Buffer Capacity, Ecosystem Feedbacks, and Seawater Chemistry under Global Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ecosystem Description
2.2. Modeling Approach


| Parameters | Parameter value | Units | Source |
|---|---|---|---|
| pmax | |||
| 400 µatm, 26 °C | 90 | mmol C m−2 h−1 | [15,17] |
| 600 µatm, 27.5 °C | 96.9 | Q10 from [18] | |
| 900 µatm, 29 °C | 103.6 | Q10 from [18] | |
| rdark | |||
| 400 µatm, 26 °C | 35 | mmol C m−2 h−1 | [10,14] |
| 600 µatm, 27.5 °C | 40.2 | Q10 from [18] | |
| 900 µatm, 29 °C | 46.1 | Q10 from [18] | |
| Imax | 1000 | µmol photons m−2 s−1 | [14] and Kāne‘ohe Bay Monitoring Program |
| Ik | 586 | µmol photons m−2 s−1 | [19] |
| kD | 1 | mmol C m−2 h−1 | [17,20] |
| bD | -6 | mmol C m−2 h−1 | [10,19] |
| kLE | |||
| 400 µatm, 26 °C | 9.1 | mmol C m−2 h−1 | [10] |
| 600 µatm, 27.5 °C | 8.463 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| 900 µatm, 29 °C | 6.552 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| bLE | |||
| 400 µatm, 26 °C | 9.1 | mmol C m−2 h−1 | [10] |
| 600 µatm, 27.5 °C | 8.463 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| 900 µatm, 29 °C | 6.552 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| kdark | |||
| 400 µatm, 26 °C | 3.4 | mmol C m−2 h−1 | [10] |
| 600 µatm, 27.5 °C | 3.162 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| 900 µatm, 29 °C | 2.448 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| bdark | |||
| 400 µatm, 26 °C | 3.4 | mmol C m−2 h−1 | [10] |
| 600 µatm, 27.5 °C | 3.162 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| 900 µatm, 29 °C | 2.448 | mmol C m−2 h−1 | Temperature-calcification equation from [21] |
| Ca | |||
| 400 µatm, 26 °C | 1.0 | Dimensionless | Estimated in conjunction with [22] |
| 600 µatm, 27.5 °C | 0.5 | Dimensionless | Estimated in conjunction with [22] |
| 900 µatm, 29 °C | 0.01 | Dimensionless | Estimated in conjunction with [22] |
2.3. Photosynthesis and Respiration



2.4. Calcification and Carbonate Dissolution






2.5. Ecosystem Feedback Scenarios
- (1)
- No feedbacks: This scenario assumes that reef metabolism is not influenced by seawater chemistry or temperature and that community structure is stable under all global change scenarios. We do not consider this scenario likely but rather use it as a means to distinguish the influence of ecosystem feedbacks from the pure chemical effects imposed by global change on seawater chemistry. For this scenario reef metabolic rates were calculated as if Ωarag were constant at Ωarag = 2.85 and temperature were constant at 26 °C (present-day mean summertime values), but including the influence of irradiance on photosynthesis and calcification. This scenario allows us to examine how global change affects seawater chemistry given the same metabolic forcing and provides a baseline with which to examine the effects of the other ecosystem feedbacks;
- (2)
- Calcification and dissolution feedback: This scenario allows calcification and dissolution to vary dynamically depending on changes in seawater chemistry and temperature (in addition to irradiance), however, it assumes that temperature and pCO2 have no effect on photosynthesis or respiration and that calcifier abundance is constant;
- (3)
- Calcification and dissolution + calcifier abundance feedbacks: In this scenario calcification and dissolution were allowed to vary dynamically and calcifier abundance was reduced for the future scenarios. Temperature and pCO2 were assumed to have no effect on photosynthesis or respiration;
- (4)
- Calcification and dissolution + photosynthesis and respiration feedbacks: Like the scenario above, calcification and dissolution were allowed to vary dynamically but temperature and pCO2 were also allowed to affect photosynthesis and respiration. Calcifier abundance was assumed to be constant;
- (5)
- Calcification and dissolution + calcifier abundance + photosynthesis and respiration feedbacks: Calcification, dissolution, and photosynthesis were all allowed to vary dynamically based on changes in chemistry and irradiance while calcification, photosynthesis, and respiration were allowed to change based on temperature. Calcifier abundance was also allowed to decrease for the future scenarios.
2.6. Reef Edge vs. Reef Flat Calcification, and Ecosystem Calcification Thresholds
3. Results and Discussions
3.1. Model Validation

3.2. Reef Flat Water Chemistry
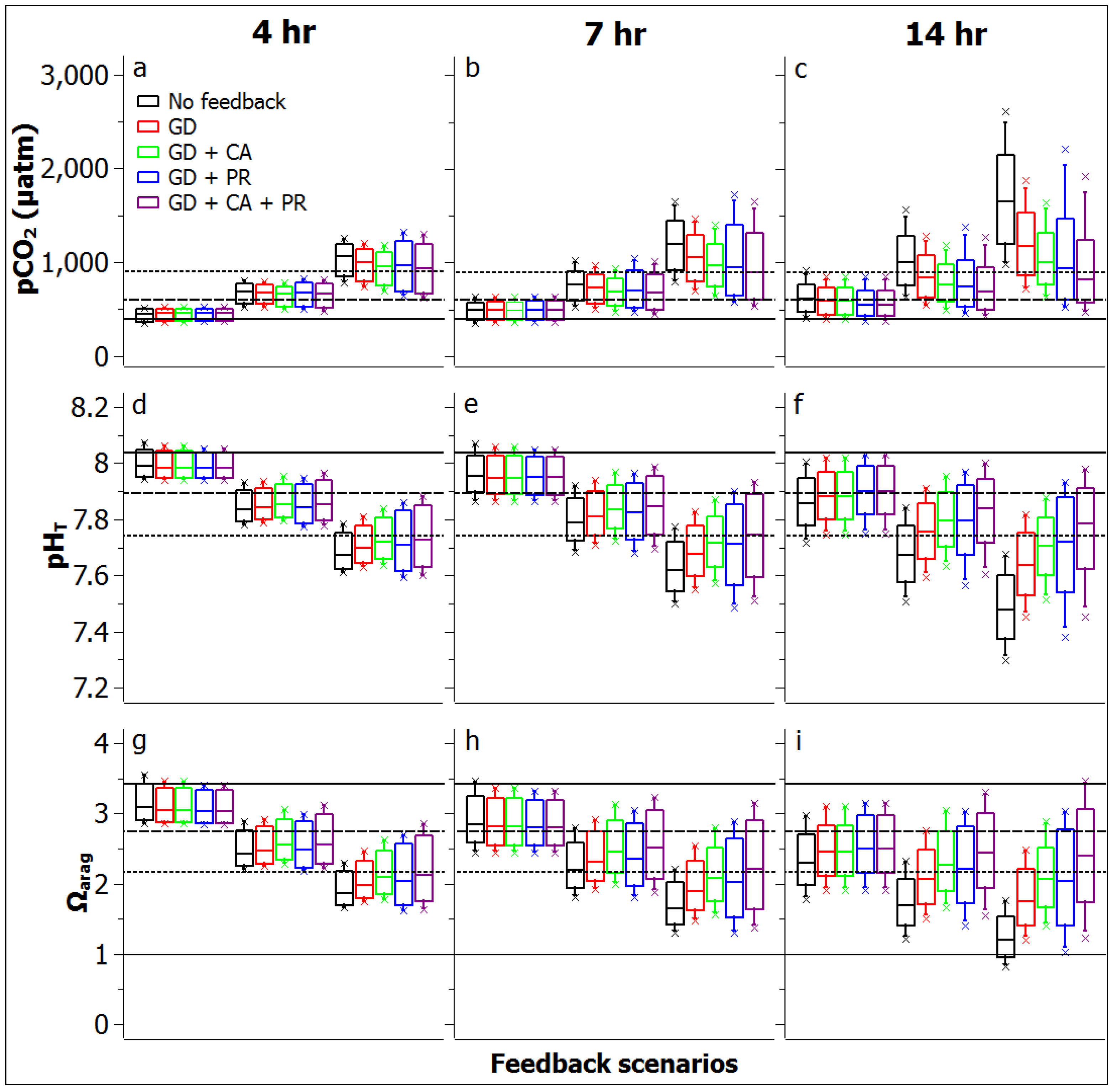
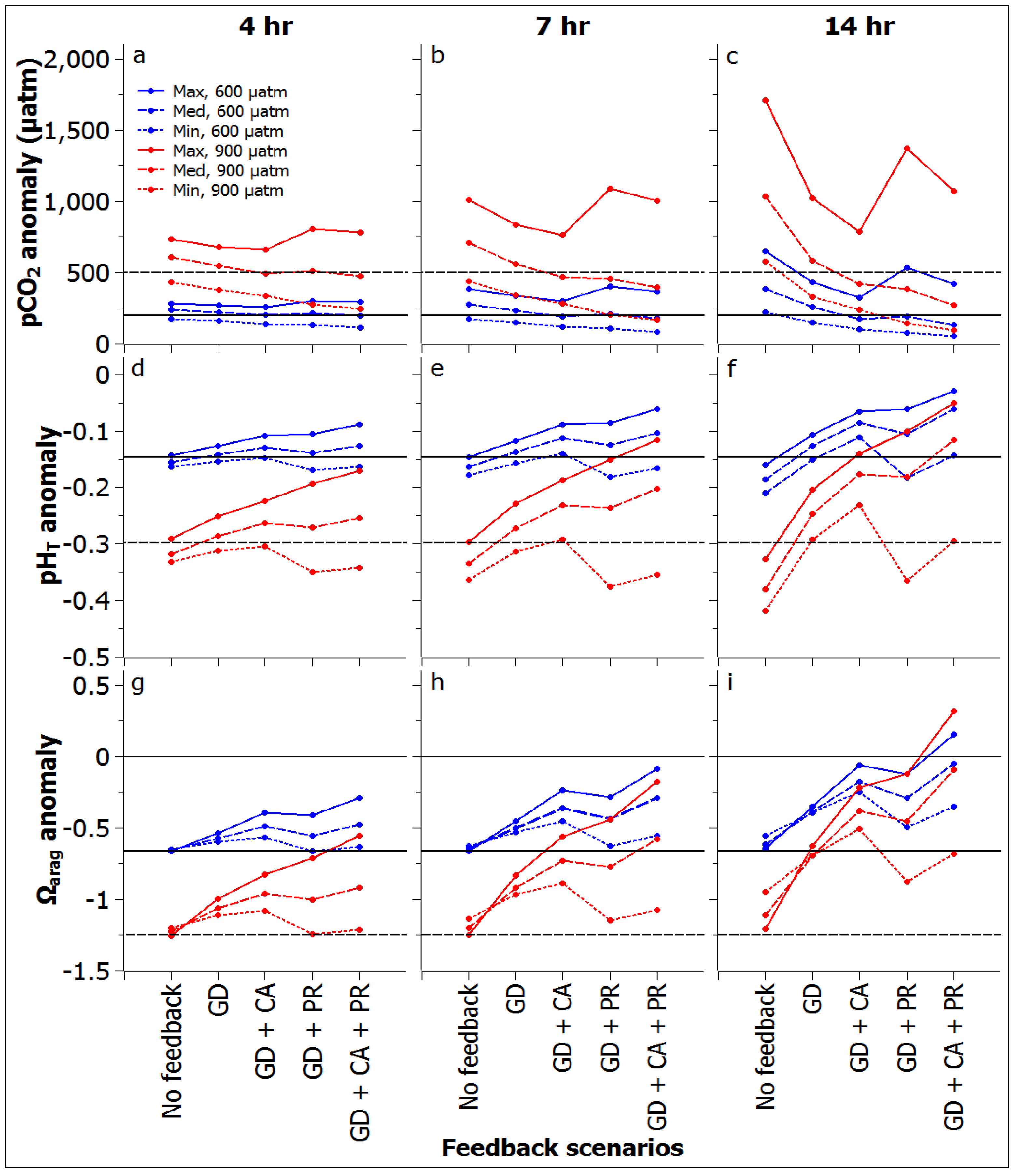
3.3. Reef Edge vs. Reef Flat Calcification, and Ecosystem Calcification Thresholds
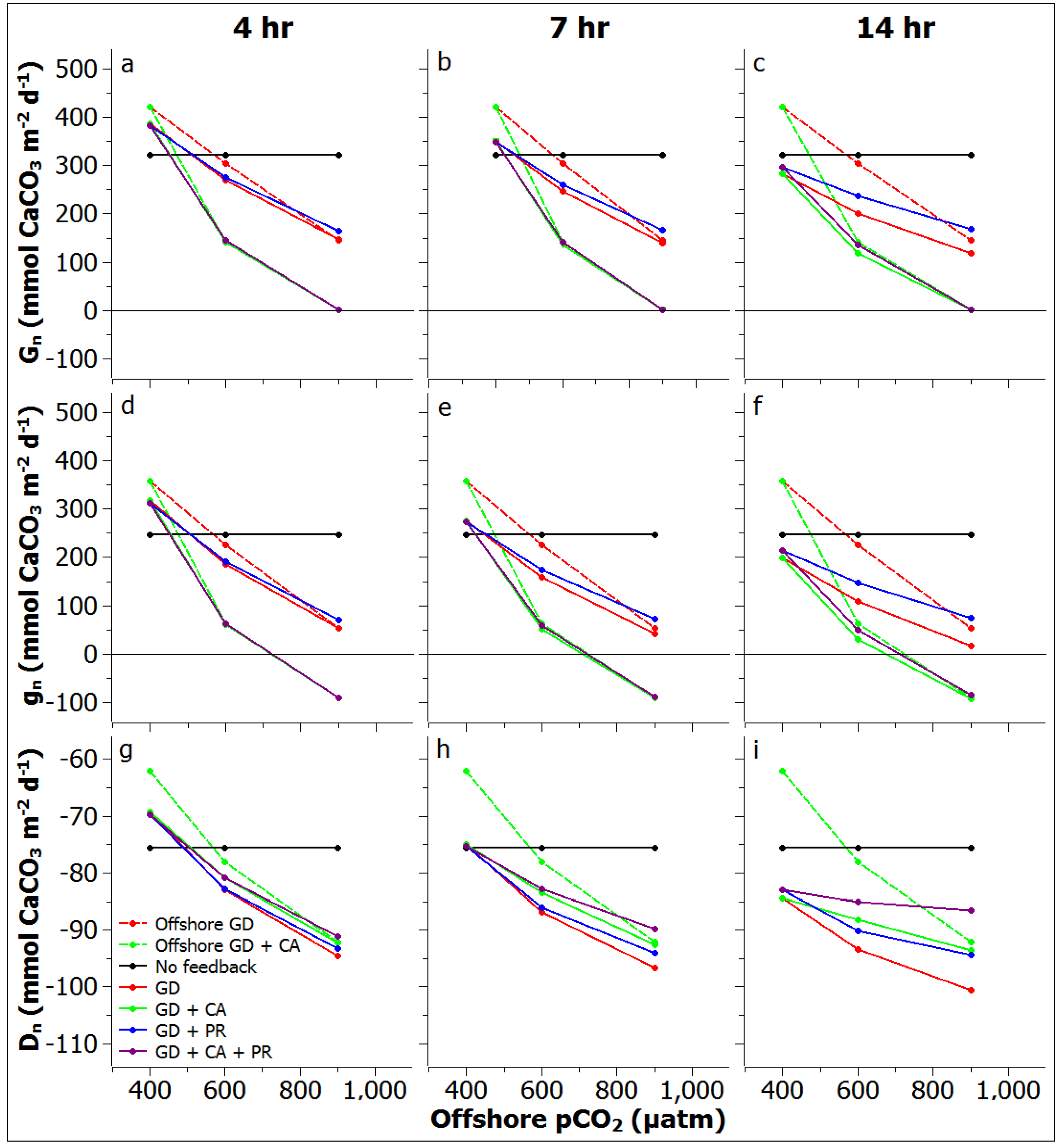
3.4. Comparisons to Other Systems
3.5. Biological Implications
3.6. Ecosystem Calcification Thresholds
4. Conclusions
Acknowledgments
References
- Revelle, R.; Suess, H.E. Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades. Tellus 1957, 9, 18–27. [Google Scholar] [CrossRef]
- Broeker, W.S.; Takahashi, T.; Simpson, H.J.; Peng, T.H. Fate of fossil fuel carbon dioxide and the global carbon budget. Science 1979, 206, 409–418. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Sundquist, E.T.; Plummer, L.N.; Wigley, T.M.L. Carbon dioxide in the ocean surface: The homogenous buffer factor. Science 1979, 204, 1203–1205. [Google Scholar]
- Fraginoulle, M. A complete set of buffer factors for acid/base CO2 system in seawater. J. Mar. Syst. 1994, 5, 111–118. [Google Scholar] [CrossRef]
- Egleston, E.S.; Sabine, C.L.; Morel, F.M.M. Revelle revisted: Buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Donner, S.D. Coping with commitment: Projected thermal stress on coral reefs under different future scenarios. PLoS ONE 2009, 4, e5712. [Google Scholar] [CrossRef]
- Borges, A.V.; Gypens, N. Carbonate chemistry in the coastal zone responds more strongly to eutrophication than ocean acidification. Limnol. Oceanogr. 2010, 55, 346–353. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6, e28983. [Google Scholar] [CrossRef]
- Shamberger, K.E.F.; Feely, R.A.; Sabine, C.L.; Atkinson, M.J.; DeCarlo, E.H.; Mackenzie, F.T.; Drupp, P.S.; Butterfield, D.A. Calcification and organic production on a Hawaiian coral reef. Mar. Chem. 2011, 127, 64–75. [Google Scholar] [CrossRef]
- Hoppe, C.J.M.; Langer, G.; Rokitta, S.D.; Wolf-Gladrow, D.A.; Rost, B. Implications of observed inconsistencies in carbonate chemistry measurement for ocean acidification studies. Biogeosci. Discuss. 2012, 9, 2401–2405. [Google Scholar] [CrossRef] [Green Version]
- Falter, J.F.; Lowe, R.J.; Atkinson, M.J.; Cuet, P. Seasonal coupling and de-coupling of net calcification rates from coral reef metabolism and carbonate chemistry at Ningaloo Reef, Western Australia. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.W.R. Program Developed for CO2 System Calculations, ORNL/CDIAC-105; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Falter, J.L.; Lowe, R.J.; Atkinson, M.J.; Monismith, S.G.; Schar, D.W. Continuous measurements of net production over a shallow reef community using a modified Eulerian approach. J. Geophys. Res. Oceans 2008, 113. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Anthony, K.R.N.; Gattuso, J.-P. Coral reefs modify their seawater carbon chemistry—Case study from a barrier reef (Moorea, French Polynesia). Glob. Chang. Biol. 2011, 17, 3667–3678. [Google Scholar] [CrossRef]
- Carpenter, R.C.; Williams, S.L. Mass transfer limitation of photosynthesis of coral algal turf communities. Mar. Biol. 2007, 151, 435–450. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kleypas, J.A.; Gattuso, J.-P. Coral reefs modify their seawater carbon chemistry—Implications for impacts of ocean acidification. Glob. Change Biol. 2011, 17, 3655–3666. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Caffrey, J.M.; Cescatti, A.; Dossena, M.; del Giorgio, P.; Gasol, J.M.; Montoya, J.M.; Pumpanen, J.; Staehr, P.A.; Trimmer, M.; et al. Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 2012, 487, 472–476. [Google Scholar] [CrossRef]
- Langdon, C.; Atkinson, M.J. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 2005, 110. [Google Scholar] [CrossRef]
- Andersson, A.J.; Kuffner, I.B.; MacKenzie, F.T.; Jokiel, P.L.; Rodgers, K.S.; Tan, A. Net loss of CaCO3 from coral reef communities due to human induced seawater acidification. Biogeosci.Discuss. 2009, 6, 2163–2182. [Google Scholar] [CrossRef]
- Buddemeier, R.W.; Jokiel, P.L.; Zimmerman, K.M.; Lane, D.R.; Carey, J.M.; Bohling, G.C.; Martinich, J.A. A modeling tool to evaluate regional coral reef responses to change in climate and ocean chemistry. Limnol. Oceanogr. Methods 2008, 6, 395–411. [Google Scholar] [CrossRef]
- Hoeke, R.K.; Jokiel, P.L.; Budemeier, R.W.; Brainard, R.E. Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS ONE 2011, 6, e18038. [Google Scholar]
- Langdon, C.; Broecker, W.S.; Hammond, D.E.; Glenn, E.; Fitzsimmons, K.; Nelson, S.G.; Peng, T.-H.; Hajdas, I.; Bonani, G. Effect of elevated CO2 on the community metabolism of an experimental coral reef. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- 21Long-Term Kaneohe Bay Monitoring Project Homepage. Available online: http://www.hawaii.edu/cisnet/index.htm (accessed on 26 August 2013).
- Silverman, J.; Lazar, B.; Cao, L.; Caldeira, K.; Erez, J. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys. Res. Lett. Oceans 2009, 36. [Google Scholar] [CrossRef]
- Yates, K.K.; Halley, R.B. CO32− concentration and pCO2 thresholds for calcification and dissolution on the Molokai reef flat, Hawaii. Biogeosci. Discuss. 2006, 3, 357–369. [Google Scholar] [CrossRef]
- Jury, C.P.; Whitehead, R.F.; Szmant, A.M. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (=Madracis mirabilis sensu Wells, 1973): Bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 2010, 15, 1632–1644. [Google Scholar]
- Jokiel, P.L. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bull. Mar. Sci. 2011, 87, 639–657. [Google Scholar] [CrossRef]
- Jokiel, P.L. The reef coral two compartment proton flux model: A new approach relating tissue-level physiological processes to gross corallum morphology. J. Exp. Mar. Biol. Ecol. 2011, 409, 1–12. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Boyd, P.W.; Hurd, C.L. Before ocean acidification: Calcifier chemistry lessons. J. Phycol. 2012, 48, 840–843. [Google Scholar] [CrossRef]
- Comeau, S.; Carpenter, R.C.; Edmunds, P.J. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. R. Soc. B 2012, 28. [Google Scholar] [CrossRef]
- Chalker, B.E.; Taylor, D.L. Light-enhanced calcification, and the role of oxidative phosphorylation in calcification of the coral Acropora cervicoris. Proc. R. Soc. B 1975, 190, 323–331. [Google Scholar] [CrossRef]
- De Beer, D.; Larkum, A.W.D. Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors. Plant Cell Environ. 2001, 24, 1209–1217. [Google Scholar] [CrossRef]
- Colombo-Pallotta, M.F.; Rodríguez-Román, A.; Iglesias-Prieto, R. Calcification in bleaching and unbleached Montastraea faveolata: Evaluating the role of oxygen and glycerol. Coral Reefs 2010, 29, 899–907. [Google Scholar] [CrossRef]
- Coles, S.L.; Brown, B.E. Coral bleaching—Capacity for acclimatization and adaptation. Adv. Mar. Biol. 2003, 46, 183–223. [Google Scholar]
- Maynard, J.A.; Anthony, K.R.N.; Marshall, P.A.; Masiri, I. Major bleaching events can lead to increased thermal tolerance in corals. Mar. Biol. 2008, 155, 173–182. [Google Scholar] [CrossRef]
- Guest, J.R.; Baird, A.H.; Maynard, J.A.; Muttaqin, E.; Edwards, A.J.; Campbell, S.J.; Yewdall, K.; Affendi, Y.A.; Chou, L.M. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 2012, 7, e33353. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh–Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar]
- PANGAEA Home Page. Available online: http://www.pangaea.de/ (accessed on 26 August 2013).
- Shaw, E.C.; McNeil, B.I.; Bronte, T.; Matear, R.; Bates, M.L. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob. Change Biol. 2013, 19, 1632–1641. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrel, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Rodolfo-Metalpa, R.; Martin, S.; Ransome, E.; Fine, M.; Turner, S.M.; Rowley, S.J.; Tedesco, D.; Buia, M.-C. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar] [CrossRef]
- Kerrison, P.; Hall-Spencer, J.M.; Suggett, D.J.; Hepburn, L.J.; Steinke, M. Assessment of pH variability at a coastal CO2 vent for ocean acidification studies. Estuar. Coast. Shelf Sci. 2011, 94, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Crook, E.D.; Potts, D.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 2012, 31, 239–245. [Google Scholar] [CrossRef]
- Dufault, A.M.; Cumbo, V.R.; Fan, T.-Y.; Edmunds, P.J. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B 2012, 279, 2951–2958. [Google Scholar] [CrossRef]
- Alenius, B.; Munguia, P. Effects of pH variability on the intertidal isopod, Paradella dianae. Mar. Freshw. Behav. Phy. 2012, 45, 245–259. [Google Scholar] [CrossRef]
- Shamberger, K.E.F.; Cohen, A.L.; McCorkle, D.C.; Lentz, S.; Feely, R.; Sabine, C.L.; Mcleod, E. Quantifying Ecosystem Calcification Responses to Elevated CO2 on Coral Reefs. In Proceedings of 12th International Coral Reef Symposium, Cairns, Australia, 9-12 July 2012.
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 2009, 37, 1131–1134. [Google Scholar] [CrossRef]
- Comeau, S.; Edmunds, P.J.; Spindel, N.B.; Carpenter, R.C. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 2013, 58, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Venn, A.; Tambutté, E.; Holcomb, M.; Tambutté, S. Live tissue imagining shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 2011, 6, e20013. [Google Scholar]
- Price, N.N.; Matz, T.R.; Brainard, R.E.; Smith, J.E. Diel variability in seawater pH related to calcification and benthic community structure on coral reefs. PLoS ONE 2012, 7, e43843. [Google Scholar]
- Kline, D.I.; Teneva, L.; Schneider, K.; Miard, T.; Chai, A.; Marker, M.; Headley, K.; Opdyke, B.; Nash, M.; Veletich, M.; et al. A short-term in situ CO2 enrichment experiment on Heron Island (GBR). Sci. Rep. 2012, 2. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Rodgers, K.S.; Kuffner, I.B.; Andersson, A.J.; Cox, E.F.; MacKenzie, F.T. Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs 2008, 27, 473–483. [Google Scholar] [CrossRef]
- Friedrich, T.; Timmerman, A.; Abe-Ouchi, A.; Bates, N.R.; Chikamoto, M.O.; Church, M.J.; Dore, J.E.; Gledhill, D.K.; González–Dávila, M.; Heinemann, M.; et al. Detecting regional anthropogenic trends in ocean acidification against natural variability. Nat. Clim. Chang. 2012, 2, 167–171. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jury, C.P.; Thomas, F.I.M.; Atkinson, M.J.; Toonen, R.J. Buffer Capacity, Ecosystem Feedbacks, and Seawater Chemistry under Global Change. Water 2013, 5, 1303-1325. https://doi.org/10.3390/w5031303
Jury CP, Thomas FIM, Atkinson MJ, Toonen RJ. Buffer Capacity, Ecosystem Feedbacks, and Seawater Chemistry under Global Change. Water. 2013; 5(3):1303-1325. https://doi.org/10.3390/w5031303
Chicago/Turabian StyleJury, Christopher P., Florence I.M. Thomas, Marlin J. Atkinson, and Robert J. Toonen. 2013. "Buffer Capacity, Ecosystem Feedbacks, and Seawater Chemistry under Global Change" Water 5, no. 3: 1303-1325. https://doi.org/10.3390/w5031303




