Impacts of Environmental Variables on a Phytoplankton Community: A Case Study of the Tributaries of a Subtropical River, Southern China
Abstract
:1. Introduction
2. Materials and Methods
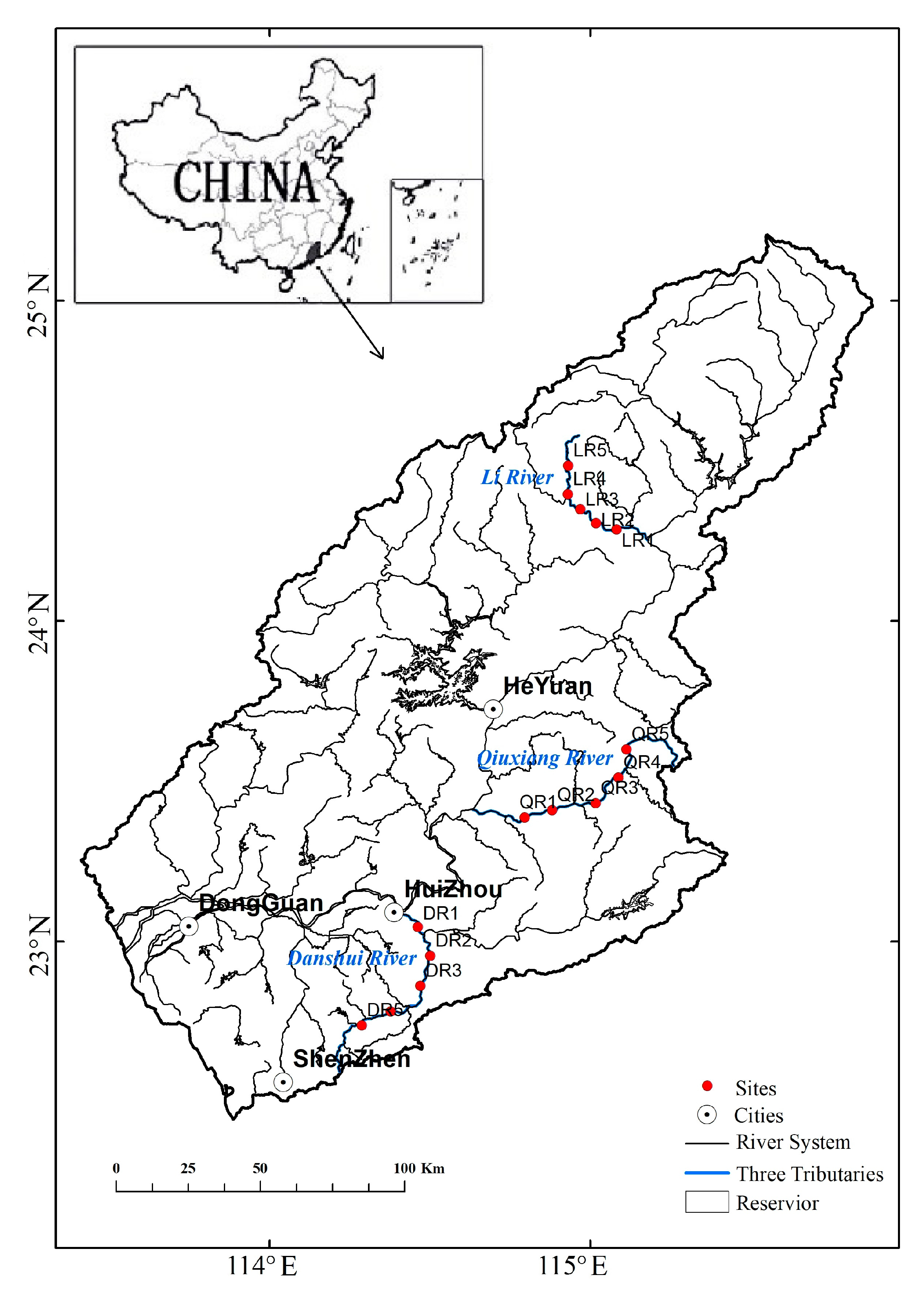
2.1. Study Area
2.2. Phytoplankton Sampling and Laboratory Procedures
2.3. Environmental Factors
2.4. Data Analysis
3. Results
3.1. Variations in Water Quality and Environmental Factors
3.2. Difference of Trophic State Index
3.3. Variations in the Phytoplankton Communities
3.3.1. Number of Taxa
3.3.2. Cell Abundance
3.3.3. Dominant Taxa
3.3.4. Community Structure Similarity
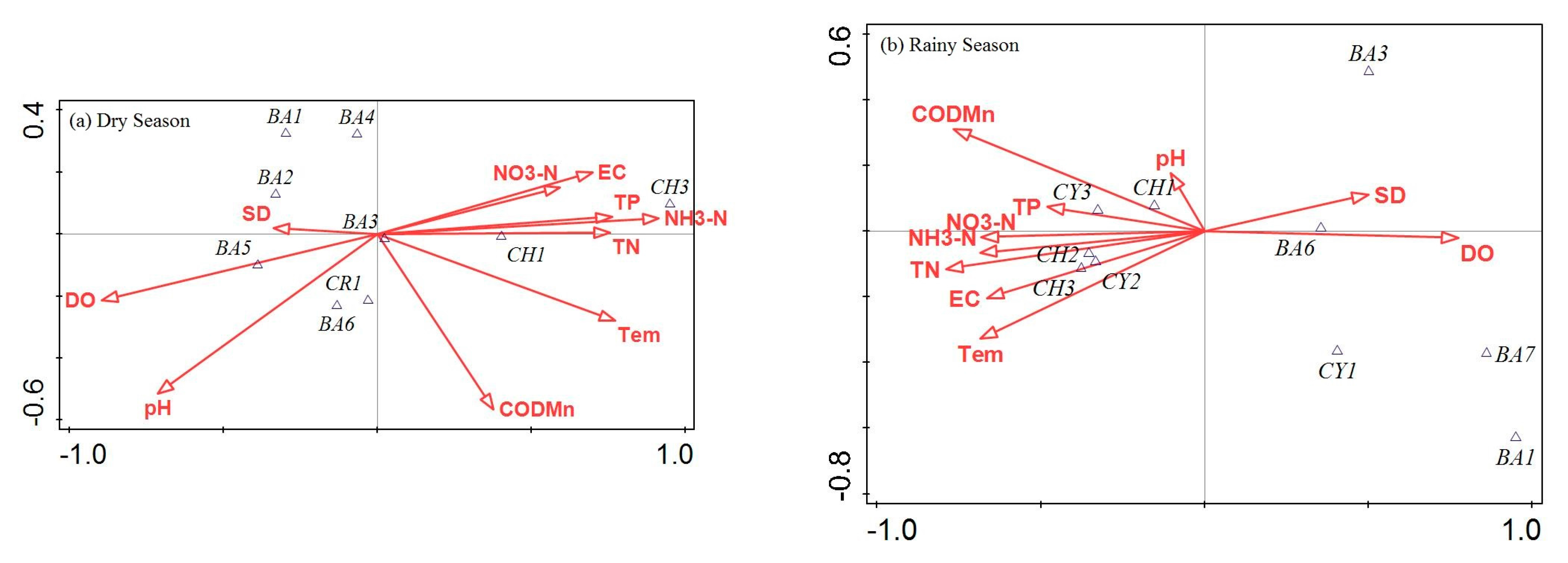
3.4. The Relationship between Environmental Factors and the Dominant Phytoplankton Taxa
4. Discussion
4.1. The Spatiotemporal Differences in Environmental Factors and Phytoplankton Community Structure
4.2. The Patterns of Variation of the Phytoplankton Communities in Rivers with Different Trophic States
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suikkanen, S.; Laamanen, M.; Huttunen, M. Long-term changes in summer phytoplankton communities of the open northern Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 71, 580–592. [Google Scholar] [CrossRef]
- Murkin, E.J.; Murkin, H.R.; Titman, R.D. Nektonic invertebrate abundance and distribution at the emergent vegetation-open water interface in the Delta Marsh, Manitoba, Canada. Wetlands 1992, 12, 45–52. [Google Scholar] [CrossRef]
- Fleming-Lehtinen, V.; Laamanen, M. Long-term changes in Secchi depth and the role of phytoplankton in explaining light attenuation in the Baltic Sea. Estuar. Coast. Shelf Sci. 2012, 102–103, 1–10. [Google Scholar] [CrossRef]
- Lundy, M.E.; Spencer, D.F.; Van Kessel, C.; Hill, J.E.; Linquist, B.A. Managing phosphorus fertilizer to reduce algae, maintain water quality, and sustain yields in water-seeded rice. Field Crop. Res. 2012, 131, 81–87. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of plankton in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- García de Emiliani, M.O. Seasonal succession of phytoplankton in a lake of the Paran River floodplain, Argentina. Hydrobiologia 1993, 264, 101–114. [Google Scholar] [CrossRef]
- De Senerpont Domis, L.N.; Elser, J.J.; Gsell, A.S.; Huszar, V.L.M.; Ibelings, B.W.; Jeppesen, E.; Kosten, S.; Mooij, W.M.; Roland, F.; Sommer, U.; et al. Plankton dynamics under different climate conditions in tropical freshwater systems (a reply to the comment by Sarmento, Amado & Descy, 2013). Freshw. Biol. 2013, 10, 2211–2213. [Google Scholar]
- Rosemond, A.D.; Mulholland, P.J.; Brawley, S.H. Seasonally shifting limitation of stream periphyton: Response of algal populations and assemblage biomass and productivity to variation in light, nutrients, and herbivores. Can. J. Fish. Aquat. Sci. 2000, 1, 66–75. [Google Scholar] [CrossRef]
- Badylak, S.; Phlips, E.J. Spatial and temporal patterns of phytoplankton composition in subtropical coastal lagoon, the Indian River Lagoon, Florida, USA. J. Plankton Res. 2004, 26, 1229–1247. [Google Scholar] [CrossRef]
- Xu, Y.; Li, A.J.; Qin, J.; Li, Q.; Ho, J.G.; Li, H. Seasonal patterns of water quality and phytoplankton dynamics in surface waters in Guangzhou and Foshan, China. Sci. Total Environ. 2017, 15, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.P.; Jiang, Y.; Xiong, X.; Dong, M.Y.; Wang, B. Characteristics of the spatial-temporal differences of land use changes in the Dongjiang River Basin from 1990 to 2009. Resour. Sci. 2011, 33, 143–152. (In Chinese) [Google Scholar]
- Ho, K.C.; Hui, K.C.C. Chemical contamination of the East River (Dongjiang) and its implication on sustainable development in the Pearl River Delta. Environ. Int. 2001, 5–6, 303–308. [Google Scholar] [CrossRef]
- Hong, H.C.; Liang, Y.; Han, B.P.; Mazumder, A.; Wong, M.H. Modeling of trihalomethane (THM) formation via chlorination of the water from Dongjiang River (source water for Hong Kong’s drinking water). Sci. Total Environ. 2007, 385, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, Y.; Liu, Q.; Hou, Z.J.; Liao, J.Y.; Fu, L.; Peng, Q.Z. Influences of the land use pattern on water quality in low-order streams of the Dongjiang River basin, China: A multi-scale analysis. Sci. Total Environ. 2016, 551–552, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xia, C.Y.; Xu, M.Y.; Guo, J.; Sun, G.P. Application of modified water quality indices as indicators to assess the spatial and temporal trends of water quality in the Dongjiang River. Ecol. Indic. 2016, 66, 306–312. [Google Scholar] [CrossRef]
- Tang, T.J.; Yang, S.; Yin, K.H.; Zou, R. Simulation of eutrophication in Shenzhen Reservoir based on EFDC model. J. Lake Sci. 2014, 26, 393–400. (In Chinese) [Google Scholar]
- Zhang, Y.; Dudgeon, D.; Cheng, D.; Thoe, W.; Fok, L.; Wang, Z.; Lee, J.H.W. Impacts of land use and water quality on macroinvertebrate communities in the Pearl River drainage basin, China. Hydrobiologia 2010, 652, 71–88. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, J.; Peng, S. Assessing the effects of landscape pattern on river water quality at multiple scales: A case study of the Dongjiang River watershed, China. Ecol. Indic. 2012, 23, 166–175. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, Y.; Ding, J.; Liu, Q.; Peng, Q.Z.; Kang, M.Y. Impacts of land use and environmental factors on macroinvertebrate functional feeding groups in the Dongjiang River basin, southeast China. J. Freshw. Ecol. 2016, 31, 21–35. [Google Scholar] [CrossRef]
- Jiang, Y; Wang, B.; Yang, H.C.; Liu, Q.R.; Zou, Y.L. Community structure of phytoplankton and its relation with water quality in Dongjiang River. Ecol. Environ. Sci. 2011, 20, 1700–1705. (In Chinese) [Google Scholar]
- Tian, S.M.; Yang, Y.; Qiao, Y.M.; He, W.X.; Lin, J.H.; Wang, D.Y. Temporal and spatial distribution of phytoplankton chlorophyll-a and its relationships with environmental factors in Dongjiang River, Pearl River basin. J. Lake Sci. 2015, 27, 31–37. (In Chinese) [Google Scholar]
- Wang, M.L.; Xia, J. Influences of land use changes and climatic vibration on water circulation in the East River Valley. Pearl River 2004, 25, 4–6. (In Chinese) [Google Scholar]
- Lv, L.T.; Peng, Q.Z.; Liao, J.Y.; Jiang, Y.; Kang, M.Y. Fluctuation and trends in precipitation and runoff in the Dongjiang River Basin over 50 years. Resour. Sci. 2013, 35, 514–520. (In Chinese) [Google Scholar]
- Ding, J.; Jiang, Y.; Fu, L.; Liu, Q.; Peng, Q.Z.; Kang, M.Y. Impacts of land use on surface water quality in a subtropical River Basin: A case study of the Dongjiang River Basin, Southeastern China. Water 2015, 8, 4427–4445. [Google Scholar] [CrossRef]
- Van Herpe, Y.; Troch, P.A. Spatial and temporal variations in surface water nitrate concentrations in a mixed land use catchment under humid temperate climatic conditions. Hydrol. Process. 2000, 14, 2439–2455. [Google Scholar] [CrossRef]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Weng, J.Z.; Xu, H.X. Atlas of Common Freshwater Algae in China; Shanghai Scientific and Technical Publishers: Shanghai, China, 2010. (In Chinese) [Google Scholar]
- National Environmental Protection Bureau. Standard Methods for the Examination of Water and Wastewater (Version 4); China Environmental Science Publish Press: Beijing, China, 2002. [Google Scholar]
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Wang, M.C.; Liu, X.Q.; Zhang, J.H. Evaluate method and classification standard on lake eutrophication. Environ. Monit. China 2002, 18, 47–49. (In Chinese) [Google Scholar]
- Liu, X.; Qian, K.; Chen, Y.; Gao, J. A comparison of factors influencing the summer phytoplankton biomass in China’s three largest freshwater lakes: Poyang, Dongting, and Taihu. Hydrobiologia 2017, 792, 283–302. [Google Scholar] [CrossRef]
- Pratt, B.; Chang, H. Effects of land cover, topography, and built structure on seasonal water quality at multiple spatial scales. J. Hazard. Mater. 2012, 209, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hively, W.D.; Hapeman, C.J.; McConnell, L.L.; Fisher, T.R.; Rice, C.P.; McCarty, G.W.; Sadeghi, A.M.; Whitall, D.R.; Downey, P.; Nino de Guzman, G.T.; et al. Relating nutrient and herbicide fate with landscape features and characteristics of 15 subwatersheds in the Choptank River watershed. Sci. Total Environ. 2011, 409, 3866–3878. [Google Scholar] [CrossRef] [PubMed]
- Braak, T.C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Tilman, G.D. Plant dominance along an experimental nutrient gradient. Ecology 1984, 65, 1445–1453. [Google Scholar] [CrossRef]
- Varol, M.; Şen, B. Assessment of surface water quality using multivariate statistical techniques: A case study of Behrimaz Stream, Turkey. Environ. Monit. Assess. 2009, 159, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Tilman, G.D.; Kilham, S.S. Phosphate and silicate growth and uptake kinetics of the diatoms Asterionella formosa and Cyclotella meneghiniana in batch and semicontinuous culture. J. Phycol. 1976, 12, 375–383. [Google Scholar] [CrossRef]
- Washington, H.G. Diversity, biotic and similarity indices: A review with special relevance to aquatic ecosystems. Water Res. 1984, 18, 653–694. [Google Scholar] [CrossRef]
- Dudgeon, D. Endangered ecosystems: A review of the conservation status of tropical Asian rivers. Hydrobiologia 1992, 3, 167–191. [Google Scholar] [CrossRef]
- Sandgren, C.D. Growth and Reproductive Strategies of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Negro, A.I.; Dehoyos, C.; Vega, J.C. Phytoplankton structure and dynamics in Lake Sanabria and Valpaiso Reservoir (NW Spain). Hydrobiologia 2000, 424, 25–37. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Oliver, R.L.; Walsby, A.E. Cyanobacterial dominance: The role of buoyancy regulation in dynamic lake environments. N. Z. J. Mar. Freshw. Res. 1987, 21, 379–390. [Google Scholar] [CrossRef]
- Song, X.L.; Liu, Z.W.; Pan, H.K.; Yang, G.J.; Chen, Y.W. Phytoplankton community structure in Meiliang Bay and Lake Wuli of Lake Taihu. J. Lake Sci. 2007, 19, 643–651. (In Chinese) [Google Scholar]
- Jensen, J.P.; Jeppesenk, E.; Olrik, K.; Kristensen, P. Impact of nutrients and physical factors on the shift Cyanobacteria to in shallow Danish lakes. J. Fish. Aquat. Sci. 1994, 51, 1692–1699. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zhang, Z.S.; Gong, J.X. New Technology of Micro Biological Monitoring; China Architecture & Building Press: Beijing, China, 1991. (In Chinese) [Google Scholar]


| Environmental Factors | Li River | Qiuxiang River | Danshui River | |||
|---|---|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | Dry Season | Rainy Season | |
| pH | 7.11 ± 0.14 a | 7.20 ± 0.33 a | 7.26 ± 0.25 a | 7.26 ± 0.47 a | 6.88 ± 0.23 a | 7.20 ± 0.64 a |
| TEM (°C) | 15.27 ± 2.58 b | 29.70 ± 1.82 a | 18.08 ± 1.38 b | 30.26 ± 1.81 a | 20.54 ± 2.53 b | 31.40 ± 1.46 a |
| DO (mg·L−1) | 8.32 ± 0.98 a | 6.51 ± 0.31 b | 7.49 ± 0.99 a | 5.49 ± 0.45 b | 4.95 ± 0.82 a | 3.17 ± 0.66 a |
| EC (μg·cm−1) | 93.12 ± 21.08 a | 88.27 ± 16.03 a | 81.65 ± 20.50 a | 87.87 ± 33.48 a | 572.6 ± 133.65 a | 372.07 ± 46.65 b |
| CODMn (mg·L−1) | 2.13 ± 0.91 a | 2.22 ± 0.72 a | 3.45 ± 0.36 a | 3.01 ± 0.96 a | 4.54 ± 0.62 a | 4.38 ± 1.18 a |
| TP (mg·L−1) | 0.08 ± 0.04 a | 0.12 ± 0.02 a | 0.11 ± 0.06 a | 0.11 ± 0.08 a | 0.65 ± 0.03 a | 0.33 ± 0.10 a |
| TN (mg·L−1) | 3.01 ± 1.13 a | 1.28 ± 0.88 a | 1.24 ± 0.67 a | 1.84 ± 0.18 a | 10.41 ± 1.18 a | 8.46 ± 1.68 b |
| NH3-N (mg·L−1) | 0.65 ± 0.47 a | 0.75 ± 0.66 a | 0.38 ± 0.19 a | 0.27 ± 0.10 a | 5.00 ± 1.95 a | 3.68 ± 1.53 a |
| NO3-N (mg·L−1) | 1.95 ± 0.41 a | 0.64 ± 0.16 a | 0.70 ± 0.39 a | 1.06 ± 0.12 a | 5.11 ± 1.55 a | 3.80 ± 1.38 a |
| SD (m) | 0.37 ± 0.26 a | 0.31 ± 0.25 a | 0.30 ± 0.22 a | 0.44 ± 0.28 a | 0.37 ± 0.33 a | 0.32 ± 0.12 a |
| Li River | Qiuxiang River | Danshui River | |
|---|---|---|---|
| Dry Season (TLI) | 44.99 ± 3.75 c | 53.83 ± 1.61 b | 66.72 ± 2.43 a |
| Rainy Season (TLI) | 46.68 ± 2.30 c | 53.73 ± 1.87 b | 64.54 ± 1.98 a |
| Trophic Level | Mesotrophic | Lightly eutrophic | Moderately eutrophic |
| Renumber | Dominant Taxa | Li River (Dry) | Li River (Rainy) | Qiuxiang River (Dry) | Qiuxiang River (Rainy) | Danshui River (Dry) | Danshui River (Rainy) |
|---|---|---|---|---|---|---|---|
| BA1 | Melosira varians | 0.074 | 0.072 | 0.026 | — | — | — |
| BA2 | Aulacoseira granulata | 0.039 | — | — | — | — | — |
| BA3 | Nitzschia spp. | 0.065 | 0.033 | 0.049 | 0.101 | 0.057 | — |
| BA4 | Cyclotella sp. | 0.021 | — | — | — | — | |
| BA5 | Gomphonema spp. | 0.030 | — | 0.031 | — | — | — |
| BA6 | Navicula spp. | 0.038 | 0.087 | 0.114 | 0.081 | 0.053 | — |
| BA7 | Synedra amphicephala | — | 0.044 | — | — | — | — |
| CH1 | Scenedesmus quadricanda | — | 0.025 | 0.024 | 0.088 | 0.036 | 0.076 |
| CH2 | Scenedesmus dimorphus | — | — | — | 0.026 | — | 0.063 |
| CH3 | Crucigenia quadrata | — | — | — | — | 0.058 | 0.064 |
| CY1 | Dolichospermum sp. | — | 0.083 | — | — | — | — |
| CY2 | Merismopedia sp. | — | — | — | 0.078 | — | 0.199 |
| CY3 | Oscillatoriales sp. | — | — | — | 0.060 | — | 0.048 |
| CR1 | Cryptomonas ovata | — | — | 0.097 | — | — | — |
| Among Tributaries | Dry Season | Rainy Season | ||
|---|---|---|---|---|
| R Statistic | p | R Statistic | p | |
| (Global R = 0.202, p = 0.001) | (Global R = 0.478, p = 0.001) | |||
| Li River vs. Qiuxiang River | 0.019 | 0.302 | 0.263 | 0.001 |
| Li River vs. Danshui River | 0.392 | 0.001 | 0.749 | 0.001 |
| Qiuxiang River vs. Danshui River | 0.208 | 0.001 | 0.392 | 0.001 |
| Axes | Dry Season | Rainy Season | ||||
|---|---|---|---|---|---|---|
| Explained Variation (%) | pseudo-F | p Value | Explained Variation (%) | pseudo-F | p Value | |
| First axis | 23.65 | 4.0 | 0.008 ** | 25.42 | 9.2 | 0.002 ** |
| Second axis | 8.35 | 1.6 | 0.934 | 9.38 | 3.9 | 0.208 |
| All axes | 51.2 | 1.4 | 0.040 * | 46.0 | 2.3 | 0.002 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Jiang, Y.; Liu, Q.; Tian, Y.; He, K.; Fu, L. Impacts of Environmental Variables on a Phytoplankton Community: A Case Study of the Tributaries of a Subtropical River, Southern China. Water 2018, 10, 152. https://doi.org/10.3390/w10020152
Hou Z, Jiang Y, Liu Q, Tian Y, He K, Fu L. Impacts of Environmental Variables on a Phytoplankton Community: A Case Study of the Tributaries of a Subtropical River, Southern China. Water. 2018; 10(2):152. https://doi.org/10.3390/w10020152
Chicago/Turabian StyleHou, Zhaojiang, Yuan Jiang, Qi Liu, Yulu Tian, Kejian He, and Lan Fu. 2018. "Impacts of Environmental Variables on a Phytoplankton Community: A Case Study of the Tributaries of a Subtropical River, Southern China" Water 10, no. 2: 152. https://doi.org/10.3390/w10020152





