Effective Removal of Lead Ions from Aqueous Solution Using Nano Illite/Smectite Clay: Isotherm, Kinetic, and Thermodynamic Modeling of Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nano I-Sm
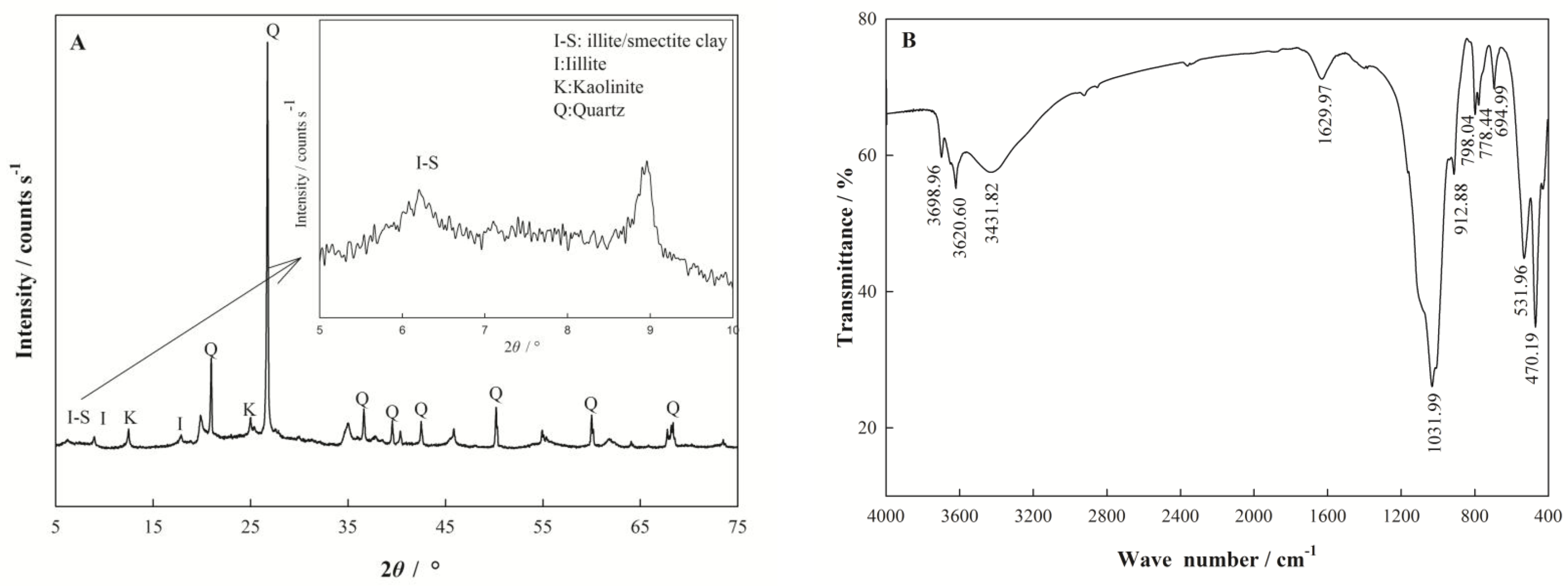
2.2. Characterization of Nano I-Sm
2.3. Batch Adsorption Experiments
2.4. Theoretical Model
2.4.1. Adsorption Kinetics Model
2.4.2. Adsorption Equilibrium
2.4.3. Adsorption Thermodynamics
3. Results
3.1. Characterization of Nano I-Sm
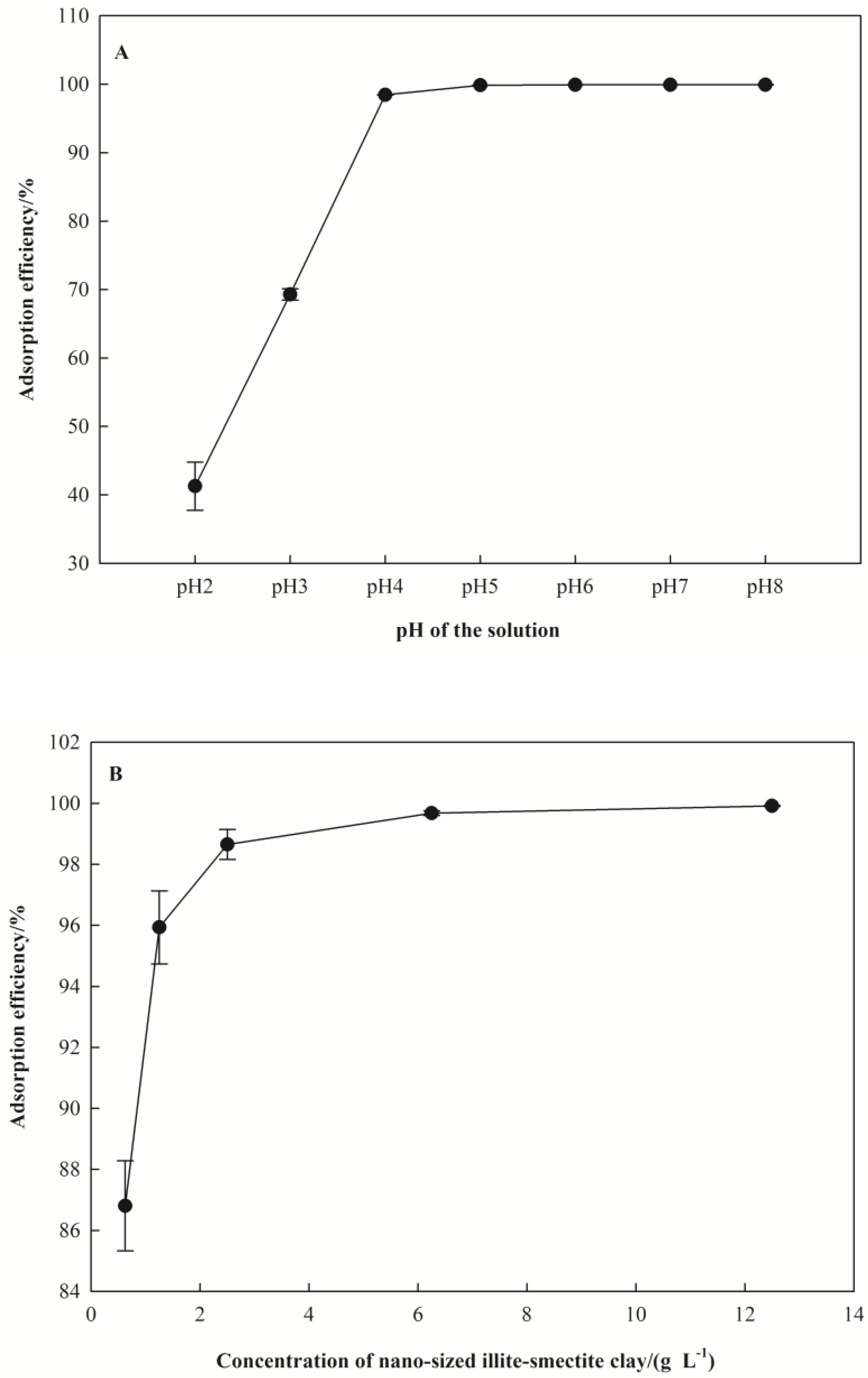
3.2. Effect of Adsorption Conditions
3.2.1. Effect of pH
3.2.2. Effect of Nano I-Sm Dosage
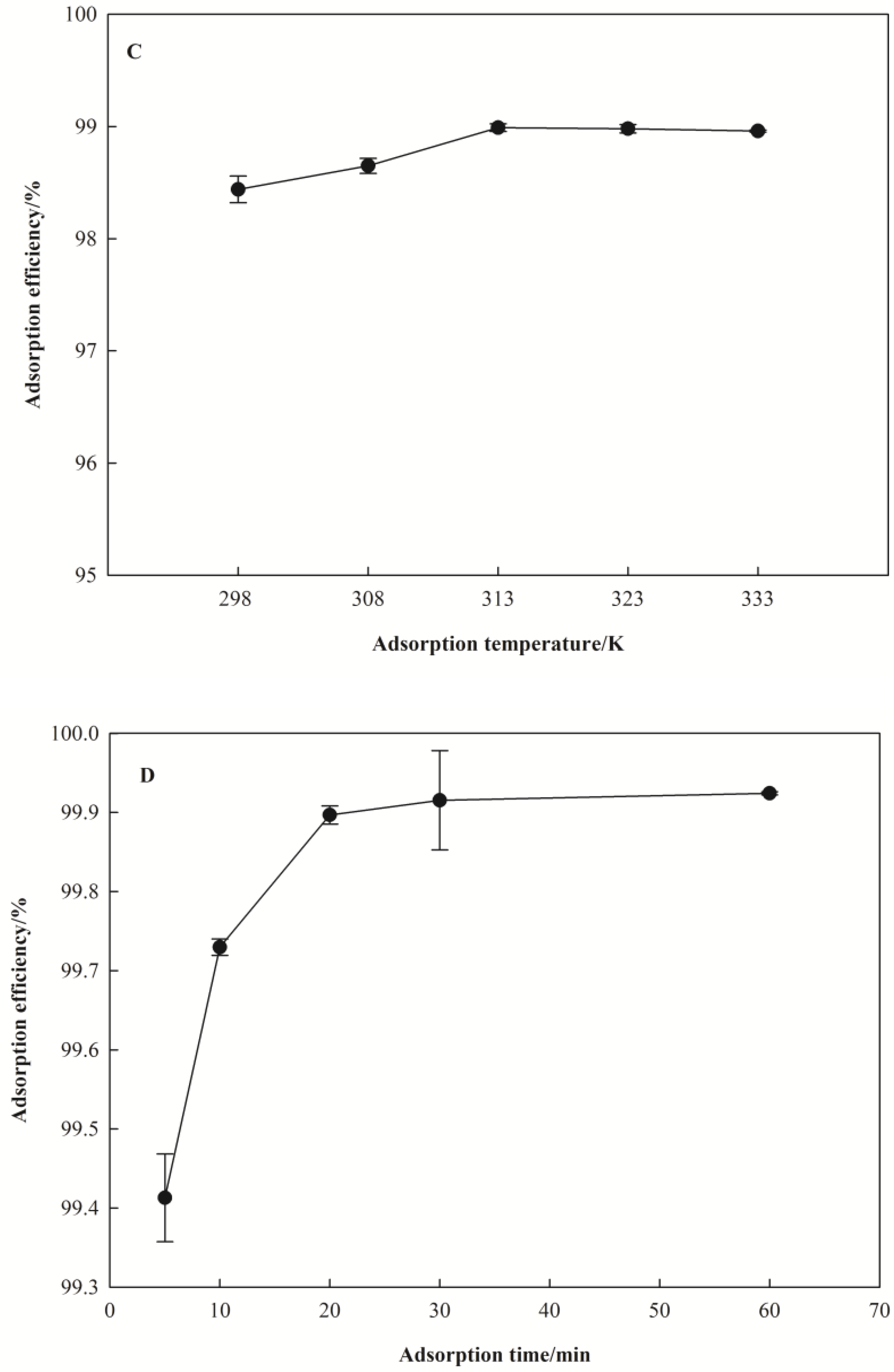
3.2.3. Effect of Adsorption Temperature
3.2.4. Effect of Adsorption Time
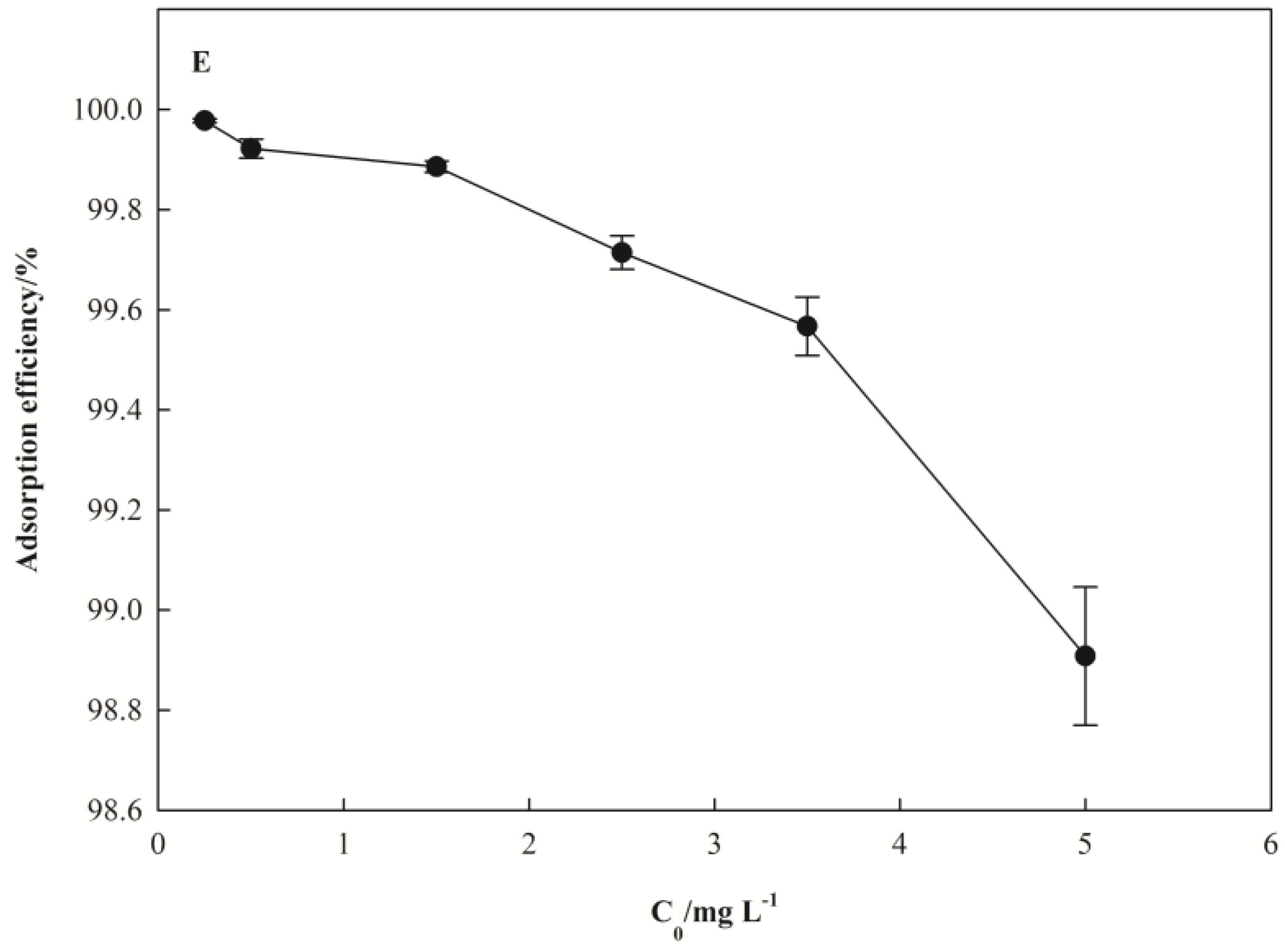
3.2.5. Effect of Initial Concentration of Pb(II)
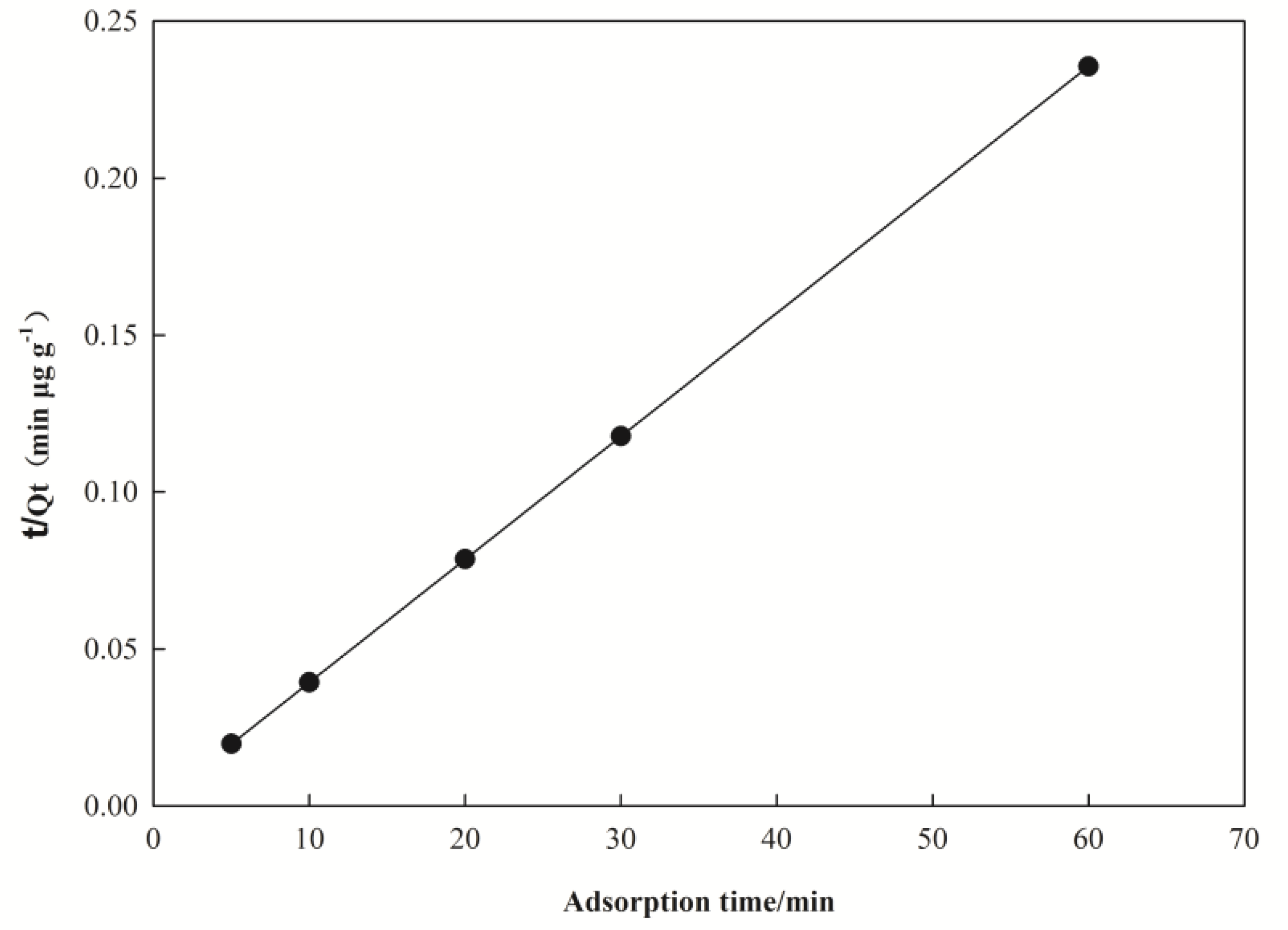
3.3. Kinetic Parameters of the Adsorption
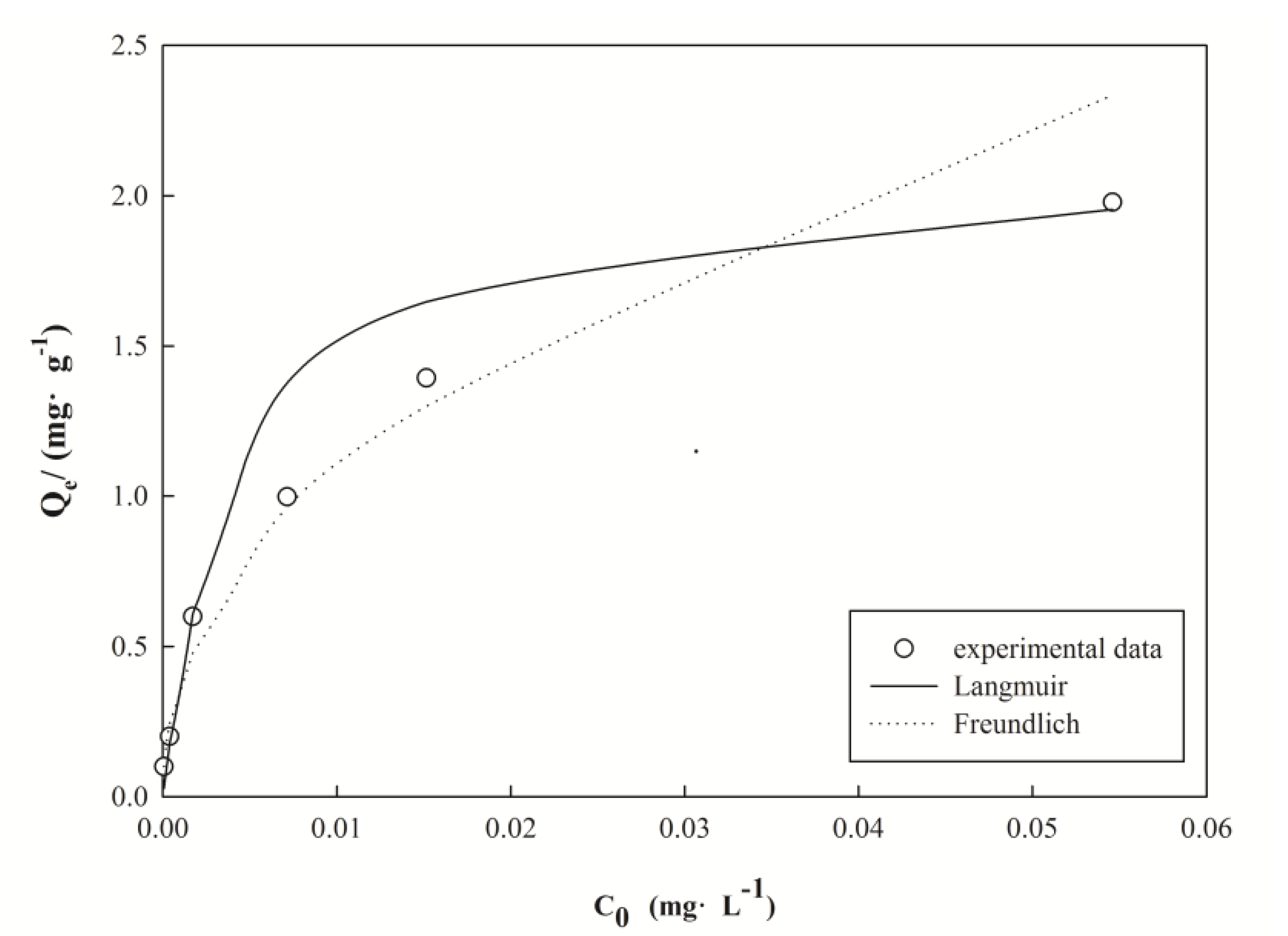
3.4. Equilibrium Parameters of the Adsorption
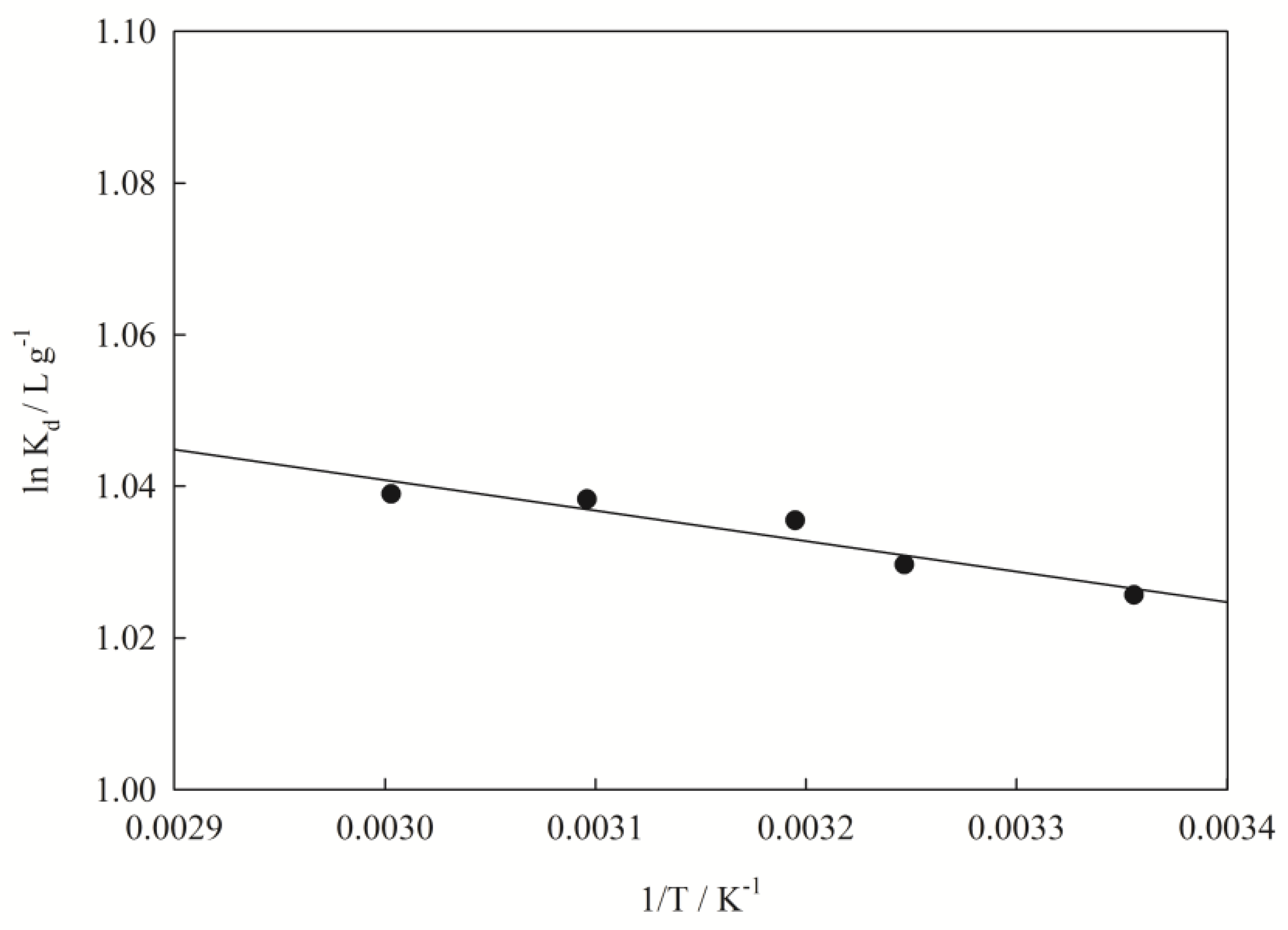
3.5. Thermodynamic Parameters of the Adsorption
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dipak, P. Research on heavy metal pollution of river Ganga: A review. Ann. Agrar. Sci. 2017, 15, 278–286. [Google Scholar] [CrossRef]
- IslamabMd, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Mamun, M.H.-A.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Xie, X.J.; Wang, F.Y.; Wang, G.J.; Mei, R.W.; Wang, C.Z. Study on Heavy Metal Pollution in Surface Water in China. Envron. Sci. Manag. 2017, 42, 31–34. [Google Scholar] [CrossRef]
- Zhang, X.W.; Yang, L.S.; Li, Y.H.; Li, H.R.; Wang, W.Y.; Ye, B.X. Impacts of lead /zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Ma, Z.W.; Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Gupta, V.K. Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Zhao, D.D.; Yu, Y.; Chen, P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res. 2016, 101, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Vergili, I.; Gönder, Z.B.; Kaya, Y.; Gürdağ, G.; Çavuş, S. Sorption of Pb (II) from battery industry wastewater using a weak acid cation exchange resin. Process Saf. Environ. Prot. 2017, 107, 498–507. [Google Scholar] [CrossRef]
- Milind, M.N.; Santosh, K.D. Lead resistant bacteria: Lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotox. Environ. Saf. 2013, 98, 1–7. [Google Scholar] [CrossRef]
- Abreham, T.B.; Abaynesh, Y.G.; Ramato, A.T.; Dawit, N.; Efrem, C.; Lidietta, G. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: A review. J. Environ. Chem. Eng. 2017, 5, 2395–2414. [Google Scholar] [CrossRef]
- Zhao, G.X.; Li, J.X.; Ren, X.M.; Chen, C.L.; Wang, X.K. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.U. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, W.M. Simultaneous removal of metal species from acidic aqueous solutions using cryptocrystalline magnesite/bentonite clay composite: An experimental and modelling approach. J. Clean. Prod. 2016, 112, 1077–1085. [Google Scholar] [CrossRef]
- Joziane, G.M.; Murilo, P.M.; Thirugnanasambandham, K.; Sergio, H.B.F.; Marcelino, L.G.; Maria, A.S.D.B.; Sivakumar, V. Preparation and characterization of calcium treated bentonite clay and its application for the removal of lead and cadmium ions: Adsorption and thermodynamic modeling. Process Saf. Environ. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Cd(II) adsorption from aqueous solution by raw and modified kaolinite. Appl. Clay Sci. 2014, 88–89, 63–72. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; ALOthman, Z.A.; Abdel-Khalek, A.A.; Tawfeek, A.M. Characterization of reactive amphiphilic montmorillonite nanogels and its application for removal of toxic cationic dye and heavy metals water pollutants. J. Ind. Eng. Chem. 2015, 31, 374–384. [Google Scholar] [CrossRef]
- Qiu, J.Y. Preparation of Illite/Smectite Clay Nano-Powder and Its Application as Rubber Filler. Master’s Thesis, South China University of Technology, Guangzhou, China, 4 June 2014. [Google Scholar]
- Yuan, S.S.; Li, Z.Y.; Pan, Z.D.; Wang, Y.M. Removal of Copper and Cadmium Ions in Aqueous Solution via Adsorption by Nano-sized Illite-Smectite Clay. J. Chin. Ceram. Soc. 2016, 44, 43–49. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yuan, Y.H.; Yan, Z.G.; Zhou, Y.Y.; Zhang, C.Y.; Huang, Y.; Xu, M. Application of nano illite/smectite clay for adsorptive removal of metals in water. Res. Environ. Sci. 2016, 29, 115–123. [Google Scholar] [CrossRef]
- Sakthivel, S.; Venkatesan, V.; Krishnan, B.; Pitchumani, B. Influence of suspension stability on wet grinding for production of mineral nanoparticles. Particuology 2008, 6, 120–124. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 152–176. ISBN 9787109066441. [Google Scholar]
- Dawodu, F.A.; Akpomie, K.G. Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution unto a Nigerian kaolinite. J. Mater. Res. Technol. 2014, 3, 129–141. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe (about the theory of so-called adsorption of soluble substances); Kungliga Svenska Vetenskapsakademiens (Royal Swedish Academy of Sciences): Stockholm, Sweden, 1898; pp. 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Wang, Q.P.; Jin, X.Y.; Chen, Z.L. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Olgun, A.; Atar, N. Equilibrium, thermodynamic and kinetic studies for the adsorption of lead (II) and nickel (II) onto clay mixture containing boron impurity. J. Ind. Eng. Chem. 2012, 18, 1751–1757. [Google Scholar] [CrossRef]
- Campo, M.D.; Bauluz, B.; Nieto, F.; Papa, C.D.; Hongn, F. SEM and TEM evidence of mixed-layer illite-smectite formed by dissolution- crystallization processes in continental Paleogene sequences in northwestern Argentina. Clay Miner. 2016, 51, 723–740. [Google Scholar] [CrossRef]
- Li, Y.J.; Zeng, L.; Zhou, Y.; Wang, T.F.; Zhang, Y.J. Preparation and Characterization of Montmorillonite Intercalation Compounds with Quaternary Ammonium Surfactant: Adsorption Effect of Zearalenone. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Deng, L.L.; Yuan, P.; Liu, D.; Liu, Z.W. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Krishna, G.B.; Susmita, S.G. Pb(II) uptake by kaolinite and montmorillonite in aqueous medium: Influence of acid activation of the clays. Colloid Surf. A 2006, 277, 191–200. [Google Scholar] [CrossRef]
- Nir, S.; Undabeytia, T.; Dana, Y.; Yasser, E.; Polubesova, T.; Serban, C.; Rytwo, G.; Lagaly, G.; Rubin, B. Optimization of adsorption of hydrophobic herbicides on montmorillonite presorbed by monovalent organic cations: Interaction between phenyl rings. Environ. Sci. Technol. 2000, 34, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Li, J.S.; Xue, Q.; Wang, P.; Li, Z.Z. Effect of lead (II) on the mechanical behavior and microstructure development of a Chinese clay. Appl. Clay Sci. 2015, 105–106, 192–199. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| SiO2 (wt %) | 64.29 |
| Al2O3 (wt %) | 20.38 |
| Fe2O3 (wt %) | 2.95 |
| K2O (wt %) | 2.74 |
| MgO (wt %) | 1.82 |
| TiO2 (wt %) | 0.82 |
| Na2O (wt %) | 0.19 |
| Loss of ignition (wt %) | 6.46 |
| BET surface area (m2∙g−1) | 39.46 |
| Micropore area (m2∙g−1) | 10.46 |
| External surface area (m2∙g−1) | 28.99 |
| Total pore volume (cm3∙g−1) | 0.011 |
| Micropore volume (cm3∙g−1) | 0.0055 |
| Adsorption average pore diameter (4 V/A by BET) | 1.07 |
| CEC (meg/100 g) | 2.11 |
| Slurry pH | 6.75 |
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Experimental Data | ||||
|---|---|---|---|---|---|---|
| k1/min−1 | Qe/(μg·g−1) | R2 | K2/(μg·g−1·min−1) | Qe/ (μg·g−1) | R2 | Qe/(μg·g−1) |
| 0.380 | 2.603 | 0.985 | 0.251 | 256.410 | 1.000 | 254.680 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Qmax/(mg∙g−1) | KL/(L∙μg−1) | R2 | Kf/(μg·g−1) | 1/n | R2 |
| 2.104 | 0.216 | 0.985 | 8.825 | 0.457 | 0.980 |
| ΔS | ΔH | ΔG/(kJ·mol−1) | ||||
|---|---|---|---|---|---|---|
| J/mol−1·K−1 | kJ·mol−1 | 298 K | 308 K | 313 K | 323 K | 333 K |
| 9.658 | 4.844 | −2.541 | −2.637 | −2.695 | −2.788 | −2.876 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Deng, C.; Yu, Z.; Wang, X.; Xu, G. Effective Removal of Lead Ions from Aqueous Solution Using Nano Illite/Smectite Clay: Isotherm, Kinetic, and Thermodynamic Modeling of Adsorption. Water 2018, 10, 210. https://doi.org/10.3390/w10020210
Yin J, Deng C, Yu Z, Wang X, Xu G. Effective Removal of Lead Ions from Aqueous Solution Using Nano Illite/Smectite Clay: Isotherm, Kinetic, and Thermodynamic Modeling of Adsorption. Water. 2018; 10(2):210. https://doi.org/10.3390/w10020210
Chicago/Turabian StyleYin, Juan, Chaobing Deng, Zhen Yu, Xiaofei Wang, and Guiping Xu. 2018. "Effective Removal of Lead Ions from Aqueous Solution Using Nano Illite/Smectite Clay: Isotherm, Kinetic, and Thermodynamic Modeling of Adsorption" Water 10, no. 2: 210. https://doi.org/10.3390/w10020210




