The Suitability of Pozzolan as Admixing Aggregate for Fe0-Based Filters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions
2.1.1. Methylene Blue
2.1.2. Iron
2.2. Solid Materials
2.2.1. Metallic Iron (Fe0)
2.2.2. Sand
2.2.3. Pozzolan
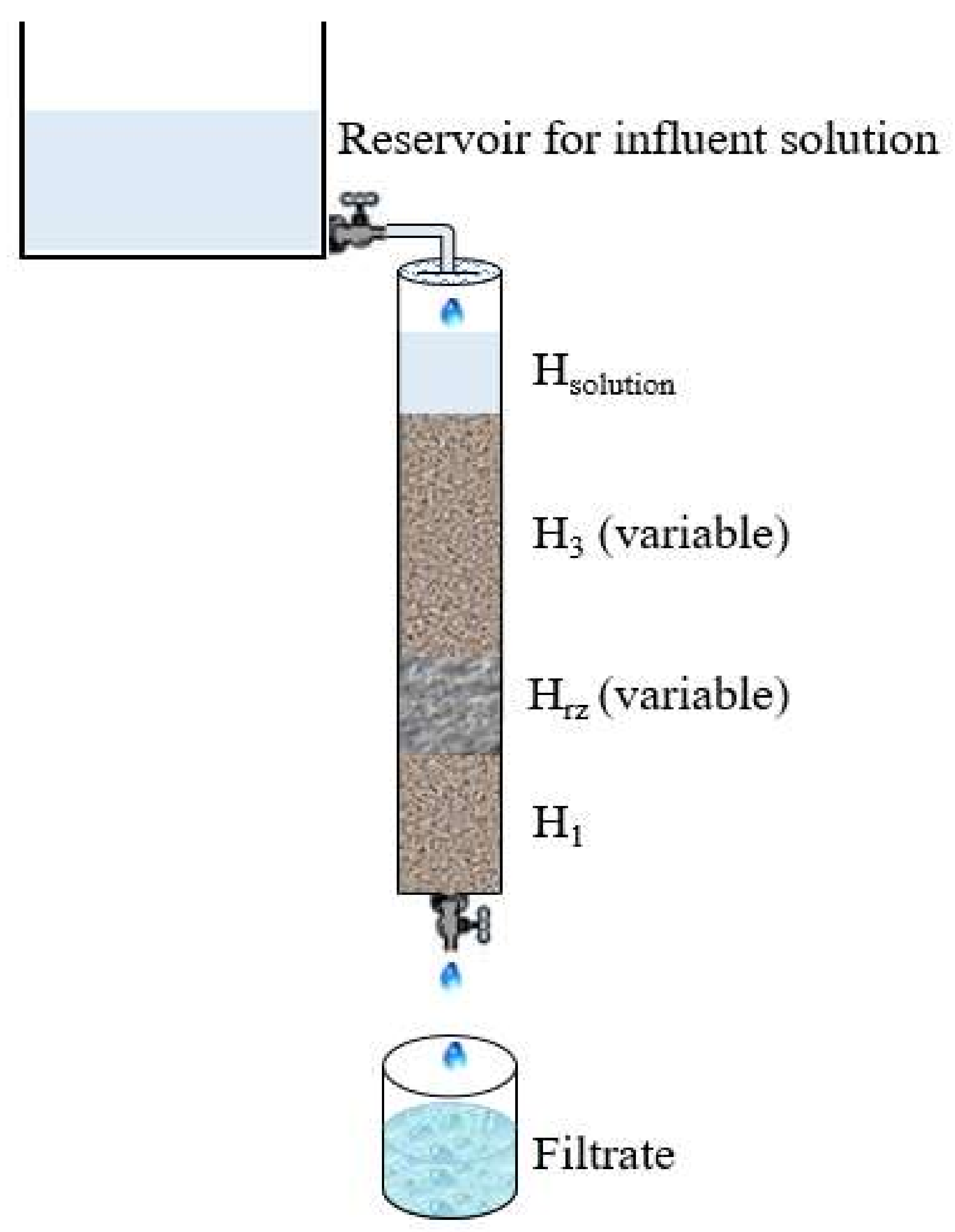
2.3. Experimental Procedure
2.4. Analytical Methods
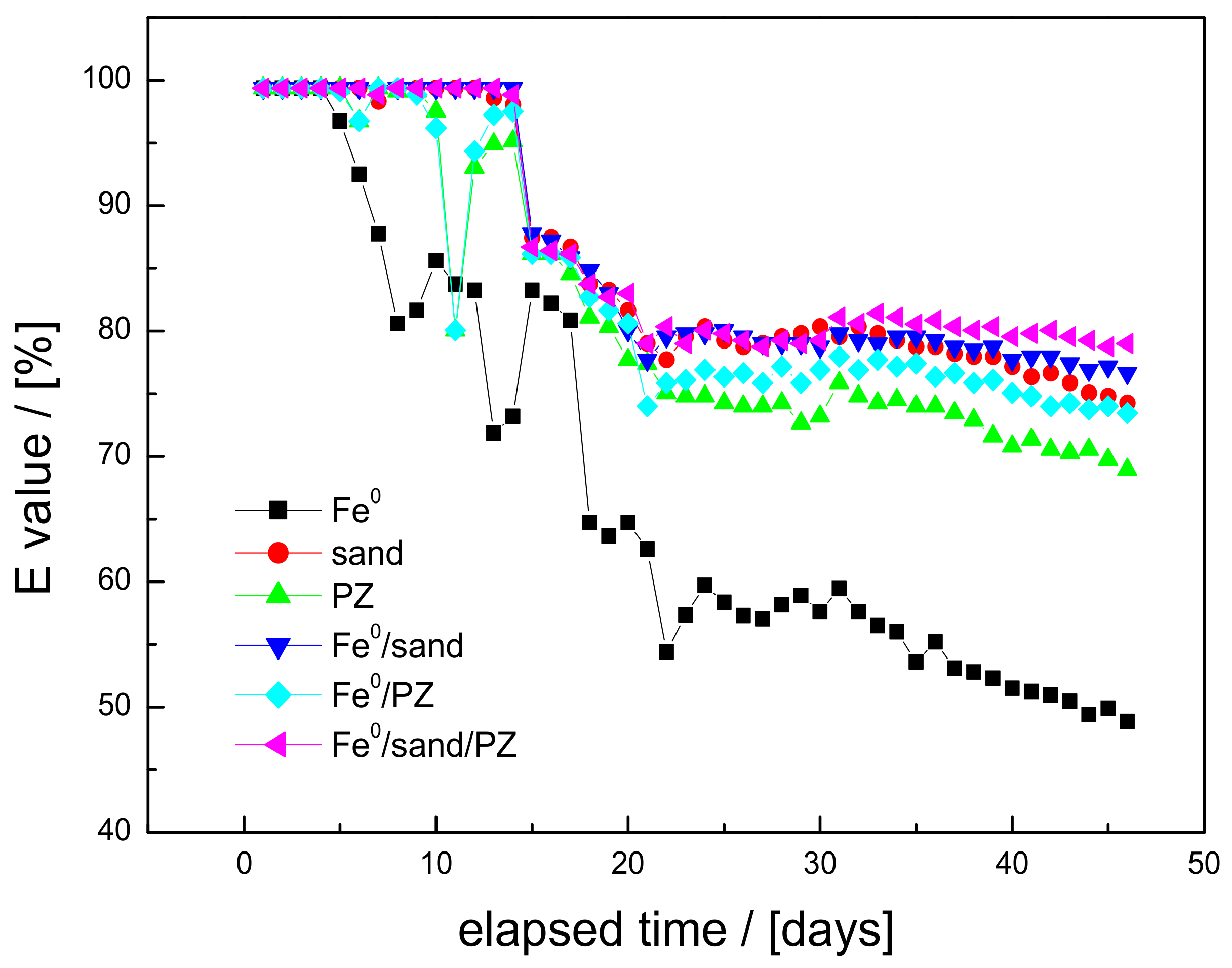
2.5. Presentation of Experimental Results: E Values
3. Results and Discussion
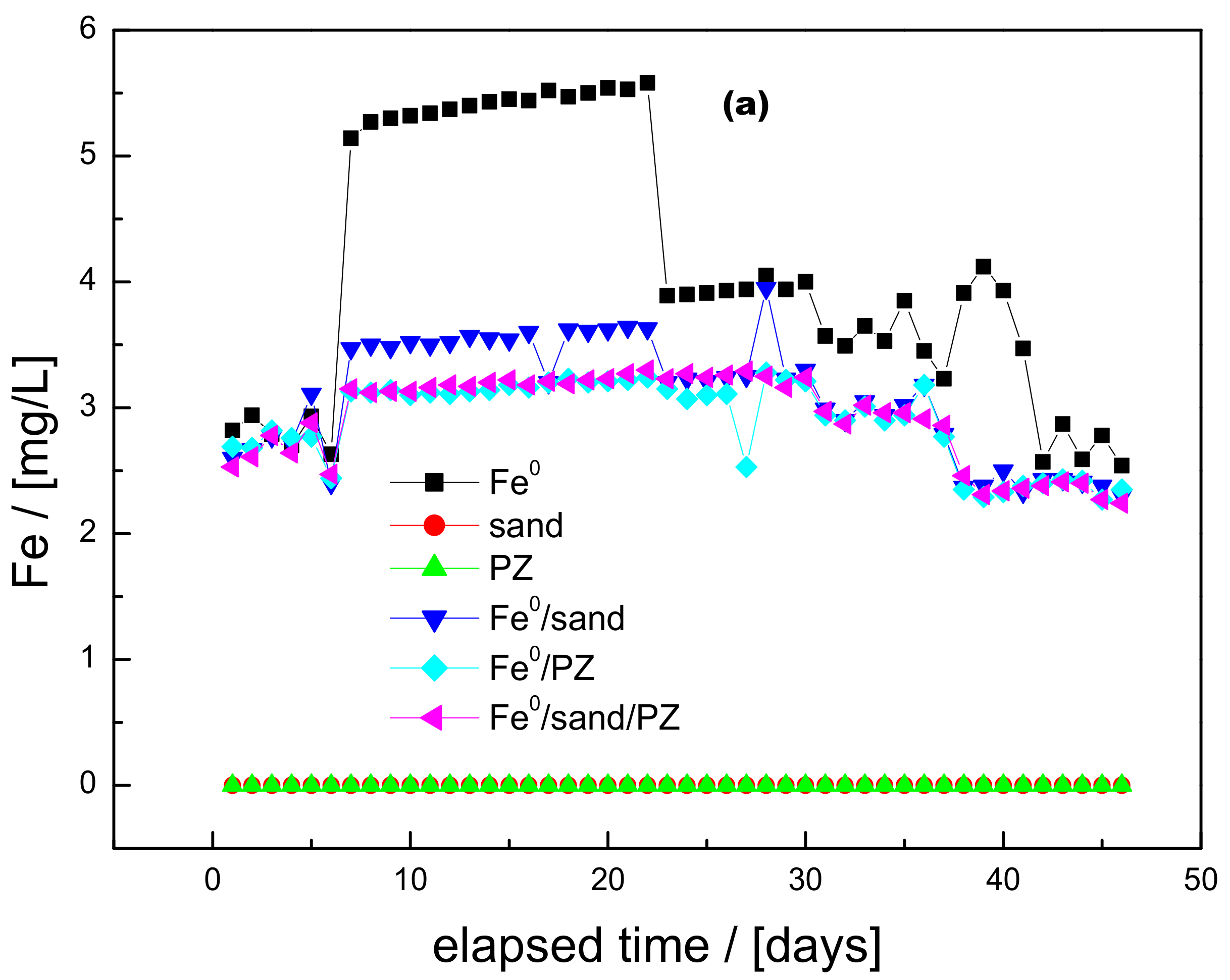
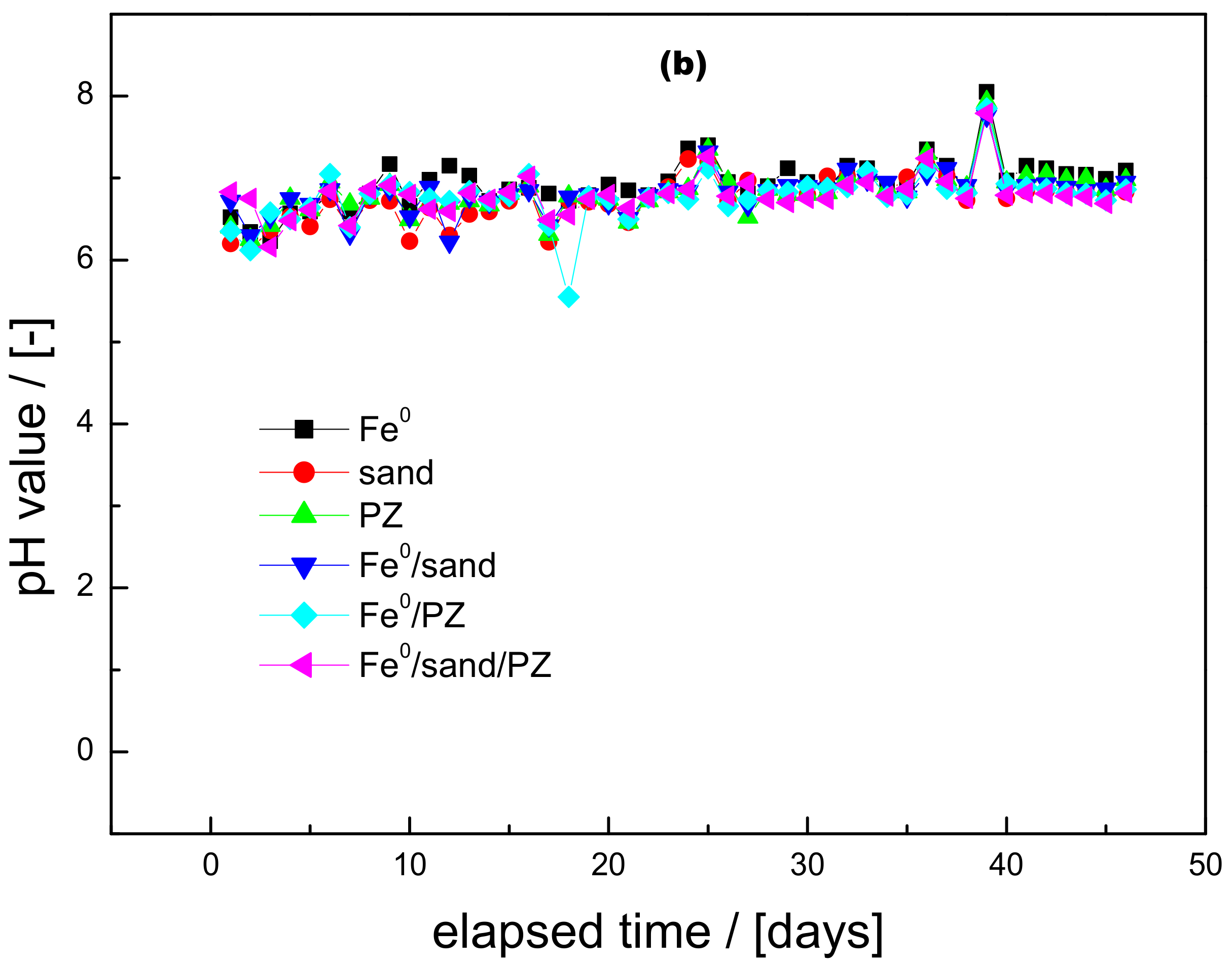
3.1. Iron Release and pH Value
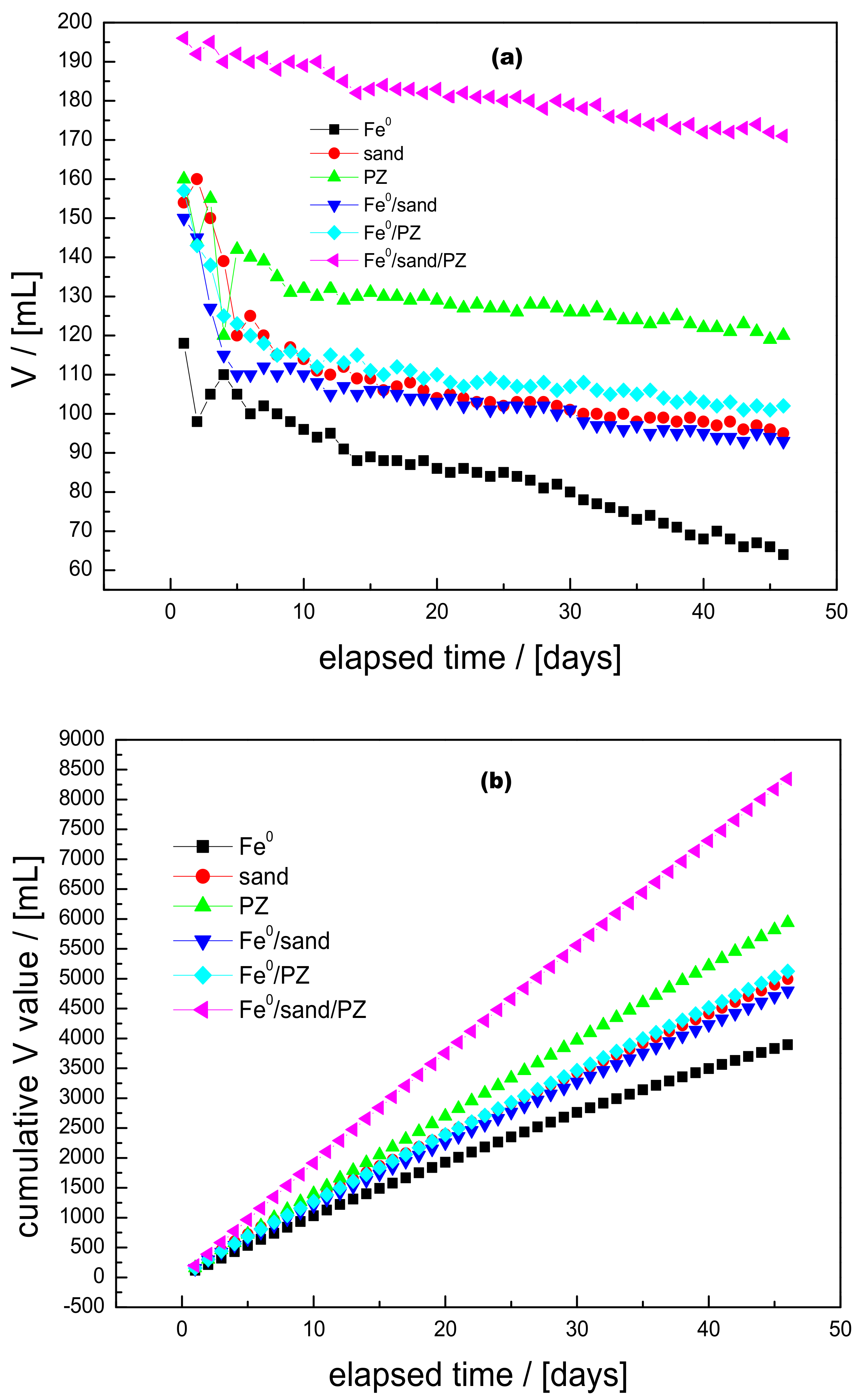
3.2. Hydraulic Conductivity
3.3. MB Discoloration
3.4. Discussion
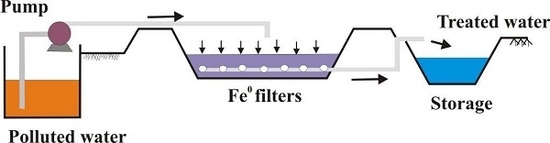
3.4.1. Significance of Results for the Design of Fe0 Filtration Systems
3.4.2. The Service Life of Fe0 Filters
3.4.3. The Particularity of the Ternary Fe0/Sand/PZ System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Groundwater 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenationof chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Schreier, C.G.; Reinhard, M. Transformation of chlorinated organic compounds by iron and manganese powders in buffered water and in landfill leachate. Chemosphere 1994, 29, 1743–1753. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 38, 80. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Noubactep, C. On the suitability of admixing sand to metallic iron for water treatment. Int. J. Environ. Pollut. Solut. 2013, 1, 22–36. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 2015, 7, 868–897. [Google Scholar] [CrossRef]
- Ulsamer, S. A Model to Characterize the Kinetics of Dechlorination of Tetrachloroethylene and Trichloroethylene by a Zero Valent Iron Permeable Reactive Barrier. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2011; p. 73. Available online: https://web.wpi.edu (accessed on 30 March 2018).
- Noubactep, C.; Caré, S. Dimensioning metallic iron beds for efficient contaminant removal. Chem. Eng. J. 2010, 163, 454–460. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Designing laboratory metallic iron columns for better result comparability. J. Hazard. Mater. 2011, 189, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Caré, S.; Moraci, N.; Noubactep, C. Improving the sustainability of granular iron/pumice systems for water treatment. J. Environ. Manag. 2013, 121, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 60. [Google Scholar]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2016, 166, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Simon, S.; Courtin-Nomade, A.; Vasiliu, A.; Sleiman, N.; Deluchat, V. Long-term influence of aeration on arsenic trapping in ZVI/sand bed reactor. RSC Adv. 2016, 6, 54479–54485. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Groundwater 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Song, D.-I.; Kim, Y.H.; Shin, W.S. A simple mathematical analysis on the effect of sand in Cr(VI) reduction using zero valent iron. Korean J. Chem. Eng. 2005, 22, 67–69. [Google Scholar] [CrossRef]
- Bi, E.; Devlin, J.F.; Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Groundw. Monit. Remed. 2009, 29, 56–62. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Impact of solids formation and gas production on the permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Met. 1923, 29, 529–591. [Google Scholar]
- Caré, S.; Nguyen, Q.T.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modelling the permeability loss of metallic iron water filtration systems. Clean Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Trois, C.; Cibati, A. South African sands as a low cost alternative solution for arsenic removal from industrial effluents in permeable reactive barriers: Column tests. Chem. Eng. J. 2015, 259, 981–989. [Google Scholar] [CrossRef]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 2016, 8, 162. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Effects of mixing granular iron with sand on the efficiency of methylene blue discoloration. Chem. Eng. J. 2012, 200, 433–438. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I. Designing Metallic Iron (Fe0)—Based Filters: Contribution to a Convenience Scale. Master’s Thesis, University of Douala, Douala, Cameroon, 20 January 2015. [Google Scholar]
- Tepong-Tsindé, R.; Phukan, M.; Nassi, A.; Noubactep, C.; Ruppert, H. Validating the efficiency of the MB discoloration method for the characterization of Fe0/H2O systems using accelerated corrosion by chloride ions. Chem. Eng. J. 2015, 279, 353–362. [Google Scholar] [CrossRef]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura Mbenguela, B. Mitigation of contamination in effluents by metallic iron: The role of iron corrosion products. Environ. Technol. Innov. 2017, 8, 71–83. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215, 959–968. [Google Scholar] [CrossRef]
- Mitchell, C.; Poole, P.; Segrove, H.D. Adsorption of methylene blue by high silica sands. Nature 1955, 176, 1025–1026. [Google Scholar] [CrossRef]
- Avom, J.; Ketcha, J.; Noubactep, C.; Germain, P. Adsorption of methylene blue from an aqueous solution onto activated carbons from palm-tree cobs. Carbon 1997, 35, 365–369. [Google Scholar] [CrossRef]
- Noubactep, C. Characterizing the discoloration of methylene blue in Fe0/H2O systems. J. Hazard. Mater. 2009, 166, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Varlikli, C.; Bekiari, V.; Kus, M.; Boduroglu, N.; Oner, I.; Lianos, P.; Lyberatos, G.; Icli, S. Adsorption of dyes on Sahara desert sand. J. Hazard. Mater. 2009, 170, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kofa, G.P.; Ndi Koungou, S.; Kayem, G.J.; Kamga, R. Adsorption of arsenic by natural pozzolan in a fixed bed: Determination of operating conditions and modelling. J. Water Process Eng. 2015, 6, 166–173. [Google Scholar] [CrossRef]
- Kofa, G.P.; Ndi Koungou, S.; Kayem, G.J.; Kamga, R. Kinetics and adsorption isotherms of arsenic (V) onto natural pozzolan. J. Eng. Appl. Sci. 2013, 8, 97–103. [Google Scholar]
- Chiew, H.; Sampson, M.L.; Huch, S.; Ken, S.; Bostick, B.C. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended biosand filters. Environ. Sci. Technol. 2009, 43, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Fortune, W.B.; Mellon, M.G. Determination of iron with o-phenanthroline: A spectrophotometric study. Ind. Eng. Chem. Anal. Ed. 1938, 10, 60–64. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Gilmore, T.J. Zero-valent iron removal rates of aqueous Cr(VI) measured under flow conditions. Water Air Soil Pollut. 2004, 155, 21–33. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water worldwide. Chem. Eng. J. 2010, 165, 740–749. [Google Scholar] [CrossRef]
- Phukan, M. Characterizing the Fe0/sand system by the extent of dye discoloration. FOG 2015, 42, 80. [Google Scholar]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu-K, B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solut. Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S.; Togue-Kamga, F.; Schöner, A.; Woafo, P. Extending service life of household water filters by mixing metallic iron with sand. Clean Soil Air Water 2010, 38, 951–959. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S.; Btatkeu-K, B.D.; Nanseu-Njiki, C.P. Enhancing the sustainability of household Fe0/sand filters by using bimetallics and MnO2. Clean Soil Air Water 2011, 40, 100–109. [Google Scholar] [CrossRef]
- Carman, P.C. Fluid flow through granular beds. Tran. Inst. Chem. Eng. 1937, 15, 150–156. [Google Scholar] [CrossRef]
- Salem, H.S.; Chilingarian, G.V. The cementation factor of Archie’s equation for shaly sand stone reservoirs. J. Pet. Sci. Eng. 1999, 23, 83–93. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Chen, H.; Heitmann, J.A. Permeability reduction phenomena in packed beds, fiber mats, and wet webs of paper exposed to flow of liquids and suspensions: A review. BioResources 2009, 4, 405–451. [Google Scholar]
- Dias, R.P.; Teixeira, J.A.; Mota, M.G.; Yelshin, A.I. Particulate binary mixtures: Dependence of packing porosity on particle size ratio. Ind. Eng. Chem. Res. 2004, 43, 7912–7919. [Google Scholar] [CrossRef] [Green Version]
- Kubare, M.; Haarhoff, J. Rational design of domestic biosand filters. J. Water Supply Res. Technol. Aqua 2010, 59, 1–15. [Google Scholar] [CrossRef]
- Heimann, S. Testing Granular Iron for Fluoride Removal. Master’s Thesis, University of Göttingen, Göttingen, Germany, 2018. In Preparation. [Google Scholar]
- Leupin, O.X.; Hug, S.J.; Badruzzaman, A.B.M. Arsenic removal from Bangladesh tube well water with filter columns containing zerovalent iron filings and sand. Environ. Sci. Technol. 2005, 39, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Avilés, M.; Garrido, S.E.; Esteller, M.V.; De La Paz, J.S.; Najera, C.; Cortés, J. Removal of groundwater arsenic using a household filter with iron spikes and stainless steel. J. Environ. Manag. 2013, 131, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.S.; Chaudhari, S.K. Arsenic removal from simulated groundwater using household filter columns containing iron filings and sand. J. Water Process Eng. 2015, 6, 151–157. [Google Scholar] [CrossRef]
- Noubactep, C. Designing metallic iron packed-beds for water treatment: A critical review. Clean Soil Air Water 2016, 44, 411–421. [Google Scholar] [CrossRef]
- Makota, S.; Nde-Tchoupe, A.I.; Mwakabona, H.T.; Tepong-Tsindé, R.; Noubactep, C.; Nassi, A.; Njau, K.N. Metallic iron for water treatment: Leaving the valley of confusion. Appl. Water Sci. 2017, 7, 4177–4196. [Google Scholar] [CrossRef]
- Moraci, N.; Lelo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for water treatment: A critical review. Clean Soil Air Water 2013, 41, 702–710. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W. On the purification of water by agitation with iron and by sand filtration. J. Soc. Arts 1886, 35, 29–38. [Google Scholar] [CrossRef]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Frechen, F.-B.; Exler, H.; Romaker, J.; Schier, W. Long-term behaviour of a gravity-driven dead end membrane filtration unit for potable water supply in cases of disasters. Water Sci. Technol. Water Supply 2011, 11, 39–44. [Google Scholar] [CrossRef]
- Nitzsche, K.S.; Lan, V.M.; Trang, P.T.K.; Viet, P.H.; Berg, M.; Voegelin, A.; Planer-Friedrich, B.; Zahoransky, J.; Müller, S.-K.; Byrne, J.M.; et al. Arsenic removal from drinking water by a household sand filter in Vietnam—Effect of filter usage practices on arsenic removal efficiency and microbiological water quality. Sci. Total Environ. 2015, 502, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Feistel, U.; Otter, P.; Kunz, S.; Grischek, T.; Feller, J. Field tests of a small pilot plant for the removal of arsenic ingroundwater using coagulation and filtering. J. Water Process Eng. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Phosphate removal from agricultural tile drainage with iron enhanced sand. Water 2017, 9, 672. [Google Scholar] [CrossRef]
- Allred, B.J. Batch test screening of industrial product/byproduct filter materials for agricultural drainage water treatment. Water 2017, 9, 791. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Caré, S.; Moraci, N.; Noubactep, C. Effect of pumice and sand on the sustainability of granular iron beds for the removal of CuII, NiII, and ZnII. Clean Soil Air Water 2013, 41, 835–843. [Google Scholar] [CrossRef]
- Moraci, N.; Calabrò, P.S. Heavy metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. J. Environ. Manag. 2010, 91, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
| Materials | Size | Source | Nature |
|---|---|---|---|
| Sand | 315 μm–630 μm | Collected (SWR *) | Adsorbent |
| Pozzolan | 315 μm–630 μm | Collected (SWR *) | Porous adsorbent |
| Fe0 | <630 μm | Donated (AC **) | Adsorbent’s generator |
| Column | Fe0 (g) | Fe0 (wt %) | Sand (g) | Sand (vol %) | PZ (g) | PZ (vol %) | Hrz (cm) | H1 (cm) | H3 (cm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.0 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 | 3.8 | 3.4 |
| 2 | 0.0 | 0.0 | 8.0 | 100 | 0.0 | 0.0 | 2.4 | 3.8 | 3.2 |
| 3 | 0.0 | 0.0 | 0.0 | 0.0 | 8.0 | 100 | 2.8 | 3.8 | 2.8 |
| 4 | 6.0 | 25 | 6.0 | 75 | 0.0 | 0.0 | 3.2 | 3.8 | 2.2 |
| 5 | 6.0 | 25 | 0.0 | 0.0 | 6.0 | 75 | 3.5 | 3.8 | 2.2 |
| 6 | 6.0 | 25 | 3.6 | 45 | 2.4 | 30 | 3.7 | 3.8 | 2.1 |
| Column | Btatkeu-K et al. [21] | ||||||||
| Fe0 (g) | Fe0 (wt %) | Sand (g) | Sand (vol %) | PM (g) | PM (vol %) | Hrz (cm) | H1 (cm) | H3 (cm) | |
| Fe0/sand | 65.0 | 50.4 | 63.9 | 49.6 | 0.0 | 0.0 | 9.8 | 8.0 | 14.7 |
| Fe0/PM | 65.0 | 73.0 | 0.0 | 0.0 | 24.1 | 27.0 | 11.0 | 8.0 | 13.5 |
| Fe0/sand/PM | 65.0 | 60.0 | 31.6 | 29.2 | 11.7 | 10.8 | 11.0 | 8.0 | 13.5 |
| Column | VT (L) | min (mg) | ΣmBM (mg) | mdiscol (mg) | ΣmFe (mg) | m1 (mg) | mrz (mg) | Es (mg·g−1) | Es1 (mg·g−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.90 | 7.80 | 2.36 | 5.44 | 16.11 | 3.13 | 2.31 | 697.44 | 0.39 |
| 2 | 4.99 | 9.98 | 1.35 | 6.63 | 0.00 | 2.94 | 3.69 | 664.33 | 0.62 |
| 3 | 5.94 | 11.88 | 2.13 | 9.75 | 0.00 | 2.57 | 7.18 | 820.71 | 1.20 |
| 4 | 4.80 | 9.60 | 1.29 | 8.31 | 14.81 | 2.02 | 6.29 | 865.63 | 1.05 |
| 5 | 5.12 | 10.25 | 1.63 | 8.62 | 14.86 | 2.02 | 6.60 | 840.98 | 1.10 |
| 6 | 8.35 | 16.69 | 2.22 | 14.47 | 24.46 | 1.93 | 12.54 | 866.99 | 2.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndé-Tchoupé, A.I.; Makota, S.; Nassi, A.; Hu, R.; Noubactep, C. The Suitability of Pozzolan as Admixing Aggregate for Fe0-Based Filters. Water 2018, 10, 417. https://doi.org/10.3390/w10040417
Ndé-Tchoupé AI, Makota S, Nassi A, Hu R, Noubactep C. The Suitability of Pozzolan as Admixing Aggregate for Fe0-Based Filters. Water. 2018; 10(4):417. https://doi.org/10.3390/w10040417
Chicago/Turabian StyleNdé-Tchoupé, Arnaud Igor, Suzanne Makota, Achille Nassi, Rui Hu, and Chicgoua Noubactep. 2018. "The Suitability of Pozzolan as Admixing Aggregate for Fe0-Based Filters" Water 10, no. 4: 417. https://doi.org/10.3390/w10040417