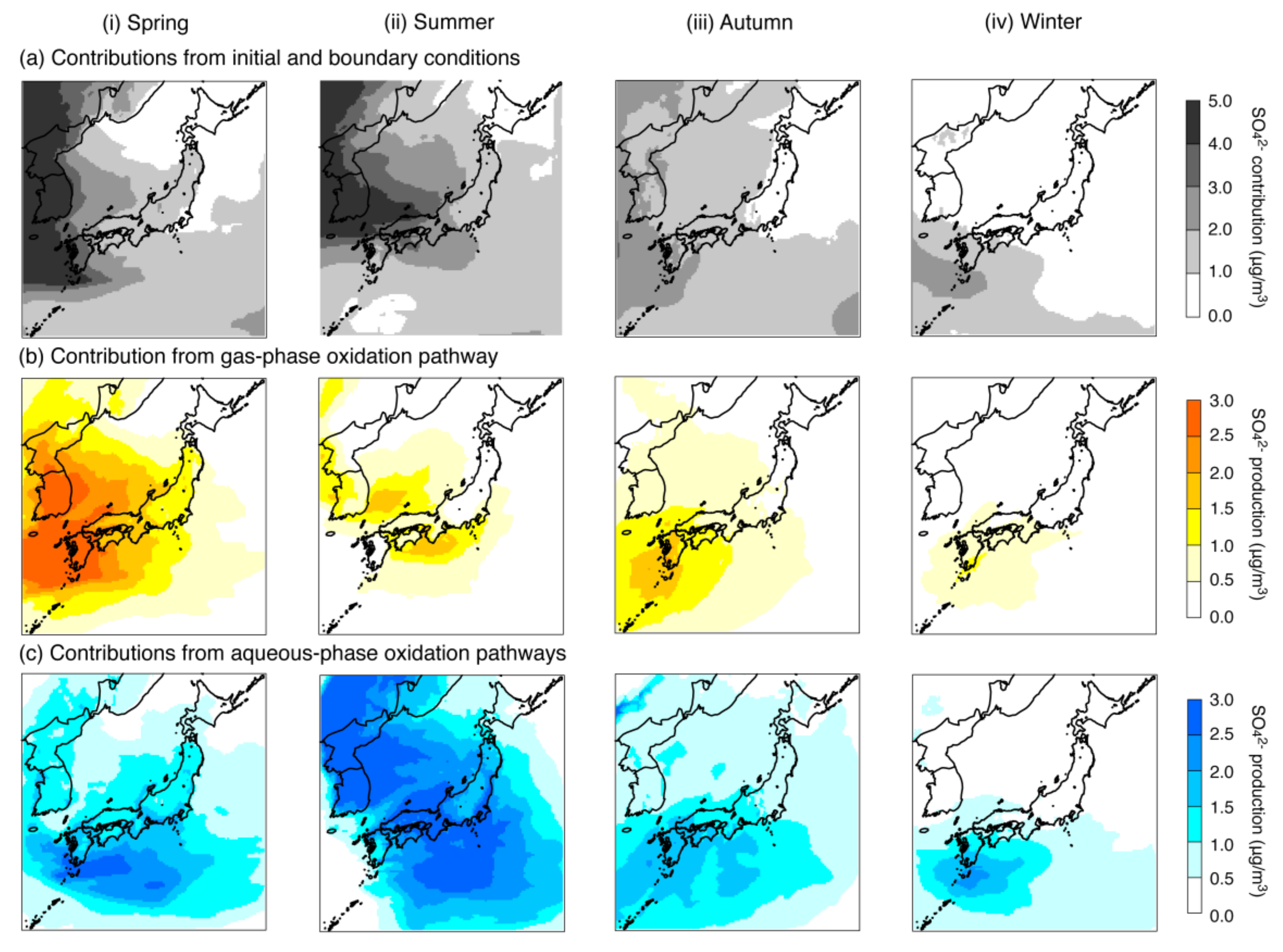
| Concentration (μg/m3) | 4.39 | 4.59 | 3.10 | 1.62 | |
| Contribution (μg/m3) | Initial and boundary conditions | 2.17 (49.3%) 1 | 2.36 (51.4%) 1 | 1.52 (49.2%) 1 | 0.79 (48.7%) 1 |
| Gas-phase oxidation pathway | 1.12 (25.4%) 1 | 0.45 (9.8%) 1 | 0.61 (19.7%) 1 | 0.31 (19.3%) 1 | |
| Aqueous-phase oxidation pathways | 1.03 (23.5%) 1 | 1.73 (37.7%) 1 | 0.91 (29.5%) 1 | 0.46 (28.7%) 1 | |
| Emissions | 0.08 (1.8%) 1 | 0.05 (1.1%) 1 | 0.05 (1.6%) 1 | 0.05 (3.3%) 1 | |
| Name | Description |
|---|---|
| Sensitivity simulation A | Fe and Mn concentrations are adjusted to observed concentrations |
| Sensitivity simulation B | Same as sensitivity simulation A, but the solubilities of Fe and Mn are increased (see Table 5) |
| Sensitivity simulation C | Same as sensitivity simulations B, but the rate constant expression of Fe- and Mn-catalyzed oxidation by O2 includes pH dependency |
| Sensitivity Simulation A | Sensitivity Simulation B | Sensitivity Simulation C | ||
|---|---|---|---|---|
| SO42− | Mean (model) (μg/m3) | 2.45 (+0.7%) 1 | 2.50 (+2.4%) 1 | 2.52 (+3.5%) 1 |
| R | 0.73 (p < 0.001) | 0.73 (p < 0.001) | 0.73 (p < 0.001) | |
| MFB (%) | –71.7 | –70.7 | –68.6 | |
| MFE (%) | +79.2 | +78.7 | +76.7 | |
| % within a factor of 2 | 41.3 | 41.5 | 43.8 | |
| % within a factor of 3 | 63.3 | 63.7 | 65.4 | |
| % within a factor of 5 | 86.9 | 87.3 | 88.6 |
| Base-Case | Sensitivity Simulation B | ||
|---|---|---|---|
| Fe | Solubility (anthropogenic) | 10% | 25% |
| Solubility (soil) | 10% | 1% | |
| Mn | Solubility | 50% | 100% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itahashi, S.; Yamaji, K.; Chatani, S.; Hayami, H. Refinement of Modeled Aqueous-Phase Sulfate Production via the Fe- and Mn-Catalyzed Oxidation Pathway. Atmosphere 2018, 9, 132. https://doi.org/10.3390/atmos9040132
Itahashi S, Yamaji K, Chatani S, Hayami H. Refinement of Modeled Aqueous-Phase Sulfate Production via the Fe- and Mn-Catalyzed Oxidation Pathway. Atmosphere. 2018; 9(4):132. https://doi.org/10.3390/atmos9040132
Chicago/Turabian StyleItahashi, Syuichi, Kazuyo Yamaji, Satoru Chatani, and Hiroshi Hayami. 2018. "Refinement of Modeled Aqueous-Phase Sulfate Production via the Fe- and Mn-Catalyzed Oxidation Pathway" Atmosphere 9, no. 4: 132. https://doi.org/10.3390/atmos9040132





