Degradation of 4-Chlorophenol by Microwave-Enhanced Advanced Oxidation Processes: Kinetics and Influential Process Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Set-Up
2.2.1. Microwave Reactor System
2.2.2. Kinetic Evaluation
2.2.3. Design of Experiments
2.3. Analytical Techniques
2.4. Principal Component Analysis (PCA) and Partial Least Square Regression (PLS)
3. Results
3.1. Preliminary Tests
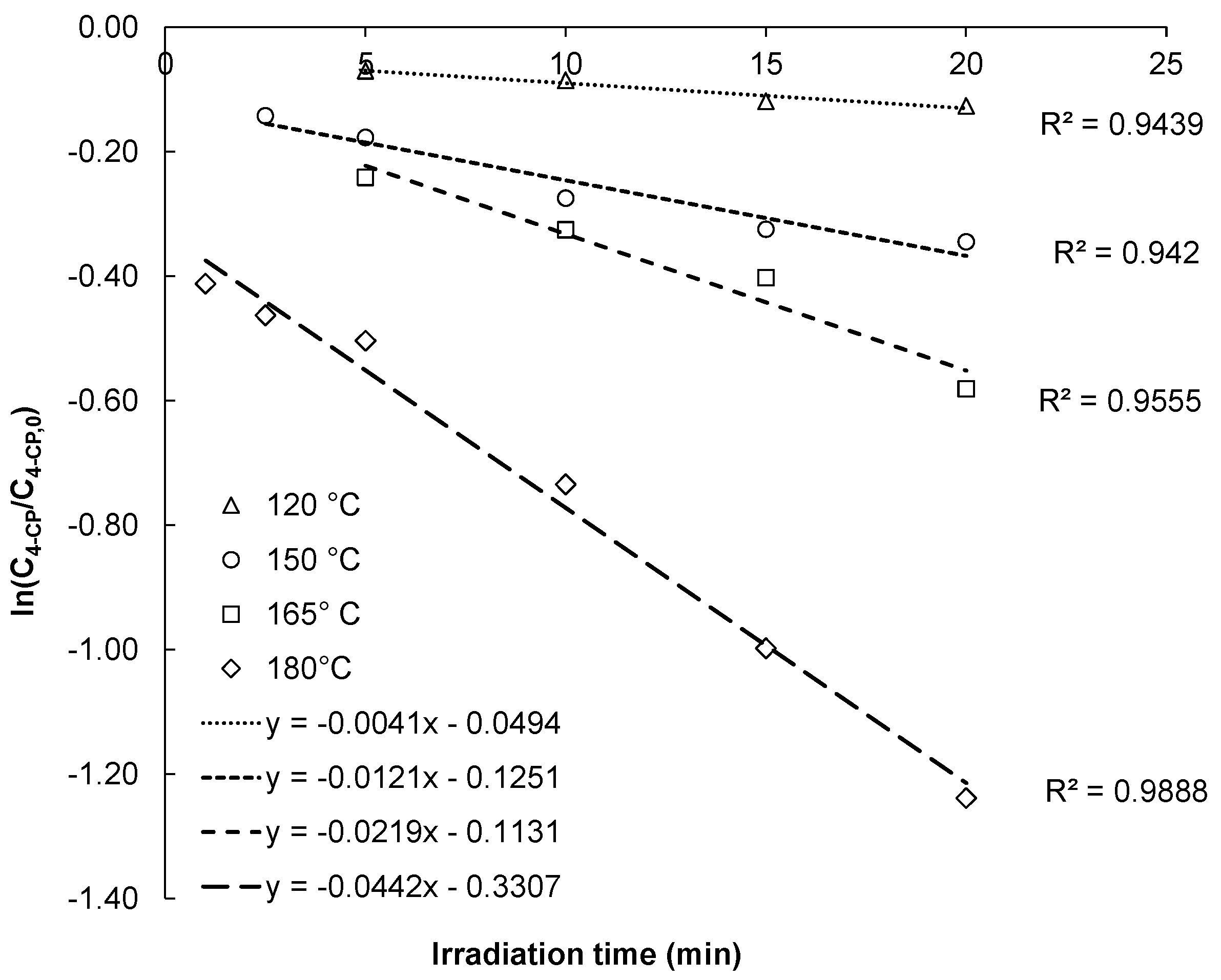
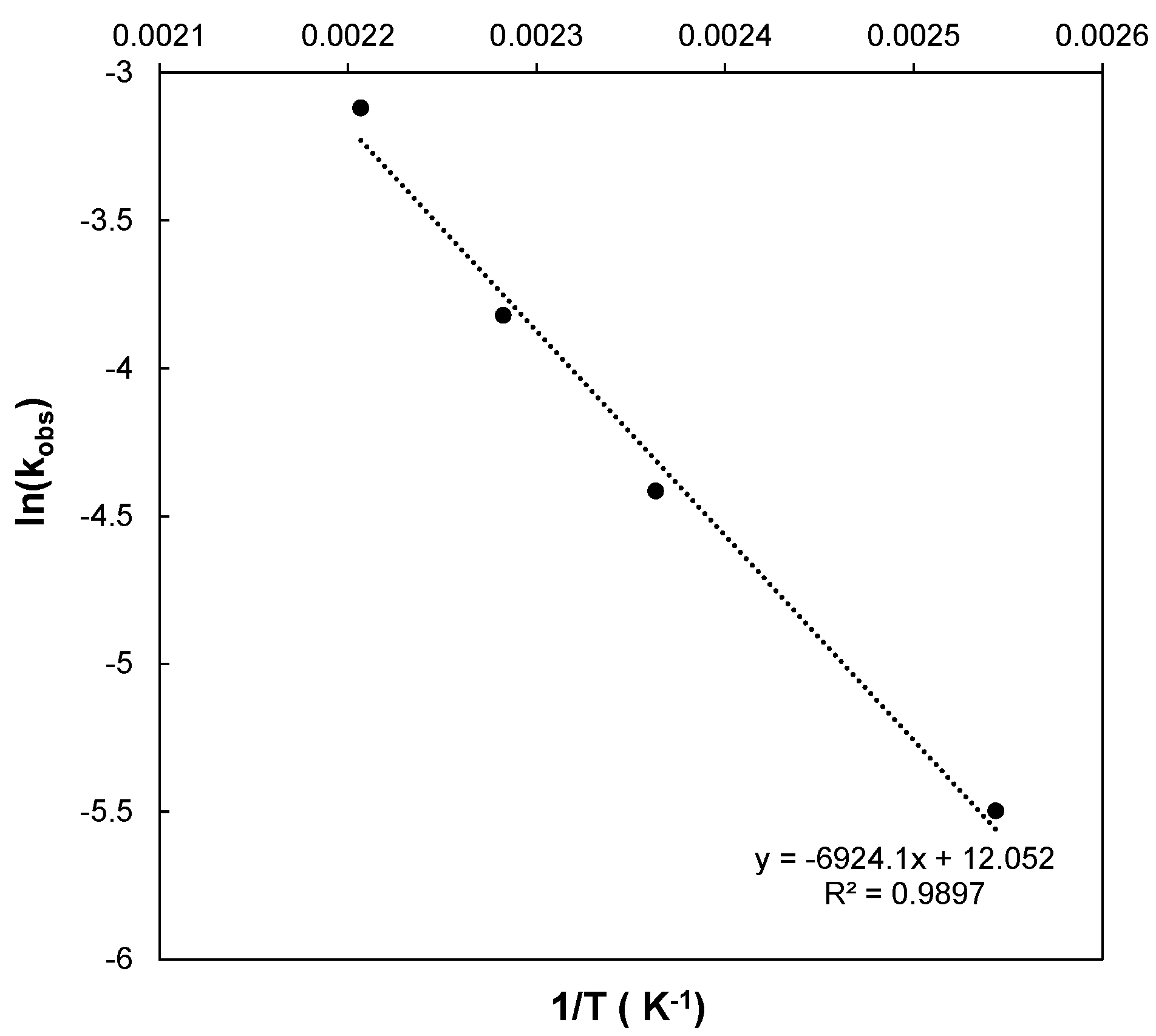
3.2. Kinetics of the MW–H2O2-Process
3.3. Influence of Process Parameters
3.4. Modeling of the MW–H2O2 Process
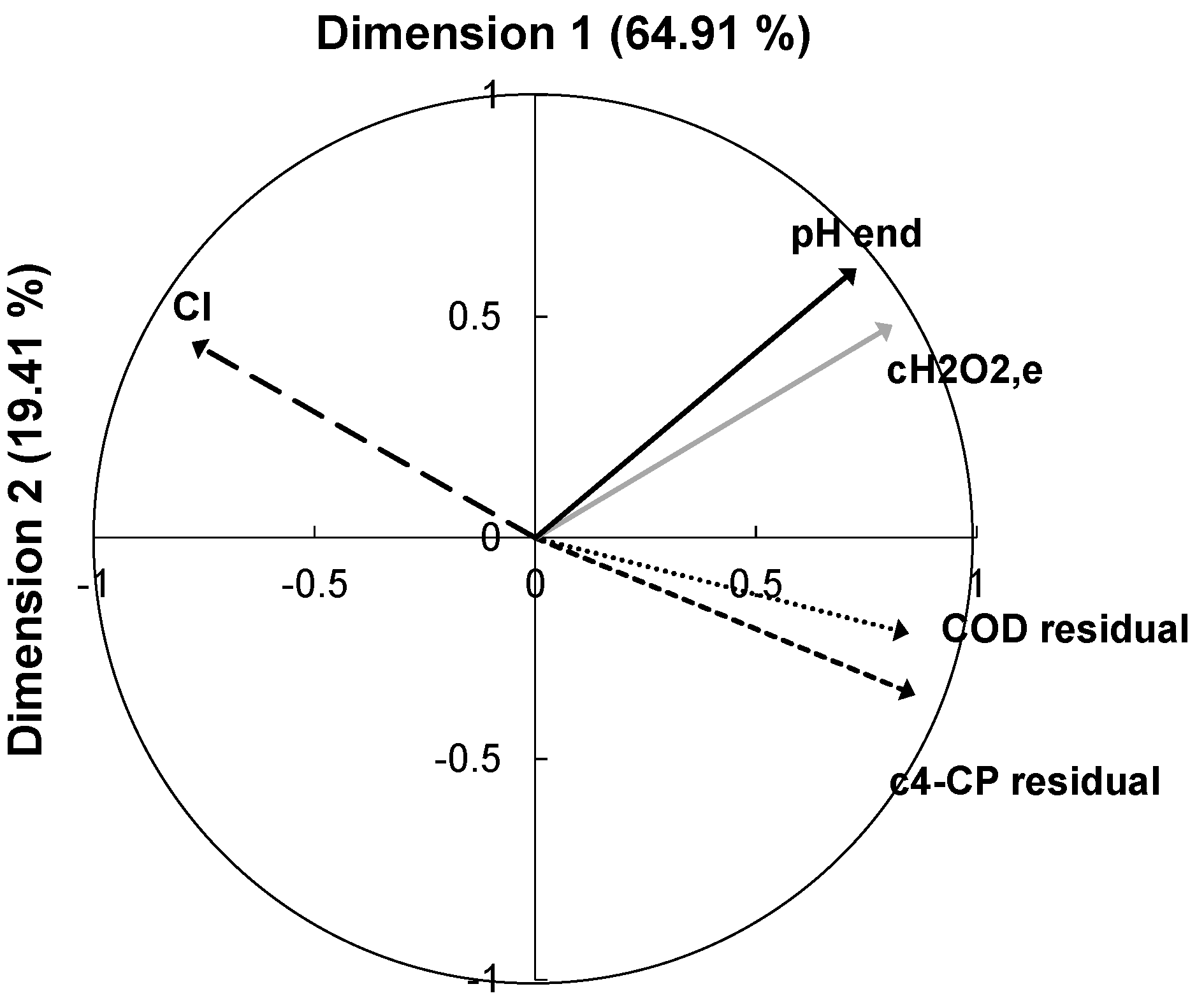
3.4.1. Obtaining the PLS Model
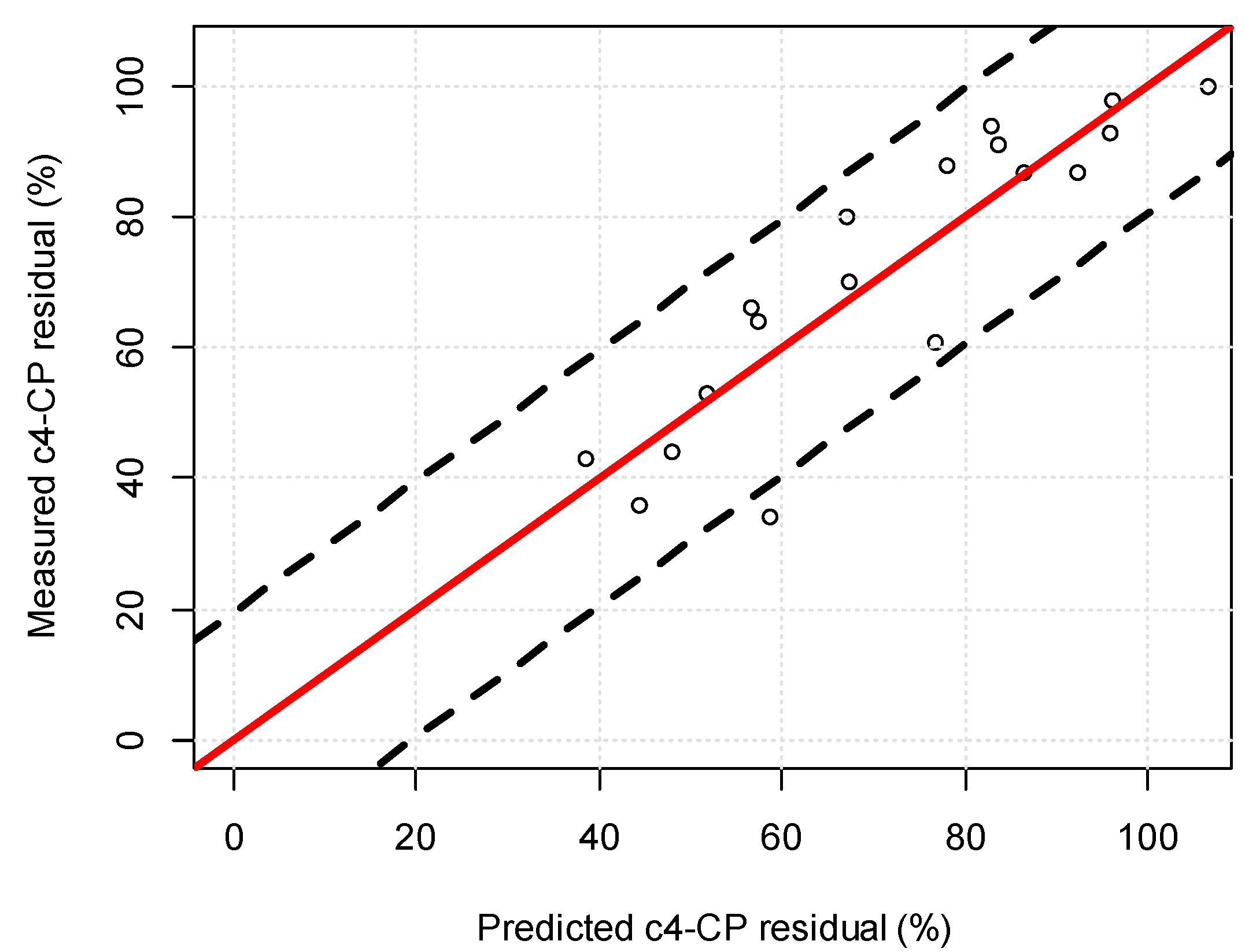
3.4.2. Model Validation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, S.; Mukhopadhyay, M.; Murthy, Z.V.P. Treatment of chlorophenols from wastewaters by advanced oxidation processes. Sep. Purif. Rev. 2013, 42, 263–295. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Garcı́a-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Zhihui, A.; Peng, Y.; Xiaohua, L. Degradation of 4-chlorophenol by microwave irradiation enhanced advanced oxidation processes. Chemosphere 2005, 60, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Remya, N.; Lin, J.G. Current status of microwave application in wastewater treatment-a review. Biochem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Homem, V.; Alves, A.; Santos, L. Microwave-assisted Fenton’s oxidation of amoxicillin. Chem. Eng. J. 2013, 220, 35–44. [Google Scholar] [CrossRef]
- Tarr, M.A. Chemical Degradation Methods for Wastes and Pollutants; CRC Press: Boca Raton, FL, USA, 2003; p. 479. [Google Scholar]
- Zhao, G.; Lv, B.; Jin, Y.; Li, D. P-chlorophenol wastewater treatment by microwave-enhanced catalytic wet peroxide oxidation. Water Environ. Res. 2010, 82, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Mudhoo, A.; Sharma, S.K. Microwave irradiation technology in waste sludge and wastewater treatment research. Crit. Rev. Environ. Sci. Technol. 2011, 41, 999–1066. [Google Scholar] [CrossRef]
- Nascimento, U.M.; Azevedo, E.B. Microwaves and Their coupling to advanced oxidation processes: Enhanced performance in pollutants degradation. J. Environ. Sci. Health A 2013, 48, 1056–1072. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Chan, W.I.; Liao, P.H.; Lo, K.V. A hydrogen peroxide/microwave advanced oxidation process for sewage sludge treatment. J. Environ. Sci. Health A 2006, 41, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yan, H.; Wei, Y.; Wang, Y.; Zeng, F.; Zheng, X. Optimization of H2O2 dosage in microwave-H2O2 process for sludge pretreatment with uniform design method. J. Environ. Sci. (China) 2012, 24, 2060–2067. [Google Scholar] [CrossRef]
- Chang, Y.M.; Tsai, K.S.; Tseng, C.H.; Chen, J.H.; Kao, C.M.; Lin, K.L. Rapid nonylphenol degradation in wastewater sludge using microwave peroxide oxidation with nitric acid. Environ. Prog. Sustain. Energy 2015, 34, 520–525. [Google Scholar] [CrossRef]
- Prasannakumar, B.R.; Regupathi, I.; Murugesan, T. An optimization study on microwave irradiated, decomposition of phenol in the presence of H2O2. J. Chem. Technol. Biotechnol. 2009, 84, 83–91. [Google Scholar] [CrossRef]
- Klán, P.; Vavrik, M. Non-catalytic remediation of aqueous solutions by microwave-assisted photolysis in the presence of H2O2. J. Photochem. Photobiol. A Chem. 2006, 177, 24–33. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric determination of hydrogen peroxide using potassium titanium (IV) oxalate. Analyst 1990, 105, 950–954. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Gins, G.; Degrève, J.; Van Impe, J.; Dewil, R. Parameter identification and modeling of the biochemical methane potential of waste activated sludge. Environ. Sci. Technol. 2011, 45, 4173–4178. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G. R package plsdepot: PLS regression 1. Retrieved Electron. May 2012, 5, 1–13. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812. [Google Scholar] [CrossRef]
- Asgari, G.; Seidmohammadi, A.; Chavoshani, A. Pentachlorophenol removal from aqueous solutions by microwave/persulfate and microwave/H2O2: A comparative kinetic study. J. Environ. Health Sci. Eng. 2014, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Shi, Z.; Zhou, S. Modeling of Fe(II)-activated persulfate oxidation using atrazine as a target contaminant. Sep. Purif. Technol. 2016, 169, 59–65. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; de Araújo Calado, V.M. The use and importance of design of experiments (DOE) in process modelling in food science and technology. Math. Stat. Methods Food Sci. Technol. 2013, 1–18. [Google Scholar] [CrossRef]

| Input Variables | Range | Output Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Symbols | Unit | Low | Medium | High | Variables | Symbols | Unit |
| Reaction temperature | T | (°C) | 60 | 120 | 180 | Residual 4-CP ratio | C4-CP residual | (%) |
| Reaction time | t | (min) | 5 | 10 | 20 | Residual COD ratio | COD residual | (%) |
| Initial 4-CP concentration | C4-CP, I | (mg/L) | 1000 | 5000 | 10,000 | Residual H2O2 ratio | (%) | |
| Initial H2O2 concentration | (g/L) | 5 | 7.5 | 11 | Chloride ratio | Cl | (%) | |
| Final pH | pHend | |||||||
| Run | Input Variables | Output Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T | t | C4-CP, I | COD Residual | C4-CP Residual | Cl | pHend | |||
| (°C) | (min) | (mg/L) | (g/L) | (%) | (%) | (%) | (%) | ||
| 1 | 60 | 5 | 1000 | 5 | 95% | 97% | 81% | 3% | 5.06 |
| 2 | 60 | 10 | 1000 | 7.47 | 100% | 100% | 79% | 1% | 4.79 |
| 3 | 60 | 20 | 1000 | 11 | 100% | 100% | 84% | 0% | 4.39 |
| 4 | 60 | 10 | 5000 | 5 | 89% | 97% | 85% | 0% | 4.64 |
| 5 | 60 | 20 | 5000 | 7.47 | 100% | 100% | 82% | 0% | 4.03 |
| 6 | 60 | 5 | 5000 | 11 | 85% | 99% | 92% | 7% | 4.37 |
| 7 | 60 | 20 | 10,000 | 5 | 100% | 97% | 77% | 2% | 2.68 |
| 8 | 60 | 5 | 10,000 | 7.47 | 96% | 100% | 76% | 1% | 3.25 |
| 9 | 60 | 10 | 10,000 | 11 | 96% | 98% | 77% | 2% | 2.56 |
| 10 | 120 | 10 | 1000 | 5 | 94% | 87% | 75% | 8% | 2.92 |
| 11 | 120 | 20 | 1000 | 7.47 | 100% | 61% | 72% | 19% | 2.53 |
| 12 | 120 | 5 | 1000 | 11 | 100% | 91% | 78% | 0% | 2.96 |
| 13 | 120 | 20 | 5000 | 5 | 94% | 87% | 72% | 2% | 2.63 |
| 14 | 120 | 5 | 5000 | 7.47 | 96% | 93% | 74% | 2% | 2.85 |
| 15 | 120 | 10 | 5000 | 11 | 95% | 94% | 78% | 2% | 2.74 |
| 16 | 120 | 5 | 10,000 | 5 | 97% | 100% | 67% | 2% | 2.52 |
| 17 | 120 | 10 | 10,000 | 7.47 | 100% | 98% | 65% | 3% | 2.21 |
| 18 | 120 | 20 | 10,000 | 11 | 93% | 88% | 63% | 4% | 2.13 |
| 19 | 180 | 20 | 1000 | 5 | 62% | 44% | 41% | 37% | 2.19 |
| 20 | 180 | 5 | 1000 | 7.47 | 79% | 64% | 60% | 17% | 2.41 |
| 21 | 180 | 10 | 1000 | 11 | 74% | 36% | 53% | 40% | 2.16 |
| 22 | 180 | 5 | 5000 | 5 | 88% | 80% | 45% | 0% | 2.06 |
| 23 | 180 | 10 | 5000 | 7.47 | 76% | 66% | 34% | 0% | 1.78 |
| 24 | 180 | 20 | 5000 | 11 | 62% | 43% | 19% | 15% | 1.58 |
| 25 | 180 | 10 | 10,000 | 5 | 89% | 70% | 10% | 23% | 1.6 |
| 26 | 180 | 20 | 10,000 | 7.47 | 90% | 53% | 8% | 22% | 1.46 |
| 27 | 180 | 5 | 10,000 | 11 | 75% | 34% | 22% | 42% | 1.39 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milh, H.; Van Eyck, K.; Dewil, R. Degradation of 4-Chlorophenol by Microwave-Enhanced Advanced Oxidation Processes: Kinetics and Influential Process Parameters. Water 2018, 10, 247. https://doi.org/10.3390/w10030247
Milh H, Van Eyck K, Dewil R. Degradation of 4-Chlorophenol by Microwave-Enhanced Advanced Oxidation Processes: Kinetics and Influential Process Parameters. Water. 2018; 10(3):247. https://doi.org/10.3390/w10030247
Chicago/Turabian StyleMilh, Hannah, Kwinten Van Eyck, and Raf Dewil. 2018. "Degradation of 4-Chlorophenol by Microwave-Enhanced Advanced Oxidation Processes: Kinetics and Influential Process Parameters" Water 10, no. 3: 247. https://doi.org/10.3390/w10030247






