Effects of Ocean Acidification and Warming on Sperm Activity and Early Life Stages of the Mediterranean Mussel (Mytilus galloprovincialis)
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Organism and Collection of Gametes
2.2. Sperm Activity
2.3. Larval Experiment
| Time (h a.f.) | Treatment | Measured parameters | Calculated parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pCO2 | T | S * | T ** (°C) | pHT | DIC (μmol kg−1) | pHT | pCO2 (ppm) | pCO2 (μatm) | AT (μeq kg−1) | ΩAr | |
| 0.5 | CSW | L | 35 | 16.8 ± 0.5 | 7.93 ± 0.03 | 2903 ± 24 | 8.06 ± 0.03 | 549 ± 43 | 539 ± 42 | 3200 ± 33 | 3.6 ± 0.3 |
| TSW | L | 7.87 ± 0.09 | 2964 ± 32 | 8.00 ± 0.09 | 662 ± 158 | 651 ± 156 | 3226 ± 68 | 3.3 ± 0.6 | |||
| CSW | H | 19.6 ± 0.2 | 7.92 ± 0.05 | 2911 ± 12 | 8.00 ± 0.05 | 661 ± 76 | 646 ± 75 | 3207 ± 29 | 3.7 ± 0.4 | ||
| TSW | H | 7.81 ± 0.07 | 2925 ± 48 | 7.88 ± 0.07 | 890 ± 163 | 870 ± 159 | 3146 ± 22 | 2.9 ± 0.4 | |||
| 47.5 | CSW | L | 35 | 16.8 ± 0.5 | 7.97 ± 0.06 | 2850 ± 91 | 8.10 ± 0.06 | 490 ± 64 | 481 ± 63 | 3173 ± 138 | 3.9 ± 0.6 |
| TSW | L | 7.60 ± 0.01 | 2859 ± 176 | 7.73 ± 0.01 | 1210 ± 73 | 1189 ± 71 | 2969 ± 181 | 1.7 ± 0.1 | |||
| CSW | H | 19.6 ± 0.2 | 8.02 ± 0.04 | 2834 ± 48 | 8.09 ± 0.04 | 504 ± 60 | 493 ± 58 | 3198 ± 21 | 4.4 ± 0.3 | ||
| TSW | H | 7.65 ± 0.01 | 2887 ± 95 | 7.71 ± 0.01 | 1293 ± 85 | 1263 ± 83 | 3018 ± 92 | 2.0 ± 0.1 | |||
2.3.1. Calcification
2.3.2. Metabolism
2.3.3. Larval Size and Survival
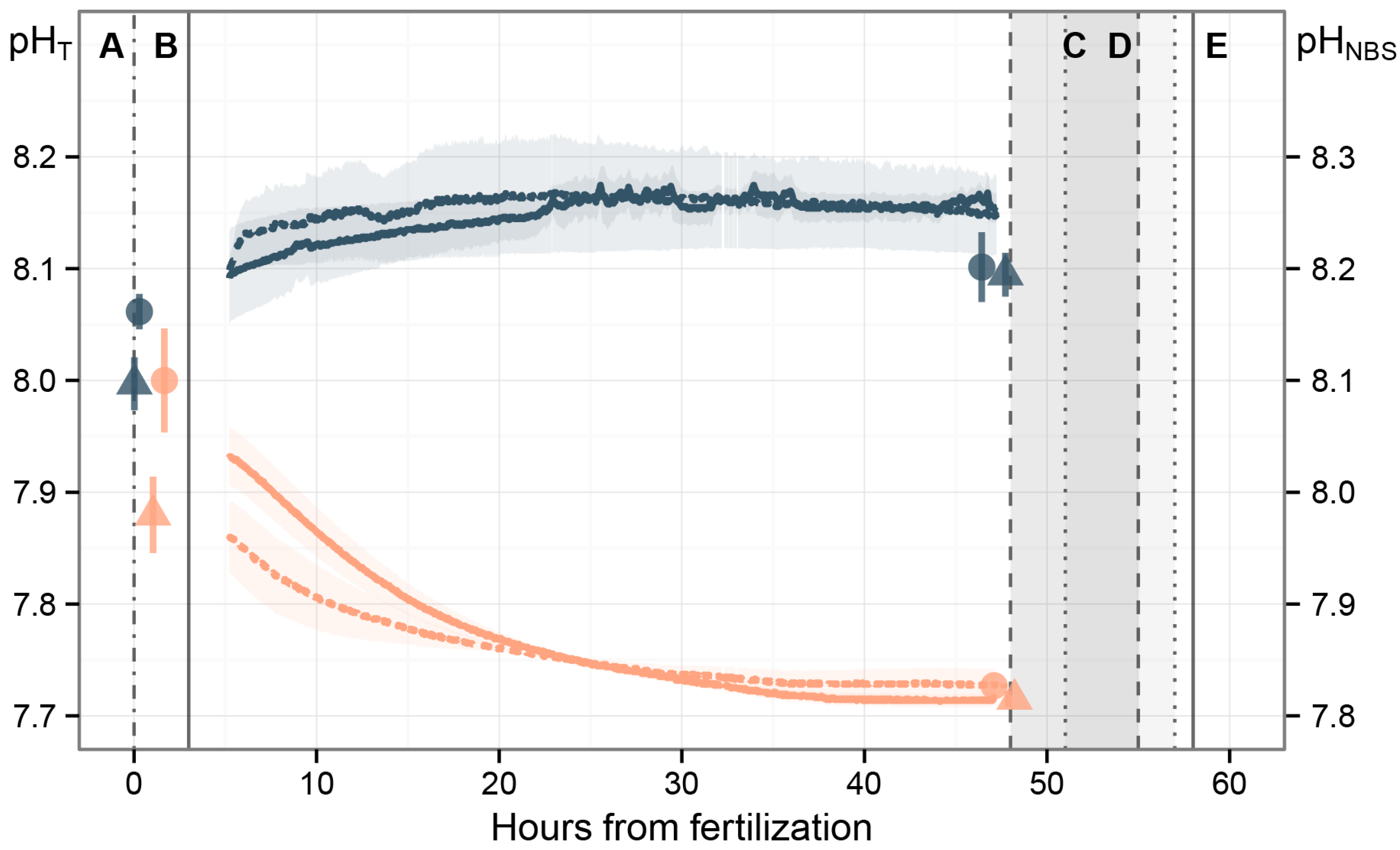
2.4. Carbonate System
2.5. Statistical Analyses
3. Results
3.1. Carbonate System
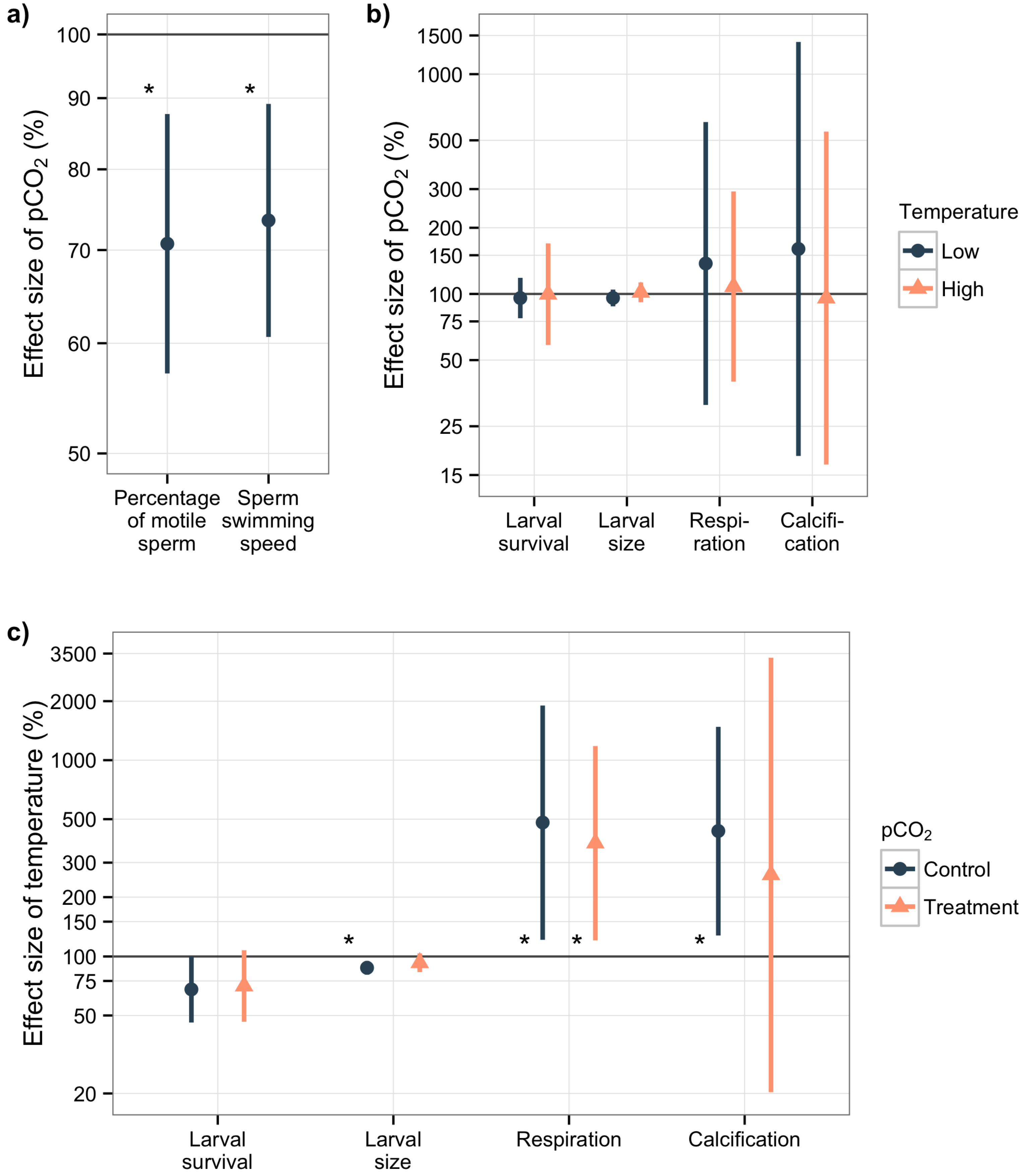
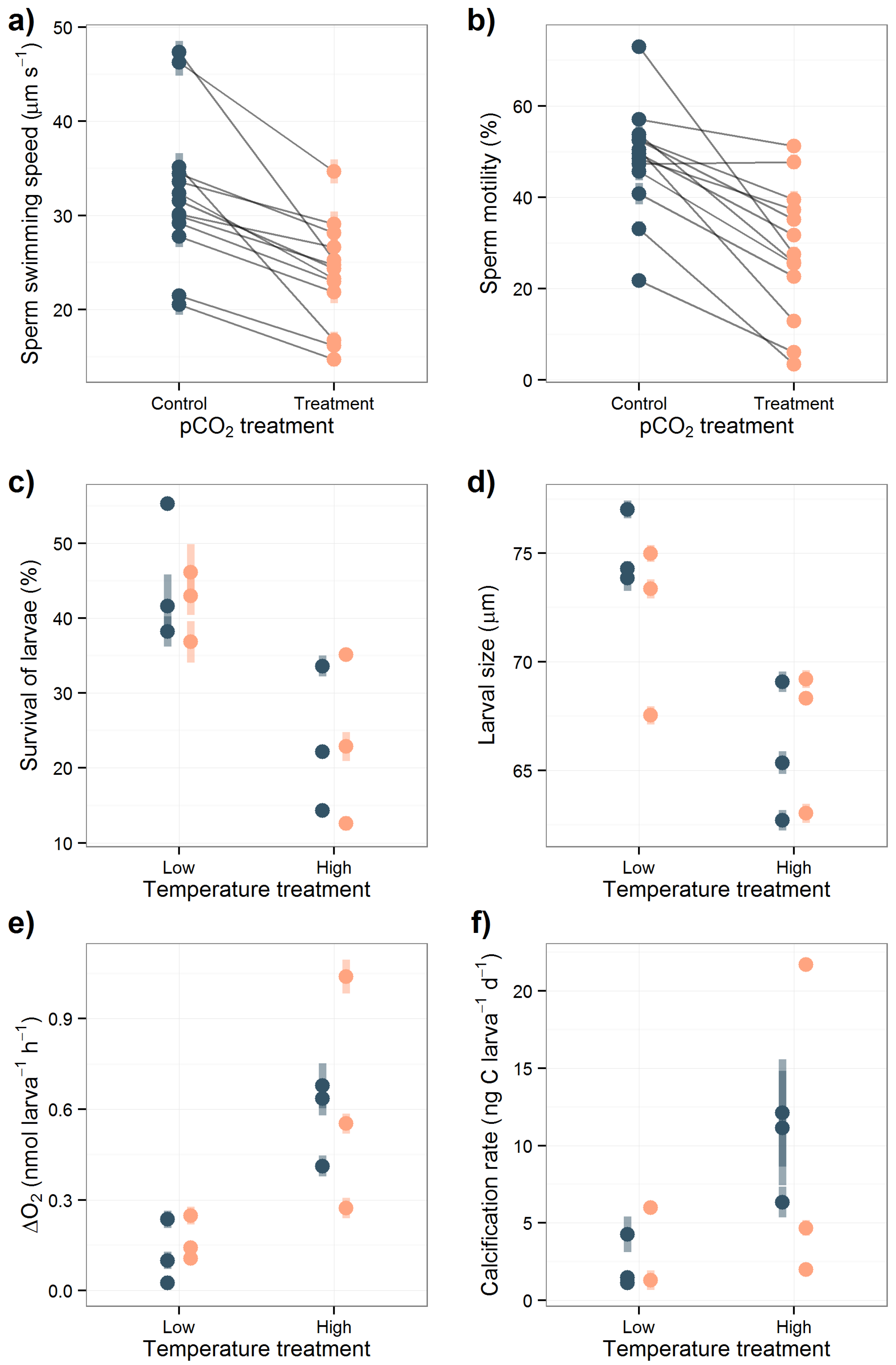
3.2. Sperm Activity
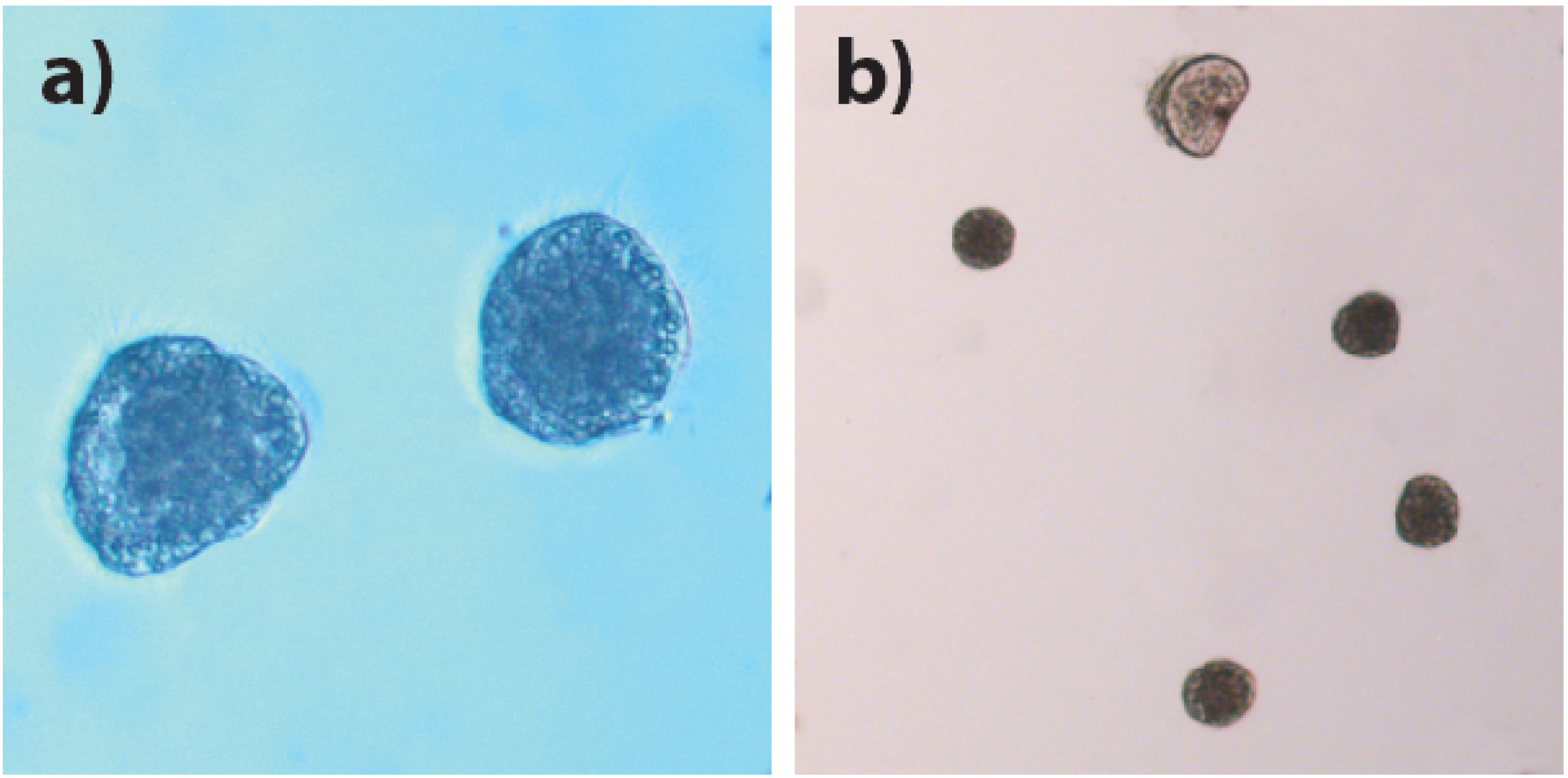
3.3. Larval Experiment
| Response | Factor | Df | Estimate | SE | t value | P | R2 |
|---|---|---|---|---|---|---|---|
| Speed | Intercept | 41.20 | 4.59 | 8.97 | <0.001 | ||
| time (h) | 1 | −3.20 | 1.52 | −2.10 | 0.047 | ||
| pCO2 (TSW) | 1 | −8.56 | 2.492 | −3.44 | 0.002 | ||
| Residuals | 23 | 6.35 | |||||
| Total | 25 | 0.002 | 0.41 | ||||
| Motility | Intercept | 56.17 | 9.73 | 5.77 | <0.001 | ||
| time (h) | 1 | −2.87 | 3.21 | −0.89 | 0.382 | ||
| pCO2 (TSW) | 1 | −19.98 | 5.28 | −3.78 | <0.001 | ||
| Residuals | 23 | 13.46 | |||||
| Total | 25 | 0.003 | 0.40 | ||||
| Intercept | 79.04 | 12.12 | 6.52 | <0.001 | |||
| time (h) | 1 | −1.62 | 4.16 | −0.39 | 0.704 | ||
| Residuals | 11 | 12.30 | |||||
| Total | 12 | 0.704 | 0.01 | ||||
| Intercept | 91.75 | 24.70 | 3.72 | 0.003 | |||
| time (h) | 1 | −12.61 | 8.48 | −1.49 | 0.165 | ||
| Residuals | 11 | 25.07 | |||||
| Total | 12 | 0.165 | 0.17 |
4. Discussion
4.1. Carbonate System
4.2. Acidification Effects on Sperm Activity
4.3. Overall Effects in the Larval Experiment
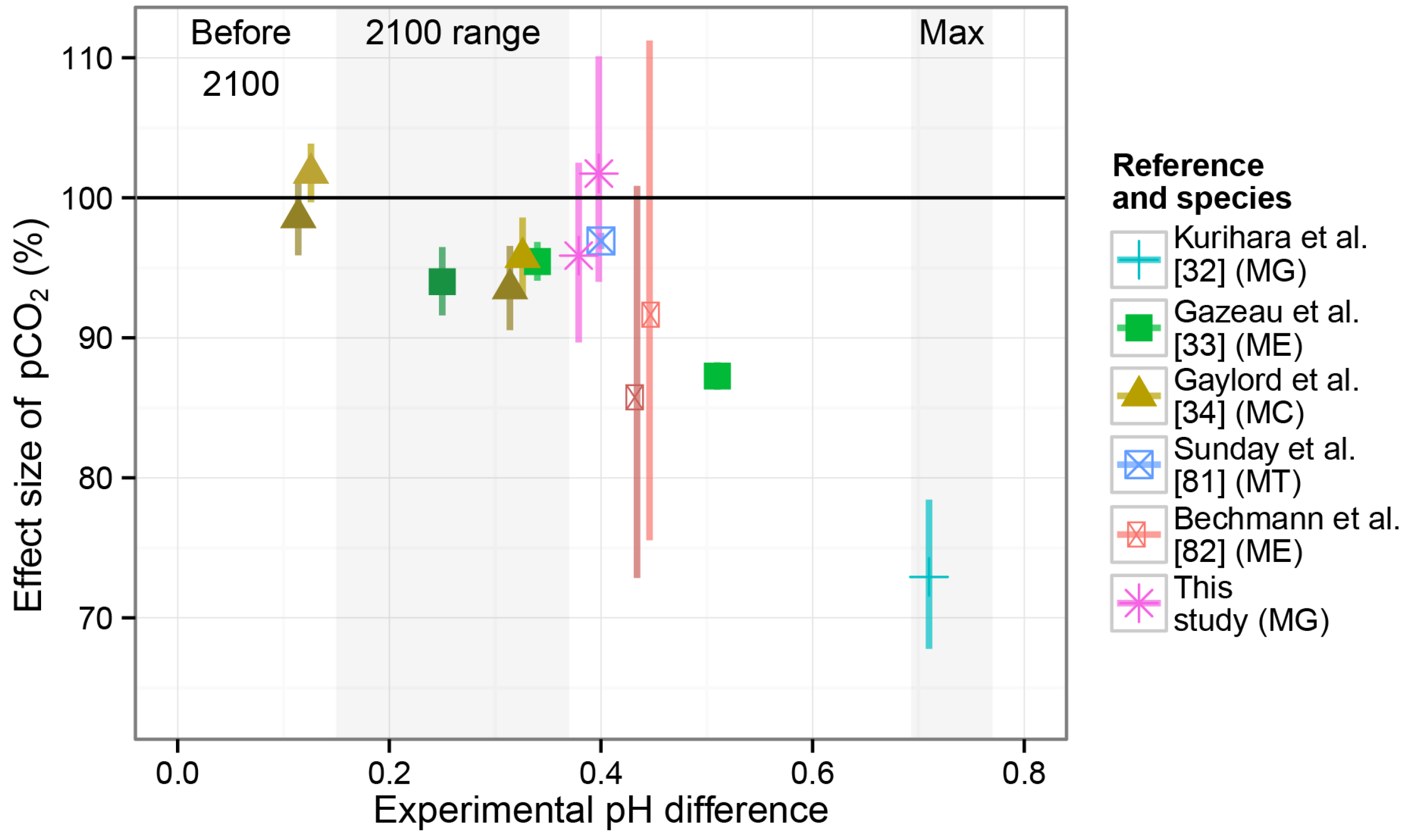
4.3.1. Acidification Effects on Larval Survival and Size
4.3.2. Temperature Effects on Metabolism, Larval Survival and Size
4.3.3. Calcification
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007; pp. 1–104. [Google Scholar]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 749–845. [Google Scholar]
- Orr, J.C. Recent and Future Changes in Ocean Carbonate Chemistry. In Ocean Acidification, 1st ed.; Gattuso, J.P., Hansson, L., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 41–63. [Google Scholar]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.; Duarte, C.; Álvarez, M. Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuar. Coast. Shelf Sci. 2010, 86, 157–164. [Google Scholar] [CrossRef]
- Dupont, S.; Dorey, N.; Thorndyke, M. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar. Coast. Shelf Sci. 2010, 89, 182–185. [Google Scholar] [CrossRef]
- Ross, P.M.; Parker, L.; O’Connor, W.A.; Bailey, E.A. The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 2011, 3, 1005–1030. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M. Impact of OceanWarming and Ocean Acidification on Marine Invertebrate Life History Stages: Vulnerabilities and Potential for Persistence in a Changing Ocean. In Oceanography and Marine Biology—An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; CRC Press: Boca Raton, FL, US, 2011; Volume 49, pp. 1–42. [Google Scholar]
- Somero, G.N. Thermal physiology and vertical zonation of intertidal animals: Optima, limits, and costs of living. Integr. Comp. Biol. 2002, 42, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. Reg. I 2007, 293, R911–R921. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.D.G.; Randall Hughes, A.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.B.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Przeslawski, R. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 2013, 53, 582–596. [Google Scholar] [CrossRef] [PubMed]
- McElhany, P.; Shallin Busch, D. Appropriate pCO2 treatments in ocean acidification experiments. Mar. Biol. 2012, 159, 1–6. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS One 2011, 6, e28983:1–e28983:11. [Google Scholar]
- Duarte, C.M.; Hendriks, I.E.; Moore, T.S.; Olsen, Y.S.; Steckbauer, A.; Ramajo, L.; Carstensen, J.; Trotter, J.A.; McCulloch, M. Is ocean scidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar. Coasts 2013, 36, 221–236. [Google Scholar] [CrossRef]
- Thomsen, J.; Gutowska, M.A.; Saphörster, J.; Heinemann, A.; Trübenbach, K.; Fietzke, J.; Hiebenthal, C.; Eisenhauer, A.; Körtzinger, A.; Wahl, M.; et al. Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 2010, 7, 3879–3891. [Google Scholar] [CrossRef] [Green Version]
- Soot-Ryen, T. A report on the family Mytilidae (Pelecypoda). Allan Hancock Pacif. Exped. 1955, 20, 1–175. [Google Scholar]
- Seed, R. Ecology. In Marine Mussels, Their Ecology and Physiology; Bayne, B.L., Ed.; Cambridge University Press: London, UK, 1976; pp. 13–66. [Google Scholar]
- Gosling, E.M. The systematic status of Mytilus galloprovincialis in Western Europe. Malacologia 1984, 25, 551–568. [Google Scholar]
- Invasive Species Specialist Group Homepage. Available online: http://www.issg.org/ (accessed on 31 March 2013).
- Dodd, J. Environmentally controlled variation in the shell structure of a pelecypod species. J. Paleontol. 1964, 38, 1065–1071. [Google Scholar]
- Fuller, S.; Lutz, R. Early shell mineralogy, microstructure, and surface sculpture in five mytilid species. Malacologia 1988, 29, 363–371. [Google Scholar]
- Andersson, A.J.; Mackenzie, F.T.; Gattuso, J.P. Effects of Ocean Acidification on Benthic Processes, Organisms, and Ecosystems. In Ocean Acidification, 1st ed.; Gattuso, J.P., Hansson, L., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 140–141. [Google Scholar]
- Navarro, J.M.; Torres, R.; Acuña, K.; Duarte, C.; Manriquez, P.H.; Lardies, M.; Lagos, N.A.; Vargas, C.; Aguilera, V. Impact of medium-term exposure to elevated pCO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere 2012, 90, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.; Melzner, F. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Mar. Biol. 2010, 157, 2667–2676. [Google Scholar]
- Fernández-Reiriz, M.J.; Range, P.; Álvarez Salgado, X.A.; Espinosa, J.; Labarta, U. Tolerance of juvenile Mytilus galloprovincialis to experimental seawater acidification. Mar. Ecol. Prog. Ser. 2012, 454, 65–74. [Google Scholar]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 2009, 37, 1131–1134. [Google Scholar] [CrossRef]
- Kurihara, H.; Asai, T.; Kato, S.; Ishimatsu, A. Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis. Aquat. Biol. 2008, 4, 225–233. [Google Scholar] [CrossRef]
- Gazeau, F.; Gattuso, J.P.; Dawber, C.; Pronker, A.E.; Peene, F.; Peene, J.; Heip, C.H.R.; Middelburg, J.J. Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences 2010, 7, 2051–2060. [Google Scholar]
- Gaylord, B.; Hill, T.M.; Sanford, E.; Lenz, E.A.; Jacobs, L.A.; Sato, K.N.; Russell, A.D.; Hettinger, A. Functional impacts of ocean acidification in an ecologically critical foundation species. J. Exp. Biol. 2011, 214, 2586–2594. [Google Scholar]
- Havenhand, J.N.; Buttler, F.R.; Thorndyke, M.C.; Williamson, J.E. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 2008, 18, 651–652. [Google Scholar]
- Foo, S.A.; Dworjanyn, S.A.; Poore, A.G.B.; Byrne, M. Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: Performance of early embryos. PLoS One 2012, 7, e42497:1–e42497:9. [Google Scholar]
- Schlegel, P.; Havenhand, J.N.; Gillings, M.R.; Williamson, J.E. Individual variability in reproductive success determines winners and losers under ocean acidification: A case study with sea urchins. PLoS One 2012, 7, e53118:1–e53118:8. [Google Scholar]
- Barros, P.; Sobral, P.; Range, P.; Chícharo, L.; Matias, D. Effects of sea-water acidification on fertilization and larval development of the oyster Crassostrea gigas. J. Exp. Mar. Biol. Ecol. 2013, 440, 200–206. [Google Scholar]
- Caldwell, G.S.; Fitzer, S.; Gillespie, C.S.; Pickavance, G.; Turnbull, E.; Bentley, M.G. Ocean acidification takes sperm back in time. Invertebr. Reprod. Dev. 2011, 55, 217–221. [Google Scholar]
- His, E.; Seaman, M.N.L.; Beiras, R. A simplification the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Res. 1997, 31, 351–355. [Google Scholar]
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 31 March 2013).
- Honkoop, P.J.C.; Luttikhuizen, P.C.; Piersma, T. Experimentally extending the spawning season of a marine bivalve using temperature change and fluoxetine as synergistic triggers. Mar. Ecol. Prog. Ser. 1999, 180, 297–300. [Google Scholar]
- Havenhand, J.N.; Schlegel, P. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences 2009, 6, 3009–3015. [Google Scholar]
- Hinting, A.; Comhaire, F.; Schoonjans, F. Capacity of objectively assessed sperm motility characteristics in differentiating between semen of fertile and subfertile men. Ferlil. Steril. 1988, 20, 635–639. [Google Scholar]
- Paasche, E.; Brubak, S. Enhanced calcification in the coccolithophorid Emiliania huxleyi (Haptophyceae) under phosphorus limitation. Phycologia 1994, 33, 324–330. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis, 1st ed.; Pergamon Press: New York, NY, USA, 1984; pp. 1–173. [Google Scholar]
- Carpenter, J.H. The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol. Oceanogr. 1965, 10, 141–143. [Google Scholar]
- Labasque, T. Spectrophotometric Winkler determination of dissolved oxygen: Re-examination of critical factors and reliability. Mar. Chem. 2004, 88, 53–60. [Google Scholar]
- Department of Energy. Recommended Standard Operating Procedures (SOPs). In Handbook of Methods for the Analysis of the Various Parameter of the Carbon Dioxide System in Sea Water, 2nd ed.; Dickson, A.G., Goyet, C., Eds.; ORNL/CDIAC-74: Oak Ridge, TN, US, 1994; pp. 30–96. [Google Scholar]
- Lewis, E.; Wallace, D. Program Developed for CO2 System Calculations; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1998; pp. 1–21. [Google Scholar]
- Gattuso, J.P.; Hansson, L. Ocean Acidification: Background and History. In Ocean Acidification, 1st ed.; Gattuso, J.P., Hansson, L., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 1–20. [Google Scholar]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. Camb. Philos. 2007, 82, 591–605. [Google Scholar]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar]
- Ahrens, W.H.; Cox, D.J.; Budhwar, G. Use of the arcsine and square root transformations for subjectively determined percentage data. Weed Sci. 1990, 38, 452–458. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009; pp. 1–211. [Google Scholar]
- Millero, F.; Morse, J.; Chen, C. The carbonate system in the western Mediterranean Sea. Deep Sea Res. 1979, 26A, 1395–1404. [Google Scholar]
- Melzner, F.; Thomsen, J.; Koeve, W.; Oschlies, A.; Gutowska, M.A.; Bange, H.W.; Hansen, H.P.; Körtzinger, A. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 2013, 160, 1875–1888. [Google Scholar] [CrossRef]
- Guide to Best Practices for Ocean Acidification Research and Data Reporting; Riebesell, U.; Fabry, V.; Hansson, L.; Gattuso, J. (Eds.) Publications Office of the European Union: Luxembourg, 2010; pp. 1–260.
- Schneider, A.; Wallace, D.W.R.; Körtzinger, A. Alkalinity of the Mediterranean Sea. Geophys. Res. Lett. 2007, 34, L15608:1–L15608:5. [Google Scholar] [CrossRef]
- Gazeau, F.; Duarte, C.M.; Gattuso, J.P.; Barrón, C.; Navarro, N.; Ruíz, S.; Prairie, Y.T.; Calleja, M.; Delille, B.; Frankignoulle, M.; et al. Whole-system metabolism and CO2 fluxes in a Mediterranean Bay dominated by seagrass beds (Palma Bay, NW Mediterranean). Biogeosciences 2005, 2, 43–60. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Lavigne, H. Technical note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 2009, 6, 2121–2133. [Google Scholar] [CrossRef]
- Range, P.; Piló, D.; Ben-Hamadou, R.; Chícharo, M.; Matias, D.; Joaquim, S.; Oliveira, A.; Chícharo, L. Seawater acidification by CO2 in a coastal lagoon environment: Effects on life history traits of juvenile mussels Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 89–98. [Google Scholar] [CrossRef]
- Pörtner, H. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. Ser. 2008, 373, 203–217. [Google Scholar] [CrossRef]
- Reuter, K.E.; Lotterhos, K.E.; Crim, R.N.; Thompson, C.A.; Harley, C.D.G. Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Glob. Chang. Biol. 2011, 17, 163–171. [Google Scholar] [CrossRef]
- Nakamura, M.; Morita, M. Sperm motility of the scleractinian coral Acropora digitifera under preindustrial, current, and predicted ocean acidification regimes. Aquat. Biol. 2012, 15, 299–302. [Google Scholar] [CrossRef]
- Morita, M.; Suwa, R.; Iguchi, A.; Nakamura, M.; Shimada, K.; Sakai, K.; Suzuki, A. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 2010, 18, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Czihak, G.; Chang, P.; Wolf, W. Fertilization kinetics of sea urchin eggs. Math. Biosci. 1982, 58, 189–216. [Google Scholar] [CrossRef]
- Styan, C.A. Polyspermy, egg size, and the fertilization kinetics of free-spawning marine invertebrates. Am. Nat. 1998, 152, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Albright, R.; Mason, B. Projected near-future levels of temperature and pCO2 reduce coral fertilization success. PLoS One 2013, 8, e56468:1–e56468:8. [Google Scholar]
- Levitan, D.R.; Petersen, C. Sperm limitation in the sea. Trends Ecol. Evol. 1995, 10, 228–231. [Google Scholar] [CrossRef]
- Levitan, D.R. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. B 2000, 267, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.T.; Jha, M.; Breton, S.; Hoeh, W.R.; Blier, P.U. No effect of sperm interactions or egg homogenate on sperm velocity in the blue mussel, Mytilus edulis (Bivalvia: Mytilidae). Can. J. Zool. 2012, 90, 1291–1296. [Google Scholar] [CrossRef]
- Williams, M.E.; Bentley, M.G. Fertilization success in marine invertebrates: The influence of gamete age. Biol. Bull. 2002, 202, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.T. The ecology of fertilization of echinoid eggs: The consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol. Bull. 1985, 169, 417–430. [Google Scholar] [CrossRef]
- Evans, J.P.; Sherman, C.D.H. Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 2013, 224, 166–183. [Google Scholar] [PubMed]
- Palumbi, S.R. All males are not created equal: Fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc. Natl. Acad. Sci. USA 1999, 96, 12632–12637. [Google Scholar] [CrossRef] [PubMed]
- Levitan, D.R.; Sewell, M.A.; Chia, F.S. How distribution and abundance influence fertilization success in the sea urchin Strongylocentotus franciscanus. Ecology 1992, 73, 248–254. [Google Scholar] [CrossRef]
- Crimaldi, J.P.; Zimmer, R.K. The physics of broadcast spawning in benthic invertebrates. Annu. Rev. Mar. Sci. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.K.; Havenhand, J.N. Effects of temperature on sperm swimming behaviour, respiration and fertilization success in the serpulid polychaete, Galeolaria caespitosa (Annelida: Serpulidae). Invertebr. Reprod. Dev. 2005, 48, 7–17. [Google Scholar] [CrossRef]
- Sunday, J.M.; Crim, R.N.; Harley, C.D.G.; Hart, M.W. Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS One 2011, 6, e22881:1–e22881:8. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, R.K.; Taban, I.C.; Westerlund, S.; Godal, B.F.; Arnberg, M.; Vingen, S.; Ingvarsdottir, A.; Baussant, T. Effects of ocean acidification on early life stages of shrimp (Pandalus borealis) and mussel (Mytilus edulis). J. Toxicol. Environ. Health A 2011, 74, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Havenhand, J.N.; Dupont, S.T.; Quinn, G.P. Designing Ocean Acidification Experiments to Maximise Inference. In Guide to Best Practices for Ocean Acidification Research and Data Reporting; Riebesell, U., Fabry, V.J., Hansson, L., Gattuso, J.P., Eds.; Publications Office of the European Union: Luxembourg, 2010; pp. 67–79. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 2005, 110, C09S04:1–C09S04:12. [Google Scholar] [CrossRef]
- Borges, A.; Frankignoulle, M. Daily and seasonal variations of the partial pressure of CO2 in surface seawater along Belgian and southern Dutch coastal areas. J. Mar. Syst. 1999, 19, 251–266. [Google Scholar] [CrossRef]
- Thomsen, J.; Casties, I.; Pansch, C.; Körtzinger, A.; Melzner, F. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: Laboratory and field experiments. Glob. Chang. Biol. 2013, 19, 1017–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godbold, J.A.; Solan, M. Long-term effects of warming and ocean acidification are modified by seasonal variation in species responses and environmental conditions. Philos. Trans. R. Soc. B 2013, 368, 20130186:1–20130186:11. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Dorey, N.; Stumpp, M.; Melzner, F.; Thorndyke, M. Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2013, 160, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A.; Borysko, L.; Raftos, D.A.; Portner, H.O. Adult exposure influences offspring response to ocean acidification in oysters. Glob. Chang. Biol. 2012, 18, 82–92. [Google Scholar] [CrossRef]
- Kennedy, V.; Mihursky, J. Effects of temperature on the respiratory metabolism of three Chesapeake Bay bivalves. Chesap. Sci. 1972, 13, 1–22. [Google Scholar] [CrossRef]
- Widdows, J. The effects of temperature on the metabolism and activity of Mytilus edulis. Neth. J. Sea Res. 1973, 7, 387–398. [Google Scholar] [CrossRef]
- Jansen, J.M.; Hummel, H.; Bonga, S.W. The respiratory capacity of marine mussels (Mytilus galloprovincialis) in relation to the high temperature threshold. Comp. Biochem. Phys. A 2009, 153, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lazo, C.; Martínez-Pita, I. Effect of temperature on survival, growth and development of Mytilus galloprovincialis larvae. Aquac. Res. 2012, 43, 1127–1133. [Google Scholar] [CrossRef]
- His, E.; Robert, R.; Dinet, A. Combined effects of temperature and salinity on fed and starved larvae of the Mediterranean mussel Mytilus galloprovincialis and the Japanese oyster Crassostrea gigas. Mar. Biol. 1989, 100, 455–463. [Google Scholar] [CrossRef]
- Beaumont, A.R.; Turner, G.; Wood, A.R.; Skibinski, D.O. Hybridisations between Mytilus edulis and Mytilus galloprovincialis and performance of pure species and hybrid veliger larvae at different temperatures. J. Exp. Mar. Biol. Ecol. 2004, 302, 177–188. [Google Scholar] [CrossRef]
- Ruiz, M.; Tarifeño, E.; Llanos-Rivera, A.; Padget, C.; Campos, B.Y. Efecto de la temperatura en el desarrollo embrionario y larval del mejillón, Mytilus galloprovincialis (Lamarck, 1819). Rev. Biol. Mar. Oceanog. 2008, 1, 51–61. [Google Scholar] [CrossRef]
- Fuentes, J.; Reyero, I.; Zapata, C.; Alvarez, G. Influence of stock and culture site on growth rate and mortality of mussels (Mytilus galloprovincialis Lmk.) in Galicia, Spain. Aquaculture 1992, 105, 131–142. [Google Scholar] [CrossRef]
- Bayne, B.L. Growth and the delay of metamorphosis of the larvae of Mytilus edulis (L.). Ophelia 1965, 2, 1–47. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Karagiannis, D.; Angelidis, P.; Staikou, A.; Michaelidis, B. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comp. Biochem. Phys. A 2010, 156, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.; Takahashi, T.; Sweeney, C.; Chipman, D.; Goddard, J.; Marubini, F.; Aceves, H.; Barnett, H.; Atkinson, M.J. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 2000, 14, 639–654. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Frankignoulle, M.; Bourge, I.; Romaine, S.; Buddemeier, R.W. Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet Chang. 1998, 18, 37–46. [Google Scholar] [CrossRef]
- Gazeau, F.; Quiblier, C.; Jansen, J.M.; Gattuso, J.P.; Middelburg, J.J.; Heip, C.H.R. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 2007, 34, L07603:1–L07603:5. [Google Scholar] [CrossRef]
- Hayakaze, E.; Tanabe, K. Early larval shell development in mytilid bivalve Mytilus galloprovincialis. Jpn. J. Malacol. 1999, 58, 119–127. [Google Scholar]
- Malone, P.G.; Dodd, R. Temperature and salinity effects on calcification rate in Mytilus edulis and its paleoecological implicatons. Limnol. Oceanogr. 1967, 12, 432–436. [Google Scholar] [CrossRef]
- Duarte, C.; Navarro, J.M.; Acuña, K.; Torres, R.; Manríquez, P.H.; Lardies, M.A.; Vargas, C.A.; Lagos, N.A.; Aguilera, V. Combined effects of temperature and ocean acidification on the juvenile individuals of the mussel Mytilus chilensis. J. Sea Res. 2014, 85, 308–314. [Google Scholar] [CrossRef]
- Pineda, J.; Porri, F.; Starczak, V.; Blythe, J. Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. J. Exp. Mar. Biol. Ecol. 2010, 392, 9–21. [Google Scholar] [CrossRef]
- Giménez, L. Marine community ecology: Importance of trait-mediated effects propagating through complex life cycles. Mar. Ecol. Prog. Ser. 2004, 283, 303–310. [Google Scholar] [CrossRef]
Appendix
| Response | Factor | Df | SS | MS | F value | P |
|---|---|---|---|---|---|---|
| Survival | pCO2 | 1 | 7.83 × 10−4 | 7.83 × 10−4 | 0.074 | 0.793 |
| T | 1 | 1.46 × 10−1 | 1.46 × 10−1 | 13.733 | 0.006 | |
| pCO2 × T | 1 | 6.50 × 10−4 | 6.50 × 10−4 | 0.061 | 0.811 | |
| Residuals | 8 | 8.52 × 10−2 | 1.06 × 10−2 | |||
| Size | pCO2 | 1 | 2.87 | 2.87 | 0.289 | 0.606 |
| T | 1 | 1.57 × 102 | 1.57 × 102 | 15.764 | 0.004 | |
| pCO2 × T | 1 | 1.35 × 101 | 1.35 × 101 | 1.354 | 0.278 | |
| Residuals | 8 | 7.96 | 9.94 | |||
| Respiration | pCO2 | 1 | 6.27 × 10−9 | 6.27 × 10−9 | 0.134 | 0.724 |
| T | 1 | 6.25 × 10−7 | 6.25 × 10−7 | 13.320 | 0.006 | |
| pCO2 × T | 1 | 0.00 | 0.00 | 0.000 | 0.995 | |
| Residuals | 8 | 3.75 × 10−7 | 4.69 × 10−8 | |||
| Calcification | pCO2 | 1 | 3.04 × 10−6 | 3.04 × 10−6 | 0.080 | 0.785 |
| T | 1 | 1.25 × 10−4 | 1.25 × 10−4 | 3.302 | 0.112 | |
| pCO2 × T | 1 | 2.11 × 10−6 | 2.11 × 10−6 | 0.056 | 0.820 | |
| Residuals | 7 | 2.65 × 10−4 | 3.79 × 10−5 |

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vihtakari, M.; Hendriks, I.E.; Holding, J.; Renaud, P.E.; Duarte, C.M.; Havenhand, J.N. Effects of Ocean Acidification and Warming on Sperm Activity and Early Life Stages of the Mediterranean Mussel (Mytilus galloprovincialis). Water 2013, 5, 1890-1915. https://doi.org/10.3390/w5041890
Vihtakari M, Hendriks IE, Holding J, Renaud PE, Duarte CM, Havenhand JN. Effects of Ocean Acidification and Warming on Sperm Activity and Early Life Stages of the Mediterranean Mussel (Mytilus galloprovincialis). Water. 2013; 5(4):1890-1915. https://doi.org/10.3390/w5041890
Chicago/Turabian StyleVihtakari, Mikko, Iris E. Hendriks, Johnna Holding, Paul E. Renaud, Carlos M. Duarte, and Jon N. Havenhand. 2013. "Effects of Ocean Acidification and Warming on Sperm Activity and Early Life Stages of the Mediterranean Mussel (Mytilus galloprovincialis)" Water 5, no. 4: 1890-1915. https://doi.org/10.3390/w5041890






