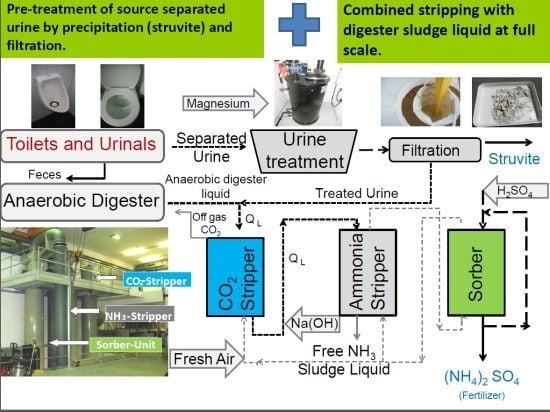
Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Urine Separation, Storage and Treatment
| Parameter | Tank 1 | Tank 2 | Tank 3 | Tank 4 | Tank 5 | Tank 6 | Tank 7 |
|---|---|---|---|---|---|---|---|
| Mg Source | MgO + (15%) HCl | MgO + (30%) HCl | MgO + H2SO4 | MgO + (50%) HCl | MgO | MgO | MgO |
| PO4−3-P (mg/L) | 166.8 ± 0.4 | 156.0 ± 0.3 | 187.2 ± 0.4 | 194.1 ± 0.3 | 167.8 ± 0.2 | 174.4 ± 1.0 | 174.4 ± 1.0 |
| NH4+-N (mg/L) | 3200 ± 50 | 3900 ± 290 | 4990 ± 237 | 4930 ± 199 | 3780 ± 133 | – | – |
| Volume (L) | 600 ± 10 | 480 ± 10 | 510 ± 10 | 510 ± 10 | 430 ± 10 | 430 ± 10 | 510 ± 10 |
2.2. Ammonia Air Stripping Process Including a CO2 Pre-Stripper



| Parameter | Value | Units |
|---|---|---|
| Column height (CO2 and Ammonia) | 10 | m |
| Packing bed height | 6 | m |
| Diameter | 0.98 | m |
| Liquid inflow | 5–12 | m3/h |
| Air flow to the ammonia stripper | 1000–6250 | Nm3/h |
| Gas flow to the CO2 stripper | 50–200 | Nm3/h |
| Average NH4+-N concentration inlet | 1000 | mg/L |
| Average NH4+-N concentration effluent | 30 | mg/L |
| Maximum removal rate | 99 | % |
| Parameter | Stage I | Stage II | Stage III | Stage IV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 0 | 25 | 43 | 75 | 89 | 95 | 125 | 162 | 178 | 179 | 195 | 210 | 211 | 235 |
| Sampling | X | X | X | X | – | X | X | X | X | – | X | X | – | X |
| Urine addition | X | X | X | X | X | X | X | X | X | X | X | X | – | – |
| Modification in the operational conditions | – | – | – | – | X | – | – | – | – | X | – | – | X | – |
| pH set point | 9.3 | 9.5 | 9.0 | 9.3 | ||||||||||
| Air flux (Nm3/h) | 3900 | 3900 | 3900 | 3900 | ||||||||||

2.3. Chemical and Crystals Analysis
2.4. Chemical Cost, Electricity and Fertilizer Price

| Year | Electricity (CHF/kWh) | NaOH (CHF/kg) | H2SO4 (CHF/kg) | HCl
(CHF/kg) | NH4NO3 (CHF/100 kg) | (NH4)2SO4 (CHF/1000 kg) |
|---|---|---|---|---|---|---|
| 2011 | 0.1 | 0.36 | 0.23 | 0.52 | – | 50.6 |
| 2010 | – | – | – | – | 169.63 | 50.0 |
| 2009 | – | – | – | – | 199.95 | – |
| 2008 | – | – | – | – | 231.83 | – |
| 2007 | – | – | – | – | 162.49 | – |
3. Results and Discussion
3.1. Magnesium Dose and Reaction Time Determination. Urine Pre-Treatment

| Sample | Composition mg/g solid | mol/molstruvite | ||||||
| NH4+ | PO43− | Mg2+ | Ca2+ | PO43− | Ca2+ | Ca3(PO4)2 | ||
| Sample 1 (Tanks 1 and 2) | 52.1 | 367.7 | 67.0 | 55.0 | 0.40 | 0.50 | 0.17 | |
| Sample 2 (Tanks 2 and 3) | 57.2 | 376.9 | 75.0 | 44.5 | 0.29 | 0.36 | 0.12 | |
| Sample 3 (Tanks 4 and 5) | 55.3 | 370.8 | 71.0 | 45.5 | 0.34 | 0.39 | 0.13 | |
| Sample 4 (Tank 6) | 63.6 | 380.0 | 80.0 | 27.0 | 0.22 | 0.21 | 0.07 | |

3.2. Ammonia Stripping with Sludge Liquid and Treated Urine
3.2.1. Experiment I
 ) in (m3/h) in the urine, liquid sludge, liquid sludge after the urine addition, effluent in the experiment I, and effluent for the removal efficiency reference (89%) [26].
) in (m3/h) in the urine, liquid sludge, liquid sludge after the urine addition, effluent in the experiment I, and effluent for the removal efficiency reference (89%) [26].
 ) in (m3/h) in the urine, liquid sludge, liquid sludge after the urine addition, effluent in the experiment I, and effluent for the removal efficiency reference (89%) [26].
) in (m3/h) in the urine, liquid sludge, liquid sludge after the urine addition, effluent in the experiment I, and effluent for the removal efficiency reference (89%) [26].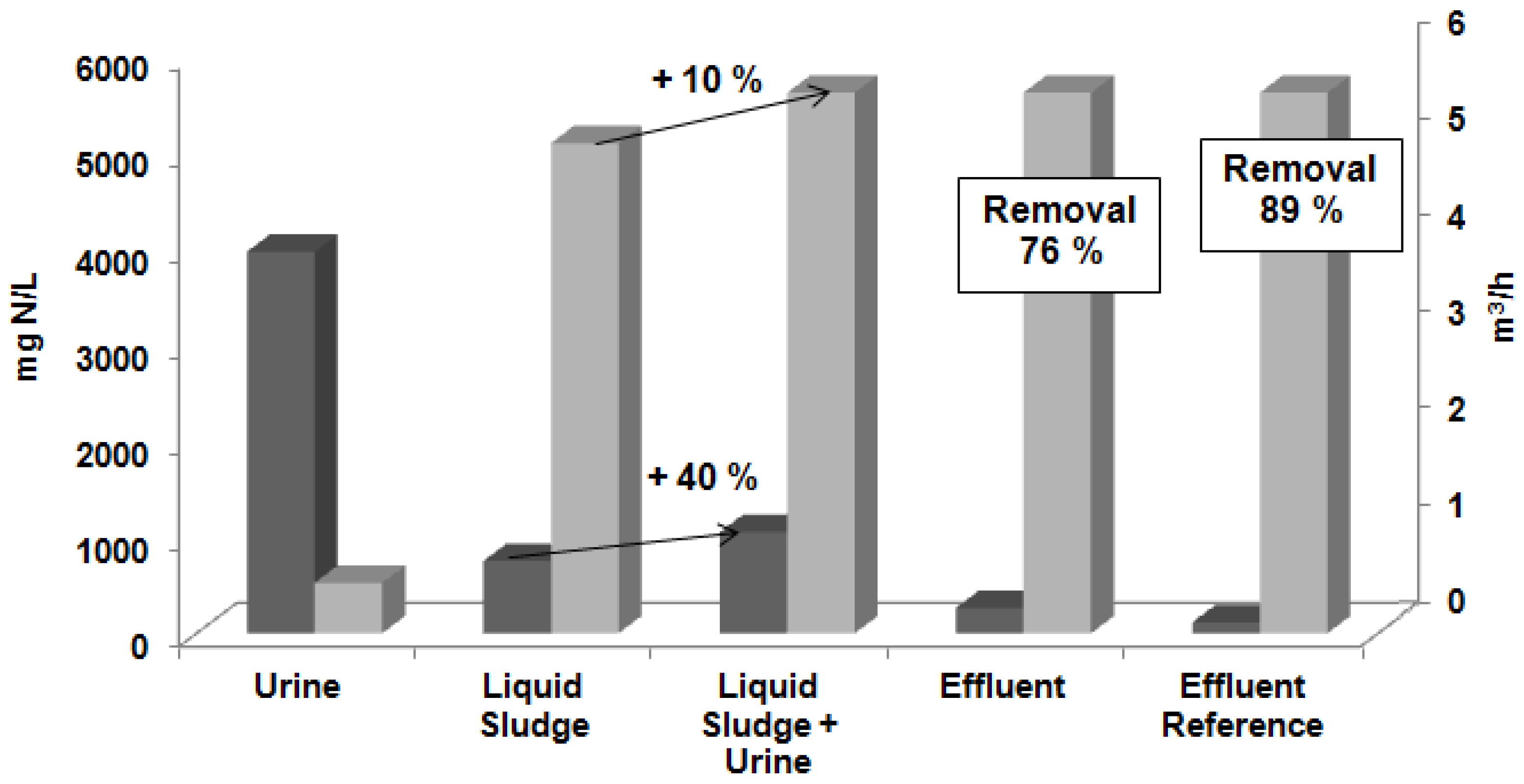
3.2.2. Experiment II
3.2.2.1 Removal Efficiency and Fertilizer Recovery
3.2.3 Chemical and Energy Cost
| Experiment (Stage) | I | II (S-I) | II (S-II) | II (S-III) |
|---|---|---|---|---|
| Temperature after the heat exchanger (°C) | 60 | 60 | 60 | 60 |
| Set point pH, after the NaOH dosage | 9.3 | 9.3 | 9.5 | 9.0 |
| Air flow in the stripping reactor (Nm3/h) | 3600 | 3900 | 3900 | 3900 |
| Air flow in the CO2 stripper (Nm3/h) | 100 | 100 | 100 | 100 |
| Sludge liquid flow (m3/h) | 5.1 | 5.1 | 5.1 | 5.1 |
| Urine flow added (L/h) | 525 | 525 | 525 | 525 |
| pH after the stripping | No data | 7.9 | 8.1 | 8.0 |
| Efficiency (%) | 76 | 65 | 73 | 65 |
| Efficiency % [26] | 89 (with 5.25 m3/h) | 89 (with 5.25 m3/h and 4000 Nm3/h) | 97 (with 5.25 m3/h and 4000 Nm3/h) | 80 (with 5.25 m3/h and 4000 Nm3/h) |
| Increase/decrease in the efficiency (%) | −13 | −24 | −24 | −13 |
4. Conclusions
- The addition of magnesium oxide in powder to the separated urine, combined with a filtration system is enough to produce and recover nutrients in form of struvite. A magnesium to phosphorus ratio of 1.4 was used in order to remove more than 95% of phosphorus from the stored urine in a short time of less than 1 hour;
- Struvite crystals with an average size of 42 to 80 µm were formed in the reactor (the urine storage tank). Nevertheless, the solids recovery system during urine pre-treatment needs to be improved in order to retrieve a higher percentage of nutrients and the separated urine collecting system should be connected to the stripping reactor in order to combine both processes and optimize the nutrients recovery system;
- The addition of 10% volume of treated urine to the sludge liquid fed in the stripping treatment system produced an increase of 40% of the ammonia concentration;
- The efficiency in the nitrogen removal was lower than the value reached during the optimization and simulations without urine addition. An increase in the NaOH dose, H2SO4 composition and air/liquid ratio was needed, due to the liquid flux increase. Nevertheless, an increase in the ammonia sulphate production rate (36%–56%) was observed during the full-scale experiments with urine addition, which demonstrated the viability of the combined system;
- Operational problems due to the treated urine addition were not reported by the WWTP operators; however, the length of the experiments was too short to discard this possibility.
Acknowledgments
Conflicts of Interest
References
- International Fertilizer Industry Association Home Page. Available online: http://www.fertilizer.org/ifa/HomePage/STATISTICS/Production-and-trade (accessed on 21 August 2013).
- Glass, A.D.M. Nitrogen use efficiency of crop plants: Physiological constraints upon nitrogen absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar]
- Steen, I.; Agro, K. Phosphorus availability in the 21st century: Management of a non-renewable resource. Phosphorus Potassium 1998, 217, 25–31. [Google Scholar]
- Roberts, T.L.; Stewart, W.M. Inorganic phosphorus and potassium production and reserves. Better Crop. 2002, 86, 6–7. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. 78th IFA Annual Conference. In Fertilizer Outlook 2010–2014; International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Environmental Protection Agency, Biological Nutrient Removal Processes and Costs; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of us freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef]
- European Commission, Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources; Office for Official Publications of the European Communities: Brussels, Belgium, 1991.
- Jenssen, P.D.; Vråle, L.; Lindholm, O. Sustainable wastewater treatment. In Proceedings of International Conference on Natural Resources and Environmental Management and Environmetal Safety and Health, Kuching, Malaysia, 27–29 November 2007.
- Jenssen, P.D.; Vatn, A. Ecologically sound wastewater treatment: Concepts and implementation. In Proceedings of the International Conference at Stensund Folk College, Stensund, Sweden, 24–28 March 1991.
- Etnier, C.; Jenssen, P.D. The Human Waste Resource in Developing Countries: Examples of Options for Reuse of Nutrients in Agriculture and Aquaculture; Centre for International Environment and Development Studies: Noragric, Norway, 1997. [Google Scholar]
- Metcalf Eddy, I.; Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Companies: NewYork, NY, USA, 2002. [Google Scholar]
- Wilsenach, J. Separate Urine Collection and Treatment: Options for Sustainable Wastewater Systems and Mineral Recovery; Stichting Toegepast Onderzoek Waterbeheer: Utrecht, The Netherlands, 2002. [Google Scholar]
- Böhler, M.; Liebi, C. Reduzierter NaOH-Verbrauch bei der Ammoniak-Strippung durch den Einsatz einer vorgeschalteten CO2-Strippung—Betriebserfahrungen auf der ARA Kloten-Opfikon (In German). Reduction of base consumption in the free ammonia stripping process by means of a pre treatment by CO2-stripping—Operational experiences at WWTP Kloten/Opfikon. In 8th Conference on Nitrogen Rejection Load—State of the Art; Markus Groemping, Atemis GmbH: Heidelberg, Germany, 2012. [Google Scholar]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Siegrist, H.; Laureni, M.; Udert, K.M. Transfer into the gas phase: Ammonia stripping. In Source Separation and Decentralization for Wastewater Management; IWA publishing: London, UK, 2013. [Google Scholar]
- Lei, X.; Sugiura, N.; Feng, C.; Maekawa, T. Pretreatment of anaerobic digestion effluent with ammonia stripping and biogas purification. J. Hazard. Mater. 2007, 145, 391–397. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Review: Phosphorus removal and recovery technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Koch, F.A. Phosphorus recovery from wastewater through struvite formation in fluidized bed reactors: A sustainable approach. Water Sci. Technol. 2008, 57, 175–181. [Google Scholar] [CrossRef]
- Shu, L.; Schneider, P.; Jegatheesan, V.; Johnson, J. An economic evaluation of phosphorus recovery as struvite from digester supernatant. Bioresour. Technol. 2006, 97, 2211–2216. [Google Scholar] [CrossRef]
- Wilsenach, J.A.; Schuurbiers, C.A.H.; van Loosdrecht, M.C.M. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Wilsenach, J.A.; van Loosdrecht, M.C.M. Integration of processes to treat wastewater and source-separated urine. J. Environ. Eng. 2006, 132, 331–341. [Google Scholar] [CrossRef]
- Böhler, M.; Büttner, S.; Morales, N.; Liebi, C.; Schachtler, M.; Siegrist, H. Recovery of nutrients from ammonia rich sludge liquids and urine for the production of fertilizer by full scale air stripping or membrane stripping. In Proceedings of the IWA World Congress on Water, Climate and Energy, Dublin, Ireland, 13–18 May 2012.
- Cohen, Y.; Kirchmann, H. Increasing the ph of wastewater to high levels with different gases CO2 stripping. Water Air Soil Pollut. 2004, 159, 265–275. [Google Scholar] [CrossRef]
- Büttner, S. Gewinnung eines düngers aus abwasser—Optimierung des verfahrens der luftstrippung der kläranlage Kloten/Opfikon [In German] (Production of a fertilizer from sewage—Optimization of the process of air stripping of the WWTP Kloten/Opfikon). Master’s Thesis, (EAWAG) Das Wasserforschungs-Institut des ETH-Bereichs, Dübendorf, Switzerland, May 2011. [Google Scholar]
- SBV Landwirtschaftliche Monatszahlen Home Page. Available online: http://www.sbv-usp.ch/de/shop/landwirtschaftliche-monatszahlen/ (accessed on 10 March 2011).
- Udert, K.M.; Larsen, T.A.; Gujer, W. Estimating the precipitation potential in urine-collecting systems. Water Res. 2003, 37, 2667–2677. [Google Scholar] [CrossRef]
- Udert, K.M.; Larsen, T.A.; Biebow, M.; Gujer, W. Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res. 2003, 37, 2571–2582. [Google Scholar] [CrossRef]
- Abegglen, C. Membrane bioreactor technology for decentralized wastewater treatment and reuse. Ph.D. Thesis, Eidgenössische Technische Hochschule, Zürich, Switzerland, November 2008. [Google Scholar]
- Tettenborn, F.; Behrendt, J.; Otterpohl, R. Resource Recovery and Removal of Pharmaceutical Residues Treatment of Separate Collected Urine; Technical University of Hamburg: Hamburg, Germany, 2007. [Google Scholar]
- Ronteltap, M.; Maurer, M.; Gujer, W. Struvite precipitation thermodynamics in source-separated urine. Water Res. 2007, 41, 977–984. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Hausherr, R.; Gujer, W. Struvite precipitation from urine—Influencing factors on particle size. Water Res. 2010, 44, 2038–2046. [Google Scholar] [CrossRef]
- Wierzbicki, A.; Sallis, J.D.; Stevens, E.D.; Smith, M.; Sikes, C.S. Crystal growth and molecular modeling studies of inhibition of struvite by phosphocitrate. Calcif. Tissue Int. 1997, 61, 216–222. [Google Scholar] [CrossRef]
- Massey, M.S.; Ippolito, J.A.; Davis, J.G.; Sheffield, R.E. Macroscopic and microscopic variation in recovered magnesium phosphate materials: Implications for phosphorus removal processes and product re-use. Bioresour. Technol. 2010, 101, 877–885. [Google Scholar] [CrossRef]
- Nelson, N.O. Phosphorus removal from anaerobic swine lagoon effluent as struvite and its use as a slow-release fertilizer. Master’s Thesis, North Carolina State University, Raleigh, USA, 2000. [Google Scholar]
- Wang, J.; Burken, J.G.; Zhang, X.; Surampalli, R. Engineered struvite precipitation: Impacts of component-ion molar ratios and pH. J. Environ. Eng. 2005, 131, 1433–1440. [Google Scholar] [CrossRef]
- Pastor, L.; Mangin, D.; Barat, R.; Seco, A. A pilot-scale study of struvite precipitation in a stirred tank reactor: Conditions influencing the process. Bioresour. Technol. 2008, 99, 6285–6291. [Google Scholar] [CrossRef]
- Etter, B.; Tilley, E.; Khadka, R.; Udert, K.M. Low-cost struvite production using source-separated urine in nepal. Water Res. 2011, 45, 852–862. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Değermenci, N.; Ata, O.N.; Yildız, E. Ammonia removal by air stripping in a semi-batch jet loop reactor. J. Ind. Eng. Chem. 2012, 18, 399–404. [Google Scholar] [CrossRef]
- Siegrist, H. Nitrogen removal from digester supernatant—Comparison of chemical and biological methods. Water Sci. Technol. 1996, 34, 399–406. [Google Scholar] [CrossRef]
- Zuleeg, S.; Wüthrich, F.; Egli, C.; Hürlimann, M.; Grömping, M.; Böhler, M.; Siegrist, H. ARA Altenrhein—Rücklaufbehandlung Variantenvergleich. (In German). In Cost Comparison on Different Side Stream Treatment Technologies for Ammonia Rich Supernatant for WWTP Altenrhein; ARA Altenrhein/Ingenieurbüro AG: St. Gallen, Switzerland, 2012. [Google Scholar]
- Fux, C.; Siegrist, H. Nitrogen removal from sludge digester liquids by nitrification/denitrification or partial nitritation/anammox: Environmental and economical considerations. Water Sci. Technol. 2004, 50, 19–26. [Google Scholar]
- Wett, B.; Buchauer, K.; Fimmi, C. Energy self-sufficiency as a feasible concept for wastewater treatment systems. In Proceedings of the Leading-Edge Technology Conference, Singapore, 4–6 June 2007.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morales, N.; Boehler, M.A.; Buettner, S.; Liebi, C.; Siegrist, H. Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale. Water 2013, 5, 1262-1278. https://doi.org/10.3390/w5031262
Morales N, Boehler MA, Buettner S, Liebi C, Siegrist H. Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale. Water. 2013; 5(3):1262-1278. https://doi.org/10.3390/w5031262
Chicago/Turabian StyleMorales, Nicolás, Marc Anton Boehler, Sandra Buettner, Christoph Liebi, and Hansruedi Siegrist. 2013. "Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale" Water 5, no. 3: 1262-1278. https://doi.org/10.3390/w5031262