1. Introduction
Metallurgy is a major industrial sector as well as one of the largest environmental polluters. The Czech Republic has a high proportion of metallurgical production in the metallurgy field, resulting in a prominent level of pollution and a large ecological impact. The production of iron and steel in the Czech Republic generated 878,675 tons of wastes in 2014, of which the proportion of waste sludge was counted as 26.633 tons [
1].
In the environmental hierarchy, metallurgy is among the major producers of massive quantities of waste in addition to one of the leading producers of air pollution, representing a wide range of substances with different consistencies, and different physical, chemical, and mineralogical compositions [
2]. Currently, the metallurgical process produces a large amount of solid waste, such as slag, dust, sludge, and sewage. A majority of this waste contains a significant amount of iron and constitutes a source of potentially hazardous metals, such as zinc, lead, cadmium, and arsenic. According to the content of hazardous pollutants, these wastes are further treated. This waste is inefficiently redesigned, causing considerable economic and environmental losses. With the development of novel science, technology, and environmental protection, the trend of improving production technologies and minimizing waste production and waste storage in landfills is increasing [
3].
Recently, significant attention is focused on the usage of industrial waste materials, since this waste is an unused resource and in many cases can cause serious liquidation problems. Waste from iron and steel production is an important secondary raw material because of its high content of iron [
4]. Currently, efforts to reuse such waste in a briquette production process as a part of the batch for iron production has been undertaken. A fine converter sludge (CS) is mixed with a converter dust, and their mixture is used in the construction industry for cement production [
5,
6].
Awareness of the environmental impact and concern for the environment has been increased in recent decades. Water management strategies of the international mining industry are used to minimize the environmental impact of mining operations, and now they are the heart of the mine development. In order to protect the environment, the quality of water leaving mine sites into downstream waters must be evaluated. Mining companies develop water management plans to minimize the potential of water contamination and to prevent the release of polluted water into the environment [
7].
A number of new technologies for the removal of heavy metals from wastewater has been developed. The predominant applied methods are coagulation, flocculation, precipitation, electrochemical processes, ion exchange, extraction, etc.; using a suitable sorbent is another favorable way of removing pollutants from wastewater. Price, availability, adsorption capacity, and strong affinity to pollutants are limiting factors for sorbent application in wastewater treatment; hence, new materials to be used as sorbents are constantly being evaluated [
6].
Adsorption has become a popular method for the removal of heavy metals from mine water and wastewater. Natural materials, waste, and residue products from industrial or agricultural activities have excellent potential as economic adsorbents for heavy metals removal from aqueous solutions. The reduction of acidity and removal of metal ions from coal mining effluents using chitosan microspheres [
8], zeolites [
9], blast furnace slag [
10], fly ash zeolite [
11], and phosphatized dolomite [
12] have been studied.
Investigating the competitive adsorption of certain metal ions on CS sorbent is rather complicated. The competitive effect of heavy metal ions on a CS sample has never been studied.
In general, the methods of mine water purification can be divided into active and passive approaches. Active methods use chemical reagents and energy. The advantage of active methods is the high efficiency of the process. The disadvantage is their financial difficulty, high consumption of chemicals, costs of operation, and maintenance of the treatment plant, as well as the costs associated with the sludge disposal [
13]. The passive method works on the principle of using naturally available energy sources in systems that require minimal maintenance; this method has lower costs, although it also has low efficiency and is time consuming. Recently, there have also been combinations of active and passive methods, mainly arising from the pursuit of ecological approaches and cost reduction. However, conventional methods such as lime-based chemical precipitation, ion exchange, and other processes have a number of shortcomings; extensive land utilization, the production of large secondary solid waste, and high capital and operating costs are among them.
The purpose of using waste materials as a sorbent brings two advantages regarding environmental pollution. The first advantage is the potential reduction of the volume of solid waste materials. The second is in the use of the waste as sorbents, which can effectively reduce wastewater toxicity at reasonably low costs [
14,
15].
This work is focused on the study of the removal of manganese, cobalt, and nickel ions contained in a mine water sample using waste materials generated in an oxygen converter of a flue gas treatment plant. The main objective of the work is to reduce the monitored parameters below the limit values for the discharge of mine water into the environment.
2. Materials and Methods
2.1. Materials
In a steel converter, oxygen is blown off by molten, carbon-rich raw iron, which reduces the carbon content of the alloy and converts it into the steel. The CS, resulting from the wet scrubbing of the gas formed, consists predominantly of iron oxides and a portion of calcium. Hematite and magnetite can be detected in CS [
16]. The CS sample used in this study was obtained from a wet purification process of combustion products of the steel production from a plant located in the Czech Republic. The CS sample consisted of a dry powder without pre-treatment. The CS sample was separated into fractions and subjugated to homogenization and sieve analysis before the primary analysis and sorption experiment. The particle size of the material used in the experiment was in the range of 100–200 μm. Subsequently, an analysis of the aqueous leachate of the CS sample was performed according to the standard EN 12457-4:2002 [
17]. For further experimental work, the materials were prepared from the original samples, in which the soluble components were removed by the process according to EN 12457-4:2002 [
17], where the methods of removing are defined. The chemical and phase composition of the CS sample were determined. Specific surface and pore sizes were also measured. The prepared aqueous leachate was analyzed and inorganic pollutants, fluorides, chlorides, sulphates, dissolved organic carbon, and the phenolic index were determined. According to EU (European Union) standard 200/532/EC [
18]., which details a list of hazardous waste, tested waste belongs to the category of waste from iron and steel industry. In the European Waste List, it is listed as No. 100214–sludge and filter cakes from gas treatment [
18]. Thus, CS was pre-treated by washing with water.
A mine water (MW) sample for laboratory experiments was obtained from a brown coal mine area, which is located in the Czech Republic. This sample of MW was collected from the surface of a mining pit area, where the water is mixed. The sample was filtered from suspended solid particles and coarse impurities by using a membrane filter with a pore size of 0.45 μm before the analyses and sorption experiment. The filtered MW sample was used to measure the concentrations of selected indicators monitored in the MW treatment plant.
2.2. Methods of Material Characterization
2.2.1. XRF Analysis
An ARL 9400 XP X-ray fluorescence (XRF) spectrometer (Thermo ARL, Pardubice, Czech Republic) was used to determine the chemical composition of the material. CS sorbent was characterized in its original state (powder). For the XRF analysis, the CS sample was taken and mixed with a wax. A pulverizing mill was utilized to obtain a homogeneous dispersion and uniform particle size of the resultant mixture (sorbent and wax). Pressed pellets for XRF analysis were prepared from the mixture by applying a hydraulic pressure of 10 metric tons to compress the sample.
The XRF spectrometer provides a fully automatic sequence for qualitative and quantitative element analysis. It is equipped with an Rh tube, 4 kW generator, crystals (TIAP—Thallium Acid Phthalate, Ge 111, LiF 200, LiF 220), and two detectors: proportional and scintillation. Standard-less analysis was made using Uniquant 4 software (Thermo ARL, Pardubice, Czech Republic).
2.2.2. XRD Analysis
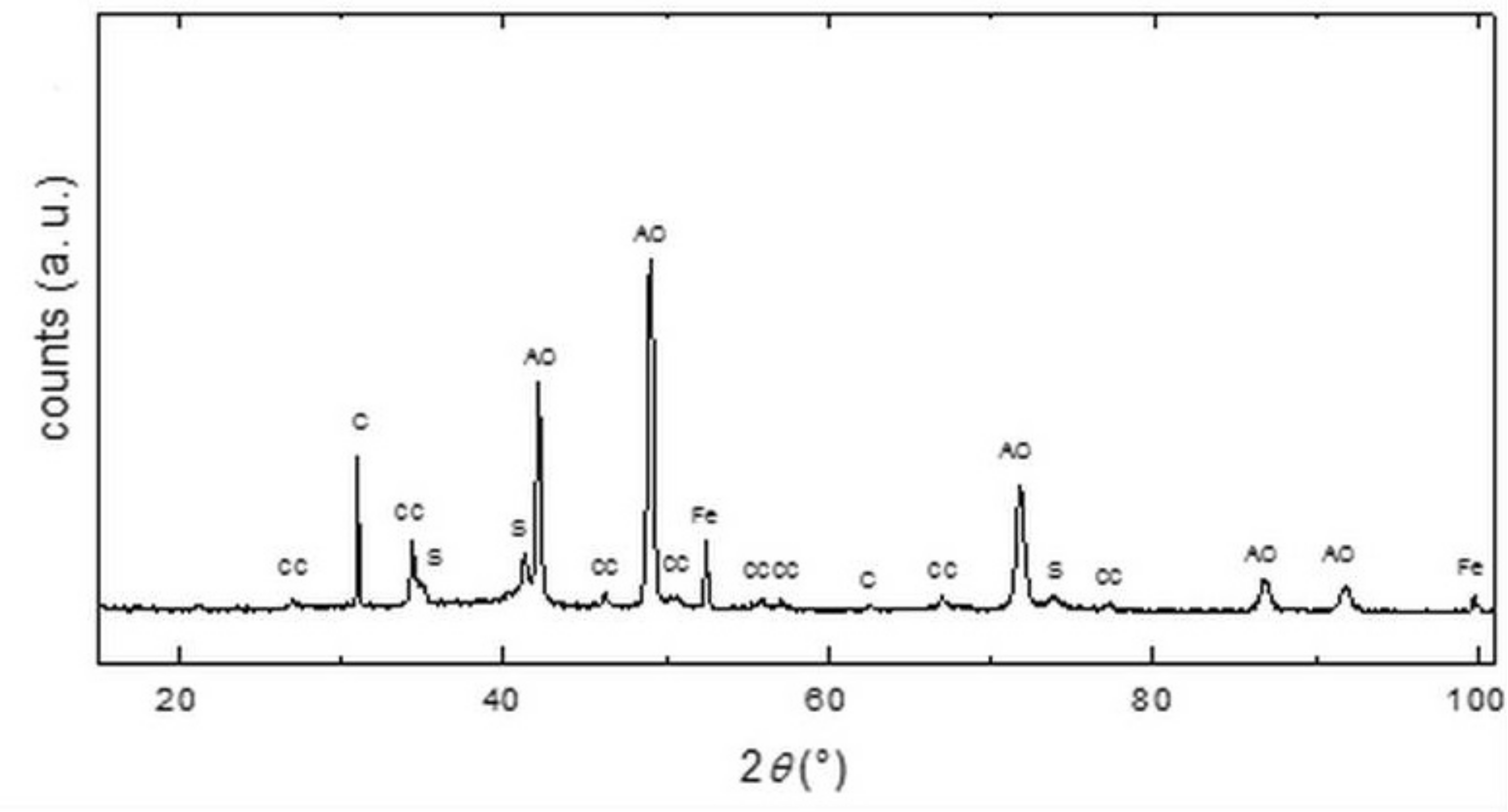
The CS samples were characterized by X-ray diffraction (XRD) analysis in a Bruker D2 Phaser diffractometer (Brucker, Vienna, Austria).in the Bragg-Brentano geometry. The XRD θ–2θ scans were acquired in an angle range of 10–100° 2θ with a step size of 0.05° 2θ. Cobalt X-ray lamp with the radiation of Cu-Kα1 (λ = 0.1789 nm) and output power of 300 W was used for diffraction. Each scan was registered over 5 h. The diffraction patterns were matched with the PDF reference database and refined by Rietveld analysis.
2.2.3. SEM
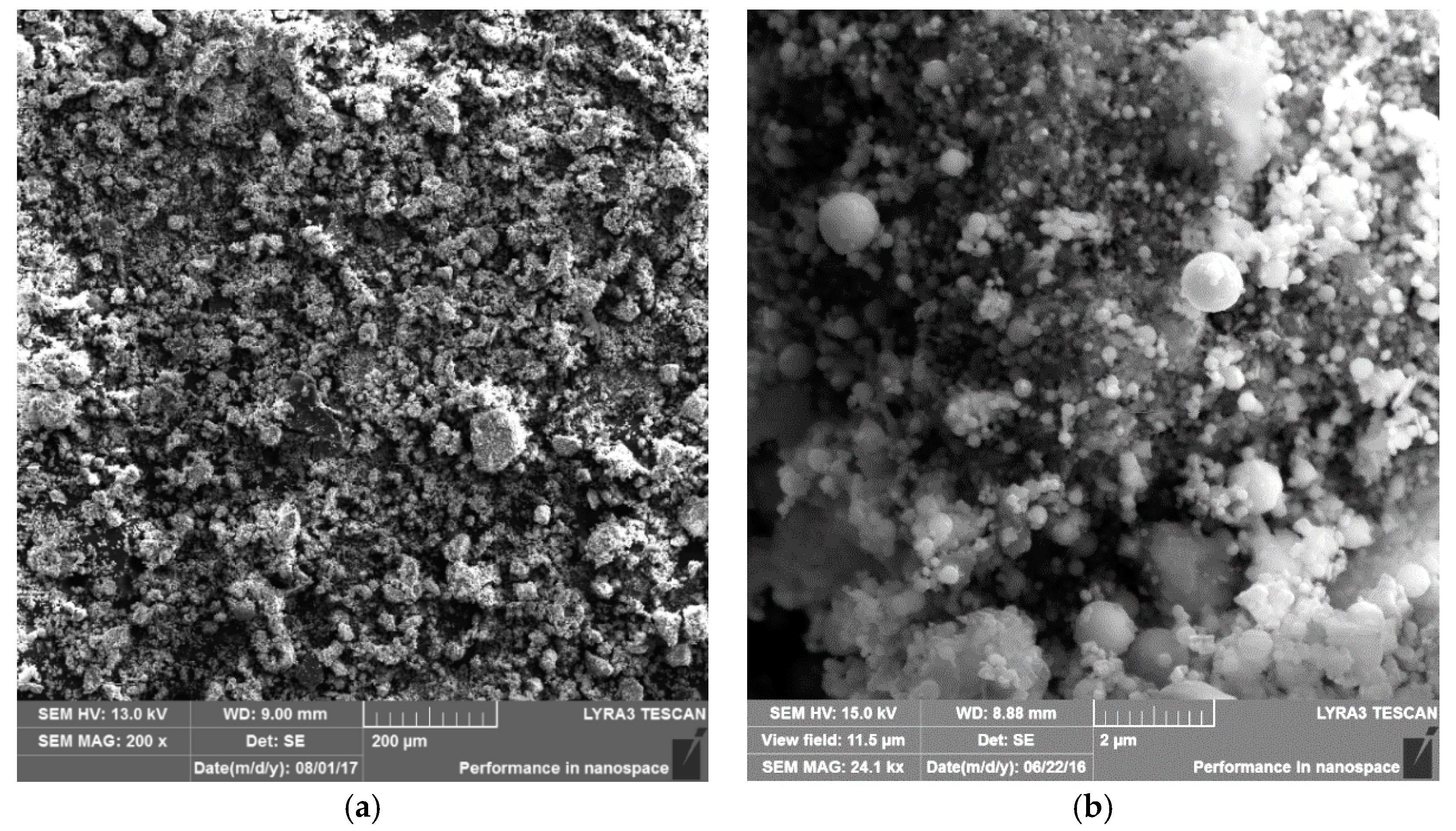
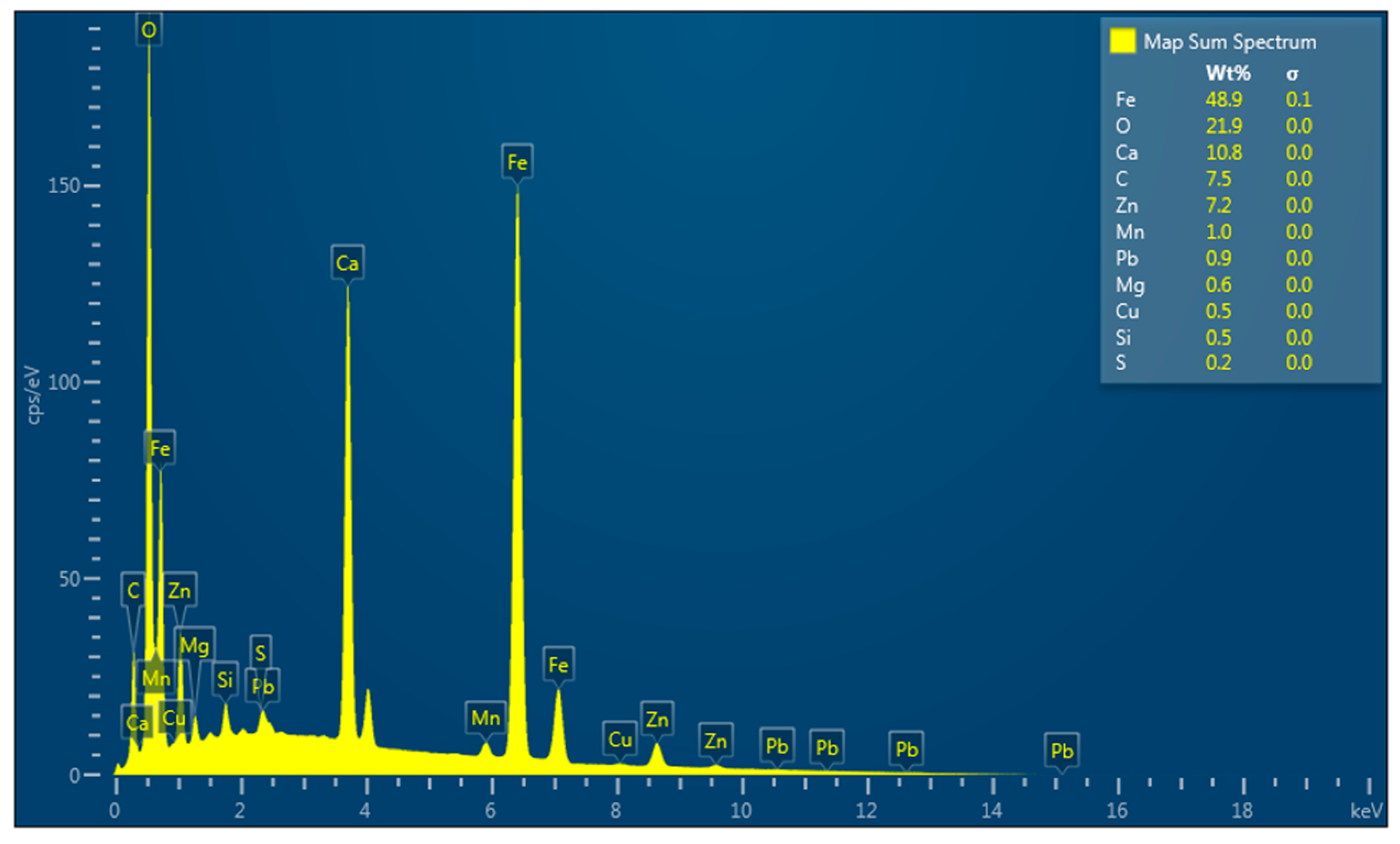
Scanning electron microscopy (SEM) LYRA 3 (TESCAN, Brno, Czech Republic) was utilized to investigate the structure of CS and to perform a detailed analysis of particles morphology and their chemical composition. SEM micrographs were taken at different magnifications. The SEM was equipped with an energy dispersive (EDX) X-ray spectrometer (TESCAN, Brno, Czech Republic), which was used for quantitative elemental analysis and for recording elementary maps of adsorbed metals. Secondary electron (SE) (TESCAN, Brno, Czech Republic) and backscattered electron (BSE) detectors (TESCAN, Brno, Czech Republic) were used for the surface analysis.2.2.4. Surface Area and Pore Size Distribution
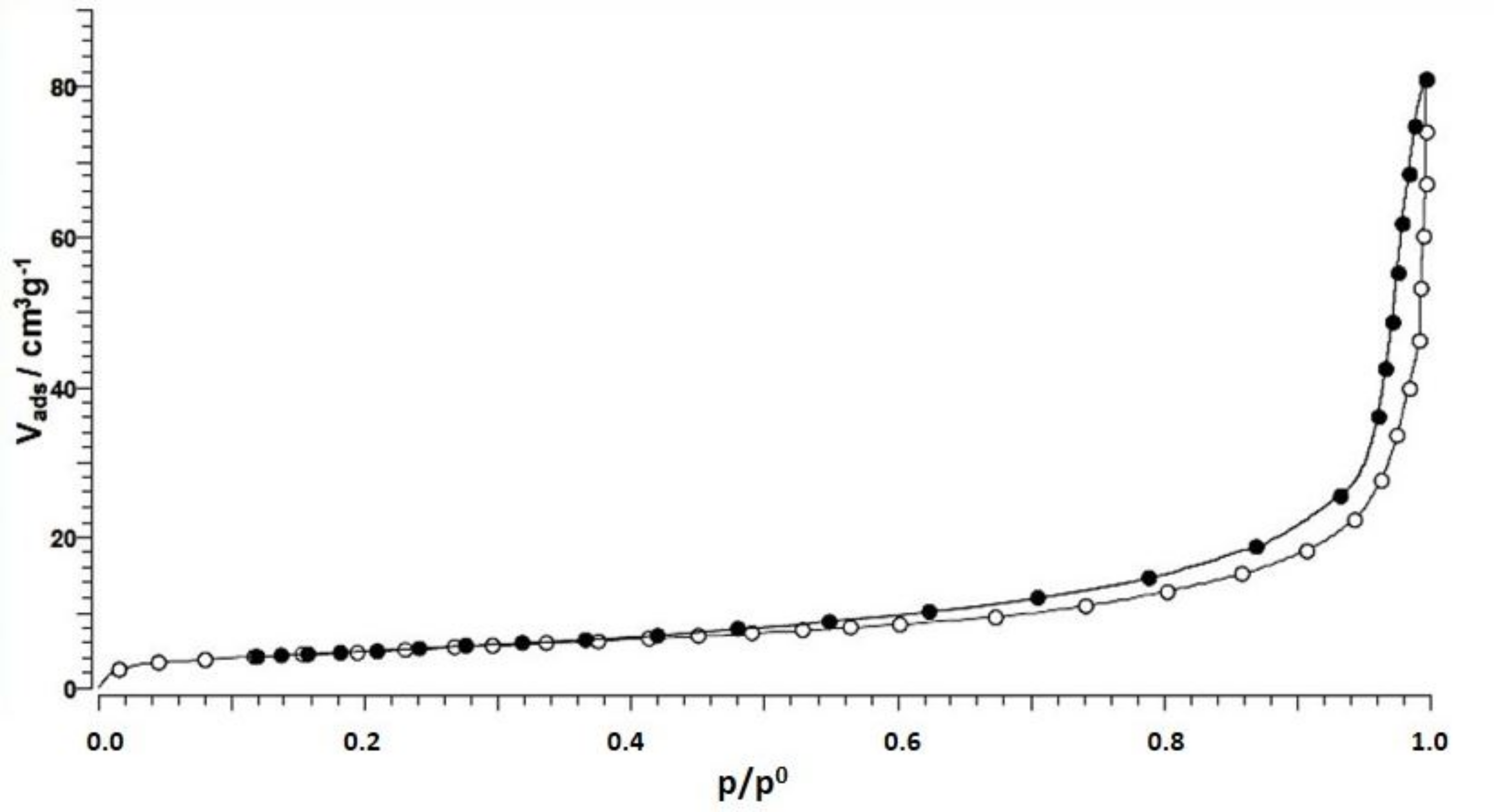
Surface area and pore size distribution were measured using a QUADRASORB EVO/SI analyzer (Quantachrome, Prague, Czech Republic). The surface area was measured by the BET (Brunauer-Emmett-Teller) nitrogen adsorption technique at −195.8 °C. The CS was outgassed in a vacuum for 72 h at 40 °C before analysis. The pore size distribution was measured by analyzing the desorption branches of the isotherm using the Barrett-Joyner-Halenda (BJH) method. The measured data were evaluated by QuadraWin software (Quantachrome, Prague, Czech Republic).
2.2.4. Concentration of Metals
The aqueous extracts were prepared according to the standard CSN EN 12457-44 [
17], and analyzed by reference methods for the water analysis. A WiseShake (WISD) horizontal circular motion shaker (Verkon, Prague, Czech Republic) was used to mix the samples. A vacuum filtration device with membrane filters with a pore size of 0.45 μm was used for the filtration of the aqueous extract. Determination of the metal ions concentration in the water solutions was performed by atomic absorption spectrometry with a flame atomization FA-AAS SOLAAR M6 (Analyte Jena AG, Jena, Germany).
2.3. Methodology of the Experiment
A sorption experiment was carried out on the CS sample by using an MW sample as an adsorbate. The CS sample after the sorption experiment was subjected to morphology studies using SEM to obtain information on the distribution of the observed elements. Simultaneously, a desorption experiment was conducted to show the strength of the adsorbent-adsorbent interaction. The experimental results were evaluated using adsorption efficiency, distribution coefficient, appropriate kinetic models, and adsorption isotherms.
Moreover, a single metal ions experiment was carried out using 100 mL distilled water and 0.25 g CS sorbent. The real experiment was performed using 100 mL real MW and 0.25 g CS sorbent. The MW sample was composed of raw water without removing the undissolved substances before the sorption experiment. The prepared suspension was shaken using a horizontal shaker at 10 RPM at laboratory temperature. After shaking time, CS sorbent was filtered through a 0.45-μm membrane filter (MILLIPORE MF) (Merck, Prague, Czech Republic). The concentration of Mn(II), Co (II), and Ni(II) ions in the filtrate was analyzed using FA-AAS (Analyte Jena AG, Jena, Germany).
The desorption experiment was evaluated on the basis of the stability and bond strength of metal ions. The experiment was assessed on the basis of the observed concentration of metal ions leached out into distilled water. The dried solid residues of the CS sorbent after sorption experiments was used for the evaluation. This dried sample was subsequently used for the formation of aqueous leachate in the same ratio-solid and liquid phase, mixing with distilled water. The resulting suspension was shaken for 168 h at room temperature. Thereafter, the solid phase was separated by filtration, using a 0.45-μm membrane filter. The concentration of the monitored ions was determined in the filtrate and the leached portion of adsorbed ions on CS was determined. The leached portion of the ions was measured using FA-AAS. The obtained data were evaluated using the calculated efficiency of the elongated fraction.
2.4. Evaluation of the Sorption Process
Evaluation of the sorption process is governed by several important processes which should be considered and can be described by adsorption efficiency, distribution coefficients, and kinetic models.
The adsorption efficiency (E) is the quantity that expresses the amount of adsorbate removed from the aqueous solution calculated from the initial concentration.
The adsorption efficiency is expressed as a percentage in Equation (1):
where
c0 is the initial concentration of adsorbate (mg·dm
−3) and
ce is the equilibrium adsorbate concentration (mg·dm
−3).
The distribution coefficient (
KD) is a constant that simplifies the relationship between the adsorbent and the adsorbed compound. This parameter is important when the assessed metal interacts and adsorbs solid materials. The values of the distribution coefficients of a number of metal ions are important for understanding the selectivity of a specific metal ion.
KD can be considered as the simplest form of isotherm which expresses the linear dependence of the concentration of the adsorbed substance on the concentration of the substance dissolved in the solution. This experimentally determined coefficient reflects the competition of solid particles in the liquid phase with impurities; however, it is not a real competition for impurities of solid particles with water [
19]. The distribution coefficient is defined as the ratio of the adsorbate adsorbed by the weight of the solid to the amount of metal in the solution. It can be described by Equation (2):
where
KD is the distribution coefficient (dm
3·g
−1),
cs is the equilibrium concentration of the adsorbed substance (mg·g
−1), and
ce is the equilibrium concentration of the substance in solution (mg·dm
−3).
Kinetic studies are important for detecting adsorption mechanisms and controlling rate possibilities, such as mass transfer or chemical reaction processes. Adsorption kinetics are most commonly described by kinetic models of the pseudo-first and pseudo-second order.
The kinetic model of the pseudo-first order, also called the Lagergen model, was firstly used to describe the adsorption at the solid-liquid interface. The basis of this model is the capacity of the solid. Derivation from the Lagergren equation requires that the concentration of the two ions be time-independent and, therefore, the constant should correspond to the linear combination of both concentration values. The Lagergren equation represents the adsorption in the case of diffusion across the liquid phase boundary to a solid sorbent. The constant of the equation varies depending on the particle size and surface film thickness, where the constant is dependent on the concentration of ions in the solution and the process temperature in the case of chemisorption. The particle size does not affect adsorption [
20]. The pseudo-first order equation can be expressed by Equation (3):
where
qe is the amount of the adsorbed metal ions in equilibrium and
qt is the amount of the adsorbed metal at a specific time
t (min) per unit mass of sorbent in mg·g
−1.
k1 is the Lagergren pseudo-first order rate constant (min
−1), and corresponds to the adsorption time (min). The speed constant
k1 determines the time factor necessary to achieve the system equilibrium [
21].
The kinetic model of the pseudo-second order is based on the assumption of chemisorption as the main mechanism of the process, and is capable of sufficiently predicting the adsorption behavior throughout the process. It is based on the assumption that chemical adsorption may limit the rate of adsorption/desorption. This model is compared to the pseudo-first order model and is considered to be more suitable to represent kinetic data because of the assumption that the chemisorption can be a step limiting the rate of adsorption. Metal ions in the chemisorption are trapped on the surface of the adsorbent and form a chemical bond [
22].
The pseudo-second order kinetic model is based on Langmuir’s adsorption model on a solid surface, where it is assumed that the adsorption rate is proportional to the concentration of the dissolved substance in the solution and the number of free adsorption sites. Desorption is the release of one substance from another, either from the surface or through the surface. Desorption can occur when an equilibrium situation is altered [
23]. The kinetic equation of the pseudo-second order is described by Equation (4):
where
qe is the amount of the adsorbate in equilibrium and
qt is the amount of the adsorbate at time
t (min) per unit weight of the sorbent (mg·g
−1).
k2 is the pseudo-second order adsorption velocity constant (g·mg
−1·min
−1).
Two known isotherm models (Langmuir and Freundlich) have been applied to describe the experimental data in a single metal ions system with distilled water. The classical non-competitive Langmuir isotherm model for the adsorption of one component is expressed by Equation (5):
where
ce (mg·dm
−3) is the equilibrium concentration of metal ions, and
q (mg·g
−1) is the amount of metal ions adsorbed per unit mass of adsorbent.
KL and
qmax (mg·g
−1) are Langmuir constants related to the rate of adsorption and adsorption capacity, respectively.
The Freundlich isotherm is expressed by Equation (6):
where
KF and
n are Freundlich isotherm constants [
6].
4. Conclusions
CS generated by the wet-gas purification process in an oxygen converter was used as a sorption material to reduce the concentrations of manganese, cobalt, and nickel ions in MW. The sorption properties of the converter sludge were verified in a real MW sample obtained from the brown coal location that was subject to the control of the pH value, undissolved substances, and manganese and iron concentrations before being discharged into the watercourse.
The CS includes a high content of iron (46.8%), which is partially bound to the zinc ferrite as one of the major crystalline phases. It also contains metallic iron, a significant proportion of graphite, and calcium carbonate, which has a positive effect on the pH value of the MW
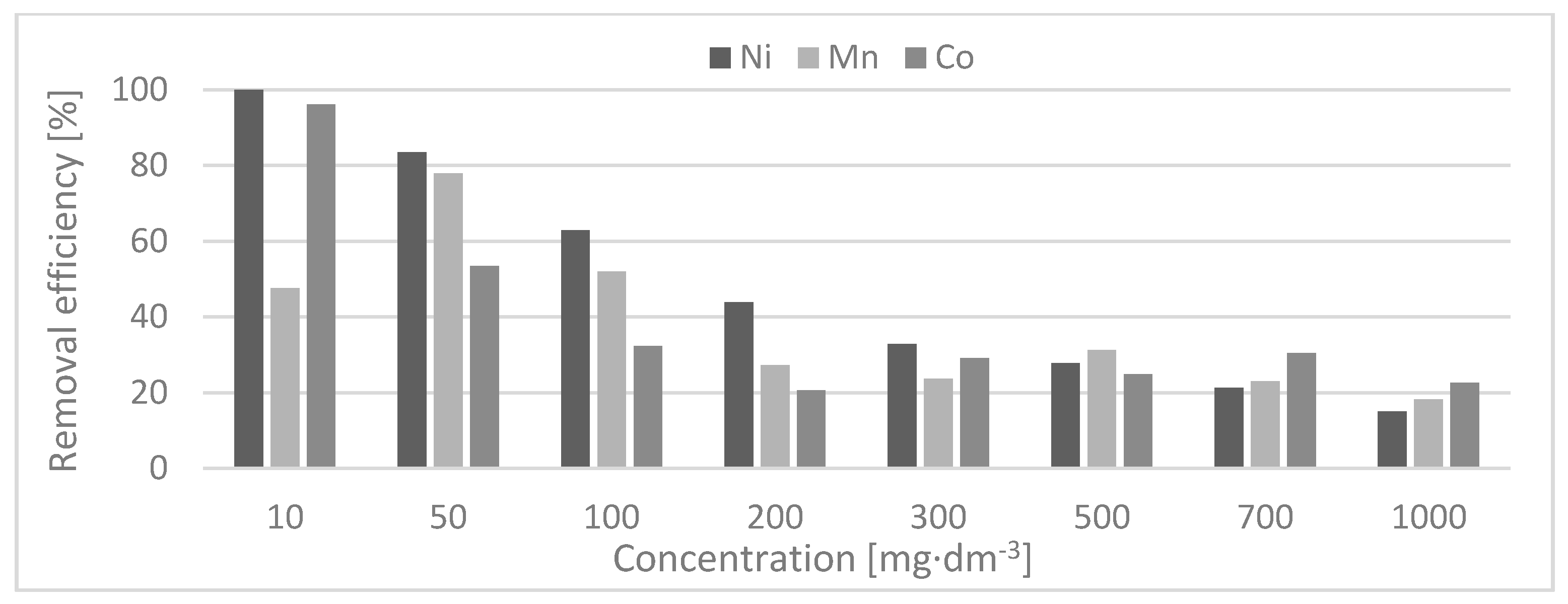
The predominant iron content of the CS plays an important role in the immobilization of the toxic elements. These results showed that the adsorption behaviors of Mn(II), Co.(II), and Ni(II) ions on the CS sorbent in a single metal ions system reach the best removal efficiency for a low initial concentration. The highest values of efficiency were reached by Ni(II), Co.(II), and Mn(II) ions, respectively. The assumption that the multi-composition of the CS creates much stronger competition on sorbent can be made based on the achieved results. Diverse binding mechanisms of heavy metal ions on different sorbents can be responsible for this effect. However, it is speculated that the “competitive effect” is not merely dependent on the total adsorption sites assigned to each fraction, but strongly depends on the surface sites that are readily “exchangeable”. The CS sorbent has to show many differences as compared to the single-component system. These differences are based on the several binding mechanisms available for heavy metals on the end-member component. High efficiency of the removal of Ni(II) and Co.(II) ions is caused by a strong affinity to the CS sorbent. On the contrary, Mn(II) does not cause such a strong affinity, however, the bound with the CS is very stable, firm, and strong. Mn ions exhibit a higher bond stability against replacement by other metals compared to the metal ions adsorbed on CS. This may be due to the more stable electron configuration of Mn(II) ions compared to those of Ni and Co.
The distribution coefficient indicates that the sorption takes place in the range of Ni > Co. > Mn. This can be explained by the size of the ionic radius. Ni(II), Co.(II), and Mn(II) occur in this range, which was confirmed by both the value of electronegativity and the distribution coefficient measurements.
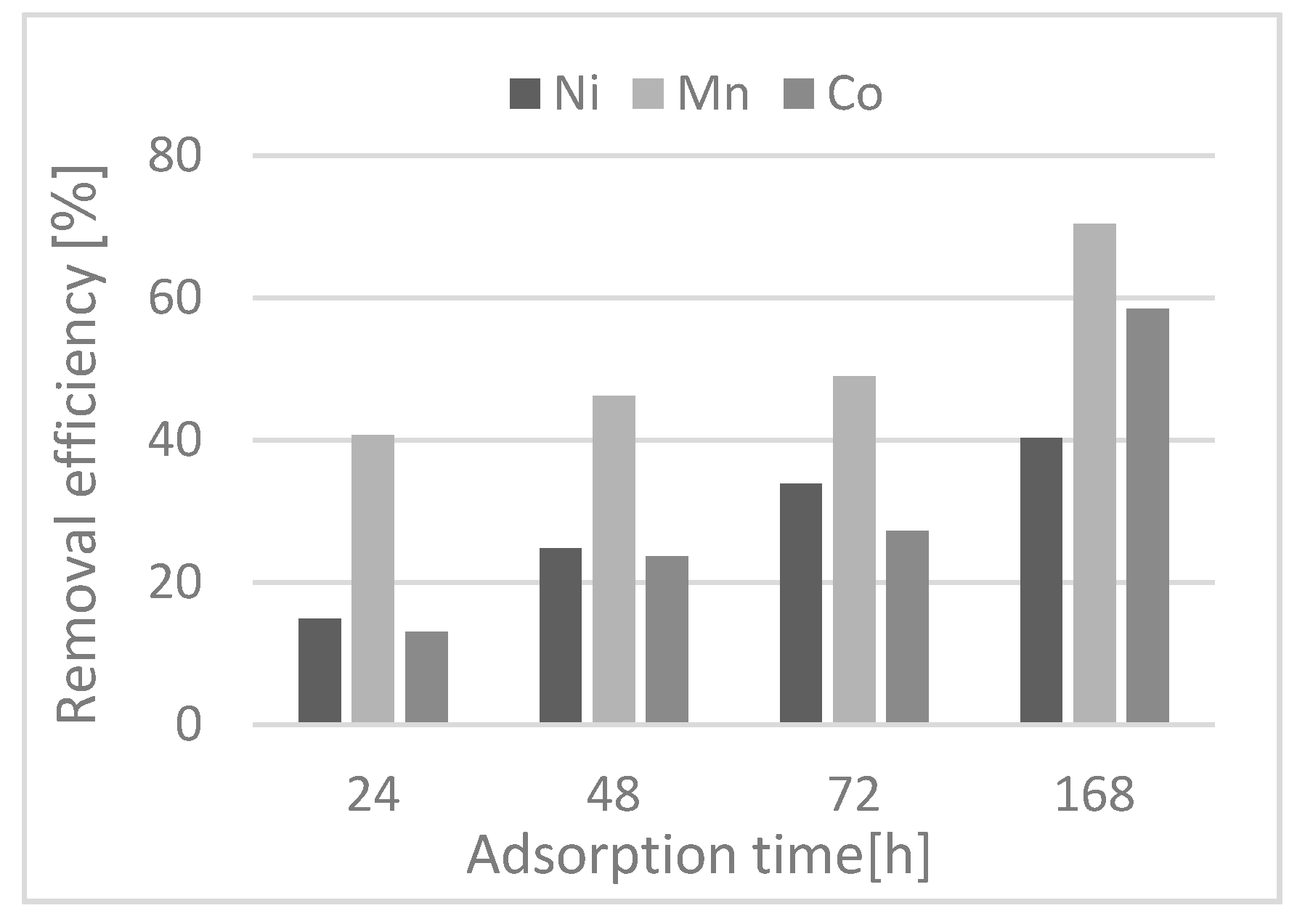
The sorption process showed that the CS reduces the content of all studied metal ions and it is not necessary to modify the pH of the MW for the optimal removal efficiency. The results of the removal efficiency of metal ions from the MW reached values of 70% for Mn(II), 59% for Co.(II), and 40% for Ni(II). The application of the kinetic models showed that the pseudo-first order model for Co.(II) and Ni(II) is more favorable for the description of this process, while for Mn(II) the pseudo-second order model is recommended.
Moreover, the high proportion of macropores and mesopores forms space for the attachment of metal ions on the surface.
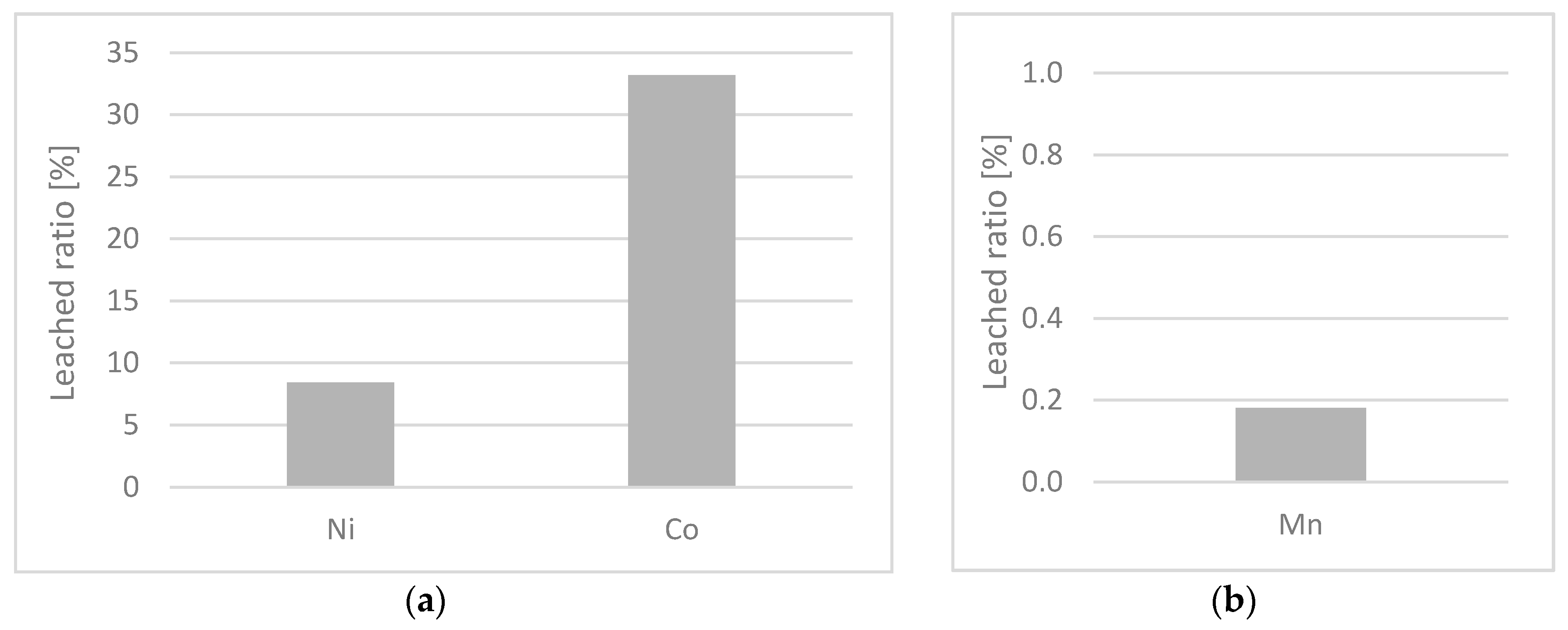
The desorption study was evaluated as an interaction strength between the adsorbent and the adsorbate, and it was evaluated after the sorption experiment with the CS + MW suspension. The results showed that manganese ions are the most strongly bound, which is the monitored parameter for the discharge of the MW into the watercourse.
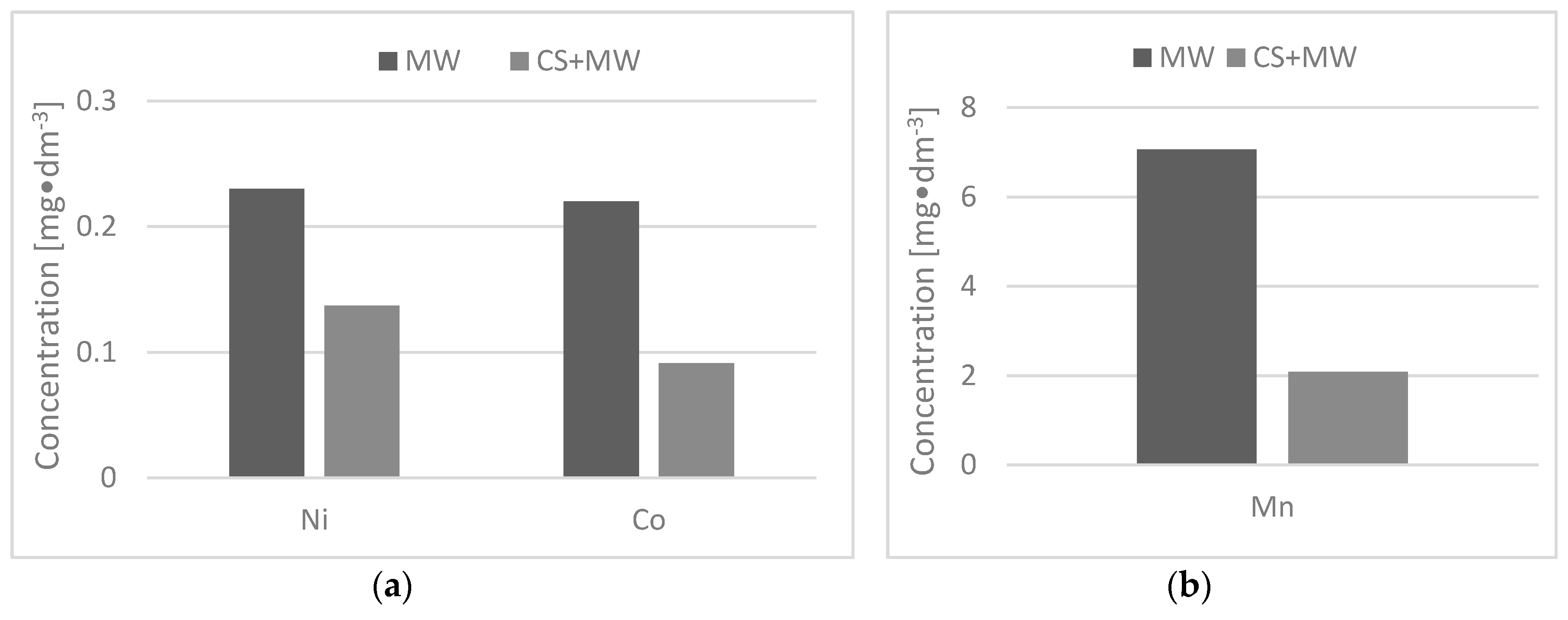
Summarizing all of the presented experimental results, it can be concluded that the CS sample has high potential for the removal of manganese, nickel, and cobalt ions from MW. The monitored concentrations of Mn(II), Co.(II), and Ni(II) as well as the pH value in the MW after the application of the CS are below the permitted limit values for the discharge of MW into the environment. The Czech Republic produces a high amount of CS, about 26,000 tons per year. The usage of CS waste would save around 22% of annual costs for mining operations compared to the existing MW treatment methods. For practical application, the low-cost and ecological aspect of the waste material utilization offers a great advantage.
The obtained results can significantly contribute to the field of chemical metallurgy by indicating the possibility of using CS as an efficient and prospective sorbent.