Defining Seasonal Functional Traits of a Freshwater Zooplankton Community Using δ13C and δ15N Stable Isotope Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
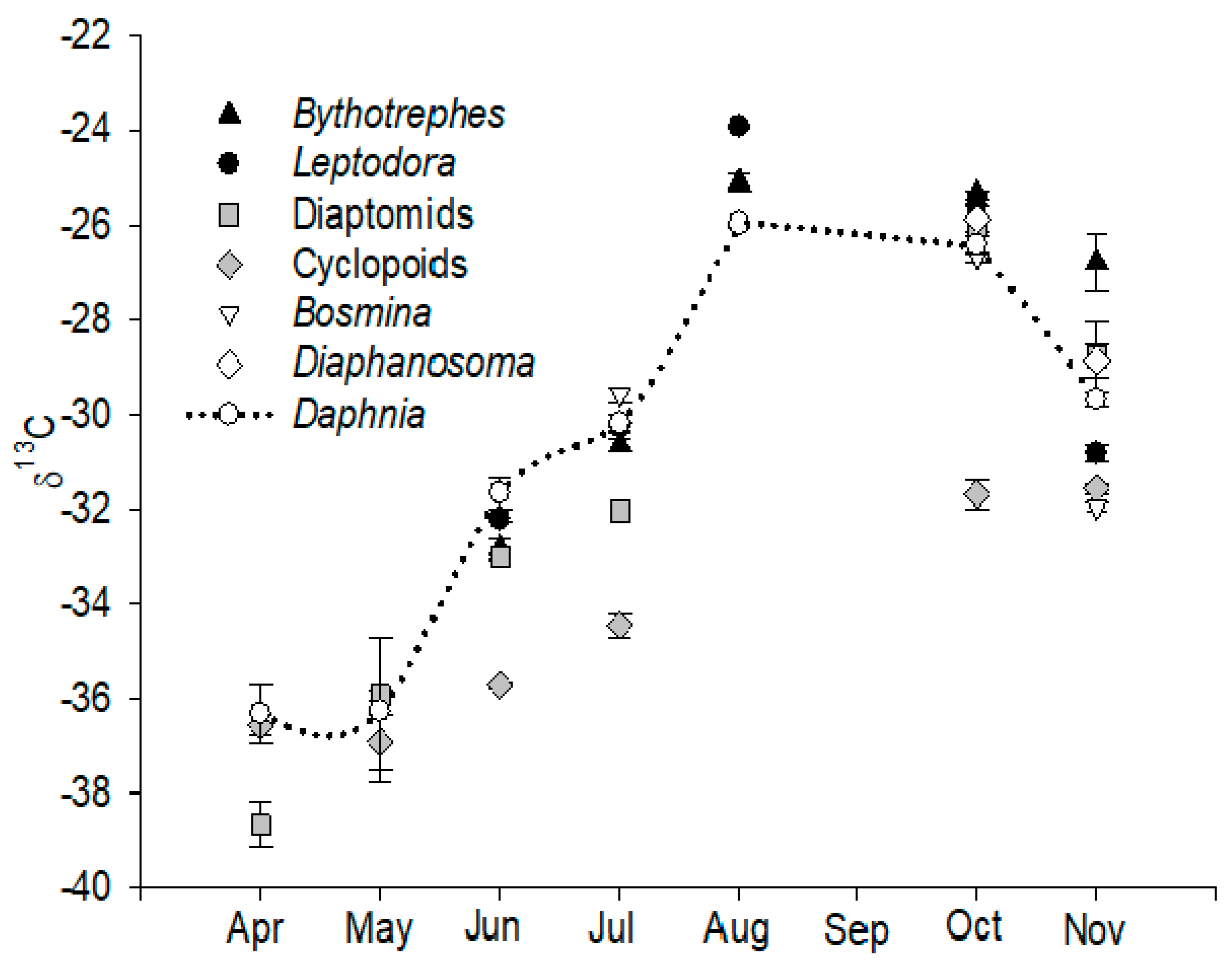
3.1. Seasonal Variation in δ13C and the Determination of Consumer Resources
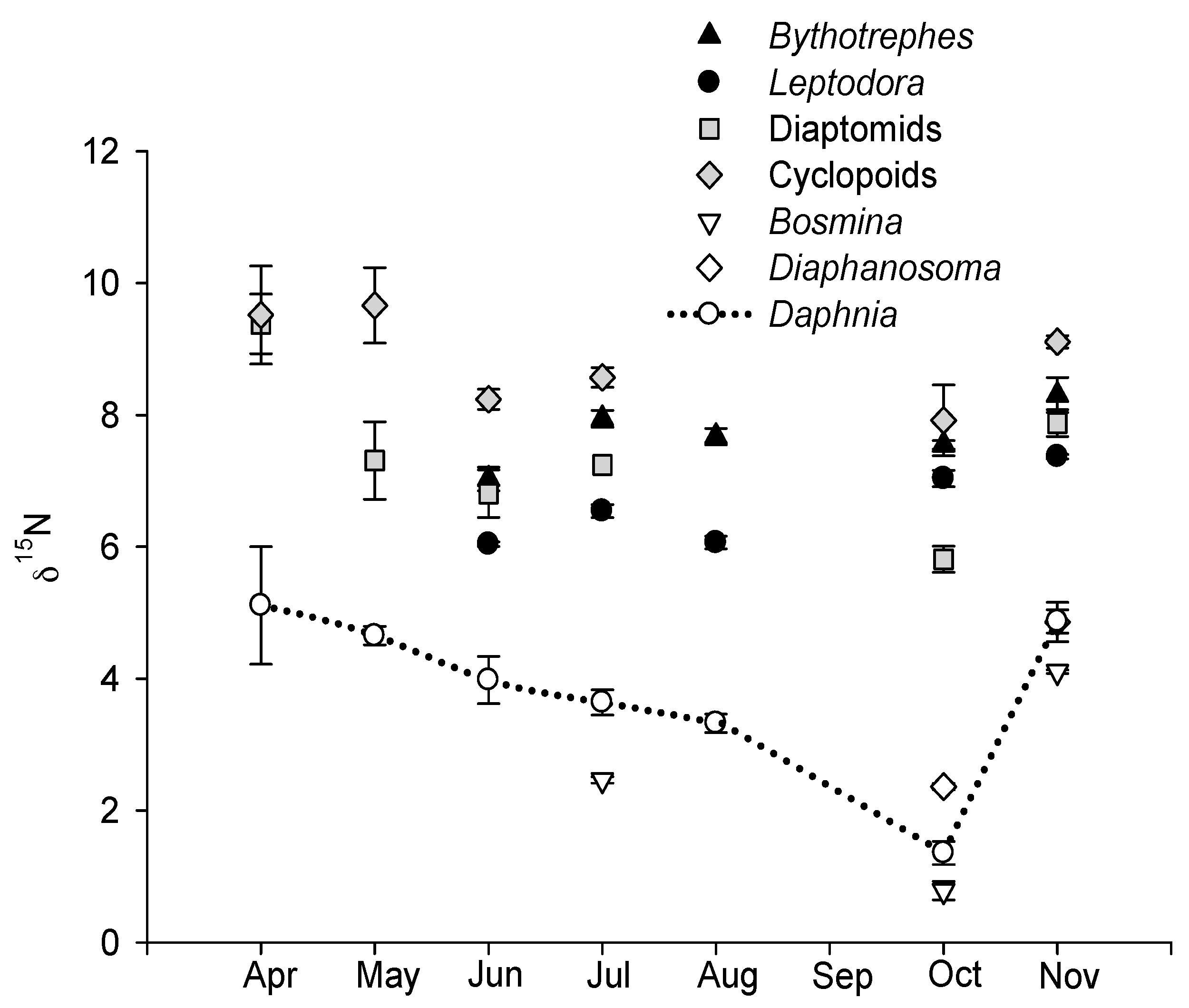
3.2. Seasonal Variation in δ15N and Determination of Trophic Levels
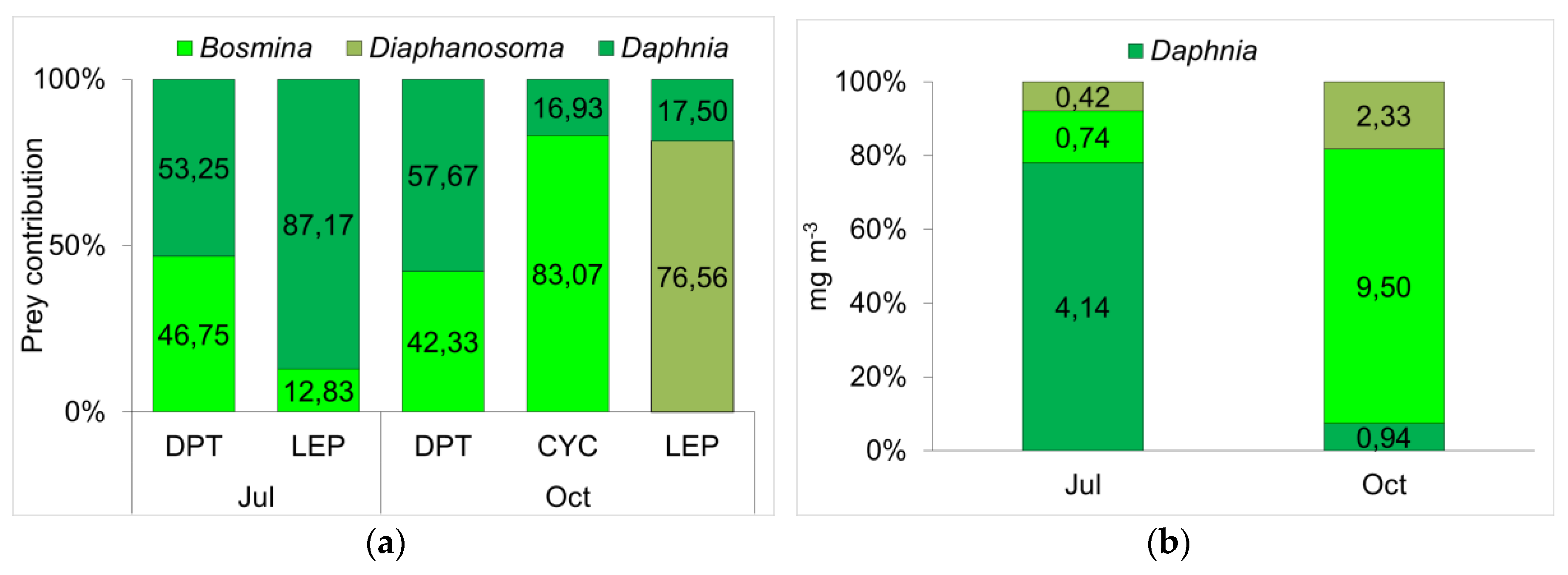
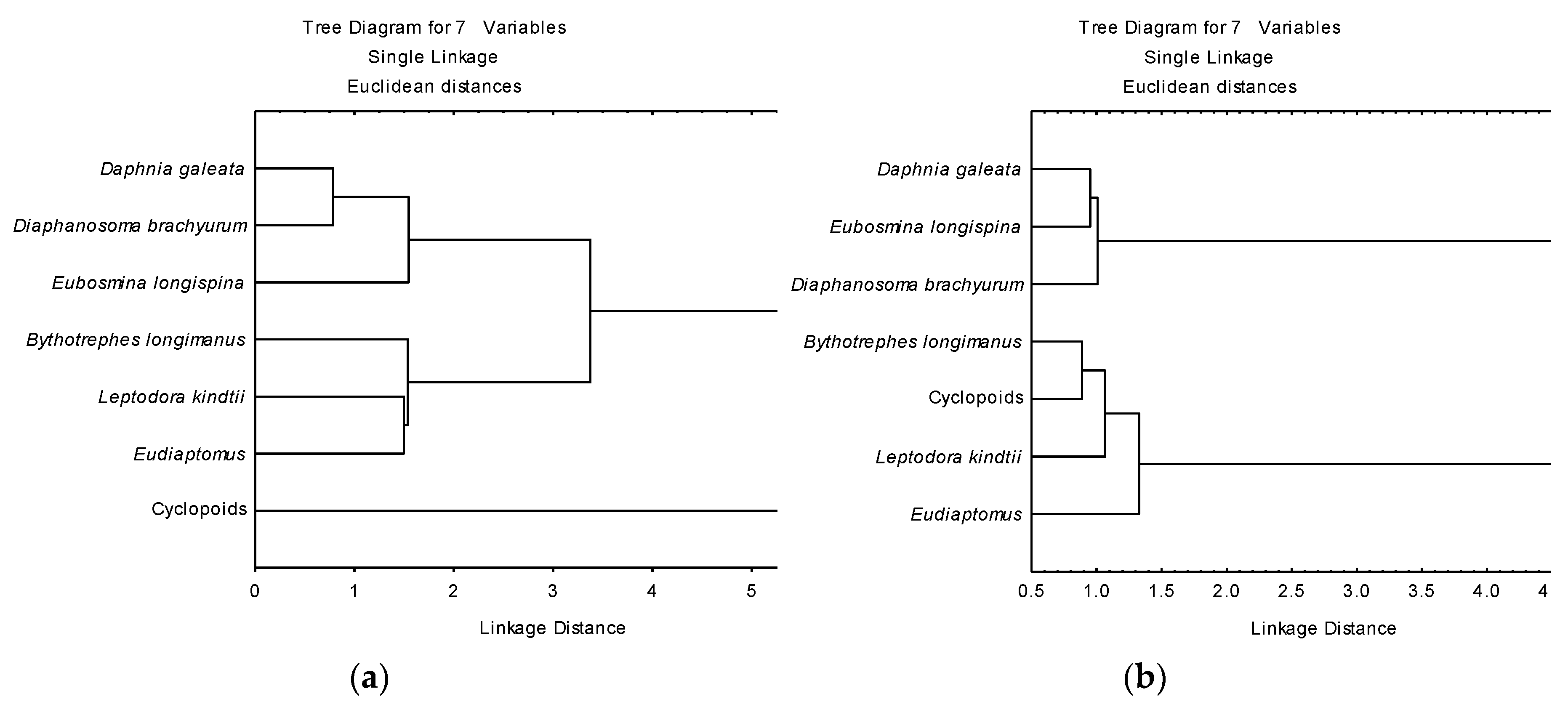
3.3. Determination of Functional Roles from δ13C and δ15N
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moss, B.R. Ecology of Fresh Waters: Man and Medium, Past to Future; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Barnett, A.J.; Finlay, K.; Beisner, B.E. Functional diversity of crustacean zooplankton communities: Towards a trait-based classification. Freshwat. Biol. 2007, 52, 796–813. [Google Scholar] [CrossRef]
- Dı́az, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Richie, P.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Walker, B.; Kinzig, A.; Langridge, J. Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosystems 1999, 2, 95–113. [Google Scholar] [CrossRef]
- Elton, C.S. Animal Ecology; University of Chicago Press: Chicago, IL, USA, 1927; p. 260. [Google Scholar]
- Leibold, M.A. The niche concept revisited: Mechanistic models and community context. Ecology 1995, 76, 1371–1382. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, M.A.; Villeger, S.; Mason, N.W.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Loseto, L.L.; Stern, G.A.; Ferguson, S.H. Size and biomagnification: How habitat selection explains beluga mercury levels. Environ. Sci. Technol. 2008, 42, 3982–3988. [Google Scholar] [CrossRef] [PubMed]
- Forest, A.; Sampei, M.; Makabe, R.; Sasaki, H.; Barber, D.G.; Gratton, Y.; Fortier, L. The annual cycle of particulate organic carbon export in Franklin Bay (Canadian Artica): Environmental control and food web implications. J. Geophys. Res. Oceans 2008, 113, C03S05. [Google Scholar]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Visconti, A.; Manca, M. Seasonal changes in the δ13C and δ15N signatures of the Lago Maggiore pelagic food web. J. Limnol. 2011, 70, 263–271. [Google Scholar] [CrossRef]
- Szepanski, M.M.; Ben-David, M.; Van Ballenberghe, V. Assessment of anadromous salmon resources in the diet of the Alexander Archipelago wolf using stable isotope analysis. Oecologia 1999, 120, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L. Mixing models in analyses of diet using multiple stable isotopes: A critique. Oecologia 2001, 127, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Gregg, J.W. Uncertainty in source partitioning using stable isotopes. Oecologia 2001, 127, 171–179. [Google Scholar] [CrossRef] [PubMed]
- De Bernardi, R.; Giussani, G.; Manca, M.; Ruggiu, D. Trophic status and the pelagic system in Lago Maggiore. Hydrobiologia 1990, 191, 1–8. [Google Scholar] [CrossRef]
- Guilizzoni, P.; Marchetto, A.; Lami, A.; Gerli, S.; Musazzi, S. Use of sedimentary pigments to infer past phosphorus concentration in lakes. J. Paleolimnol. 2011, 45, 433–445. [Google Scholar] [CrossRef]
- Manca, M.; Ruggiu, L. Consequences of pelagic food-web changes during a long-term lake oligotrophication process. Limnol. Oceanogr. 1998, 43, 1368–1373. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Matthews, B.; Mazumder, A. Temporal variation in body composition (C: N) helps explain seasonal patterns of zooplankton δ13C. Freshwat. Biol. 2005, 50, 502–515. [Google Scholar] [CrossRef]
- McConnaughey, T.; McRoy, C.P. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Mar. Biol. 1979, 53, 257–262. [Google Scholar] [CrossRef]
- Matthews, B.; Mazumder, A. Compositional and interlake variability of zooplankton effect baseline stable isotope signatures. Limnol. Oceanogr. 2003, 48, 1977–1987. [Google Scholar] [CrossRef]
- Kiljunen, M.; Grey, J.; Sinisalo, T.; Harrod, C.; Immonen, H.; Jones, R.I. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J. Appl. Ecol. 2006, 43, 1213–1222. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Dendrograms and measuring functional diversity. Oikos 2007, 116, 1422–1426. [Google Scholar] [CrossRef]
- Petchey, O.L.; Hector, A.; Gaston, K.J. How do different measures of functional diversity perform? Ecology 2004, 85, 847–857. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity–stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.; Papantoniou, G.; Edwards, F.; Lauridsen, R.B. Trophic trickles and cascades in a complex food web: Impacts of a keystone predator on stream community structure and ecosystem processes. Oikos 2008, 117, 683–692. [Google Scholar] [CrossRef]
- Petchey, O.L.; O’Gorman, E.J.; Flynn, D.F. A functional guide to functional diversity measures. In Biodiversity, Ecosystem Functioning, and Human Wellbeing. An Ecological and Economic Perspective; Oxford University Press: Oxford, UK, 2009; pp. 49–60. ISBN 9780199547951. [Google Scholar]
- Visconti, A.; Volta, P.; Fadda, A.; Di Guardo, A.; Manca, M. Seasonality, littoral versus pelagic carbon sources, and stepwise 15N-enrichment of pelagic food web in a deep subalpine lake: the role of planktivorous fish. Can. J. Fish. Aquat. Sci. 2013, 71, 436–446. [Google Scholar] [CrossRef]
- Poma, G.; Volta, P.; Roscioli, C.; Bettinetti, R.; Guzzella, L. Concentrations and trophic interactions of novel brominated flame retardants, HBCD, and PBDEs in zooplankton and fish from Lake Maggiore (Northern Italy). Sci. Total Environ. 2014, 481, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.F.; Bernasconi, S.M.; Barbieri, A.; Simona, M.; McKenzie, J.A. Interannual variation of the isotopic composition of sedimenting organic carbon and nitrogen in Lake Lugano: A long-term sediment trap study. Limnol. Oceanogr. 2004, 49, 839–849. [Google Scholar] [CrossRef]
- Caroni, R.; Free, G.; Visconti, A.; Manca, M. Phytoplankton functional traits and seston stable isotopes signature: A functional-based approach in a deep, subalpine lake, Lake Maggiore (N. Italy). J. Limnol. 2012, 71, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Grey, J.; Jones, R.I.; Sleep, D. Seasonal changes in the importance of the source of organic matter to the diet of zooplankton in Loch Ness, as indicated by stable isotope analysis. Limnol. Oceanogr. 2001, 46, 505–513. [Google Scholar] [CrossRef]
- Vuorio, K.; Meili, M.; Sarvala, J. Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshwat. Biol. 2006, 51, 807–822. [Google Scholar] [CrossRef]
- Morabito, G.; Sili, C.; Panzani, P.; Oggioni, A. Dinamica stagionale e distribuzione orizzontale di fitoplancton, carbonio organico e batterio plankton: Struttura dei popolamenti fitoplanctonici. In Ricerche Sull’evoluzione del Lago Maggiore. Aspetti Limnologici. Programma Quinquennale 2008–2012; Commissione Internazionale per la Protezione delle Acque Italo-Svizzere; ISSN 1013-8099. 2009; pp. 53–66. Available online: http://www.cipais.org/pdf/Limno_Maggiore_-_Rapporto_2009.pdf (accessed on 26 January 2018).
- Grey, J.; Jones, R.I.; Sleep, D. Stable isotope analysis of the origins of zooplankton carbon in lakes of differing trophic state. Oecologia 2000, 123, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.I.; Grey, J.; Sleep, D.; Quarmby, C. An assessment, using stable isotopes, of the importance of allochthonous organic carbon sources to the pelagic food web in Loch Ness. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 105–110. [Google Scholar] [CrossRef]
- Manca, M.; Vijverberg, J.; Polishchuk, L.; Voronov, D.A. Daphnia body size and population dynamics under predation by invertebrate and fish predators in Lago Maggiore: An approach based on contribution analysis. J. Limnol. 2008, 67, 15–21. [Google Scholar] [CrossRef]
- Matveev, V.F. Effect of Competition on the Demography of Planktonic Cladocerans: Daphnia and Diaphanosoma. Oecologia 1987, 74, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Brandl, Z. Freshwater copepods and rotifers: Predators and their prey. Hydrobiologia 2005, 546, 475–489. [Google Scholar] [CrossRef]
- Williamson, C.E.; Butler, N.M. Predation on rotifers by the suspension-feeding calanoid copepod Diaptomus pallidus. Limnol. Oceanogr. 1986, 31, 393–402. [Google Scholar] [CrossRef]
- Kling, G.W.; Fry, B.; O’Brien, W.J. Stable isotopes and planktonic trophic structure in arctic lakes. Ecology 1992, 73, 561–566. [Google Scholar] [CrossRef]
- Luecke, C.; O’Brien, W.J. The effect of Heterocope predation on zooplankton communities in arctic ponds. Limnol. Oceanogr. 1993, 28, 367–377. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Rasmussen, J.B. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 1999, 80, 1395–1404. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Chandra, S.; Park, S.K.; Vadeboncoeur, Y.; Goldman, C.R. Efficiencies of benthic and pelagic trophic pathways in a subalpine lake. Can. J. Fish. Aquat. Sci. 2006, 63, 2608–2620. [Google Scholar] [CrossRef]
- Cattaneo, A.; Manca, M.; Rasmussen, J.B. Peculiarities in the stable isotope composition of organisms from an alpine lake. Aquat. Sci. 2004, 66, 440–445. [Google Scholar] [CrossRef]
- Post, D.M.; Pace, M.L.; Hairston, N.G. Ecosystem size determines food-chain length in lakes. Nature 2002, 405, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Quay, P.D.; Emerson, S.R.; Quay, B.M.; Devol, A.H. The carbon cycle for Lake Washington—A stable isotope study. Limnol. Oceanogr. 1986, 31, 596–611. [Google Scholar] [CrossRef]
- Del Giorgio, P.A.; France, R.L. Ecosystem-specific patterns in the relationship between zooplankton and POM or microplankton δ13C. Limnol. Oceanogr. 1996, 41, 359–365. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Christoffersen, K.; Theil-Nielsen, J.; Jürgens, K. Cascading trophic interactions in the littoral zone: An enclosure experiment in shallow Lake Stigsholm, Denmark. Arch. Hydrobiol. 2002, 153, 533–555. [Google Scholar] [CrossRef]
- Barwick, M.; Maher, W. Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar. Environ. Res. 2003, 56, 471–502. [Google Scholar] [CrossRef]
- Paterson, M.J.; Rudd, J.W.; St. Louis, V. Increases in Total and Methylmercury in Zooplankton following Flooding of a Peatland Reservoir. Environ. Sci. Technol. 1998, 32, 3868–3874. [Google Scholar] [CrossRef]
- Connolly, J.P.; Pedersen, C.J. A thermodynamic-based evaluation of organic chemical accumulation in aquatic organisms. Environ. Sci. Technol. 1988, 22, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Campfens, J.; Mackay, D. Fugacity-based model of PCB bioaccumulation in complex aquatic food webs. Environ. Sci. Technol. 1997, 31, 577–583. [Google Scholar] [CrossRef]
- Morabito, G.; Mazzocchi, M.G.; Salmaso, N.; Zingone, A.; Bergami, C.; Flaim, G.; Accoroni, S.; Basset, A.; Bastianini, M.; Belmonte, G.; et al. Plankton dynamics across the freshwater, transitional and marine research sites of the LTER-Italy Network. Patterns, fluctuations, drivers. Sci. Total Environ. 2017. submitted. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visconti, A.; Caroni, R.; Rawcliffe, R.; Fadda, A.; Piscia, R.; Manca, M. Defining Seasonal Functional Traits of a Freshwater Zooplankton Community Using δ13C and δ15N Stable Isotope Analysis. Water 2018, 10, 108. https://doi.org/10.3390/w10020108
Visconti A, Caroni R, Rawcliffe R, Fadda A, Piscia R, Manca M. Defining Seasonal Functional Traits of a Freshwater Zooplankton Community Using δ13C and δ15N Stable Isotope Analysis. Water. 2018; 10(2):108. https://doi.org/10.3390/w10020108
Chicago/Turabian StyleVisconti, Anna, Rossana Caroni, Ruth Rawcliffe, Amedeo Fadda, Roberta Piscia, and Marina Manca. 2018. "Defining Seasonal Functional Traits of a Freshwater Zooplankton Community Using δ13C and δ15N Stable Isotope Analysis" Water 10, no. 2: 108. https://doi.org/10.3390/w10020108




