Tracking Inflows in Lake Wivenhoe during a Major Flood Using Optical Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods

2.1. Dissolved Organic Carbon Analysis
2.2. UV Analysis
2.3. Fluorescence Analysis

| Region | Chemical composition of organic matter |
|---|---|
| I (P1): Ex:Em 200–250:280–330 | lower molecular weight tyrosine-like aromatic amino acids |
| II (P2): Ex:Em 200–250:330–380 | low molecular weight aromatic proteins and BOD-type substances |
| III (SMP): Ex:Em 250–340:280–380 | large molecular weight peptides and proteins (microorganism related by-products) |
| IV (FA): Ex:Em 200–250:380–500 | fulvic acid type substances |
| V (HA): Ex:Em 250–500:380–500 | humic acid type substances |

3. Results and Discussion
3.1. Spatial and Vertical Variation in DOM Concentration
| Site (location) | Surface | Mid-depth | Bottom |
|---|---|---|---|
| 30004 (upstream) | 2.782 (29.8) | 2.498 (40.7) | 2.622 (191.2) |
| 30017 (upstream) | 2.624 (68.9) | 2.312 (67.7) | 2.248 (212.8) |
| 30053 (middle) | 2.129 (77.4) | 3.433 (128.2) | 2.843 (229) |
| 33140 (middle) | 2.244 (90.6) | 1.748 (107.8) | 2.218 (175.1) |
| 33137 (downstream) | 2.373 (111.3) | 2.188 (116.5) | 2.929 (210.6) |
3.2. Optical Analysis
 , where Io is the incident radiation and I is the radiation after passing through the length of solution. In fluorescence spectroscopy, the energy released by excited species to come to the ground state is also recorded. The specific excitation and emission wavelengths are unique for particular species. Two molecules may have similar excitation energies, but different emission energies.
, where Io is the incident radiation and I is the radiation after passing through the length of solution. In fluorescence spectroscopy, the energy released by excited species to come to the ground state is also recorded. The specific excitation and emission wavelengths are unique for particular species. Two molecules may have similar excitation energies, but different emission energies.3.2.1. UV Spectra
| Wavelength (nm) | Property | Reference |
|---|---|---|
| 195 | Proteins | [29] |
| 210 | Amino acids | [14,30] |
| 215 | Peptides | [30,31] |
| 230 | Proteins | [32] |
| 254 | Aromaticity | [33] |
| 260 | Hydrophobic content/COD | [16,34] |
| 265 | Relative abundance of functional group | [35] |
| 272 | Aromaticity | [36] |
| 280 | Hydrophobic carbon index | [37] |
| 285 | Humification index | [27] |
| 300 | Characterisation of humic substances | [38] |
| 310–360 | Mycosporine-like amino acids | [39,40,41] |
| 350 | Apparent molecular size | [15] |
| 365 | Aromaticity, apparent molecular weight | [42] |
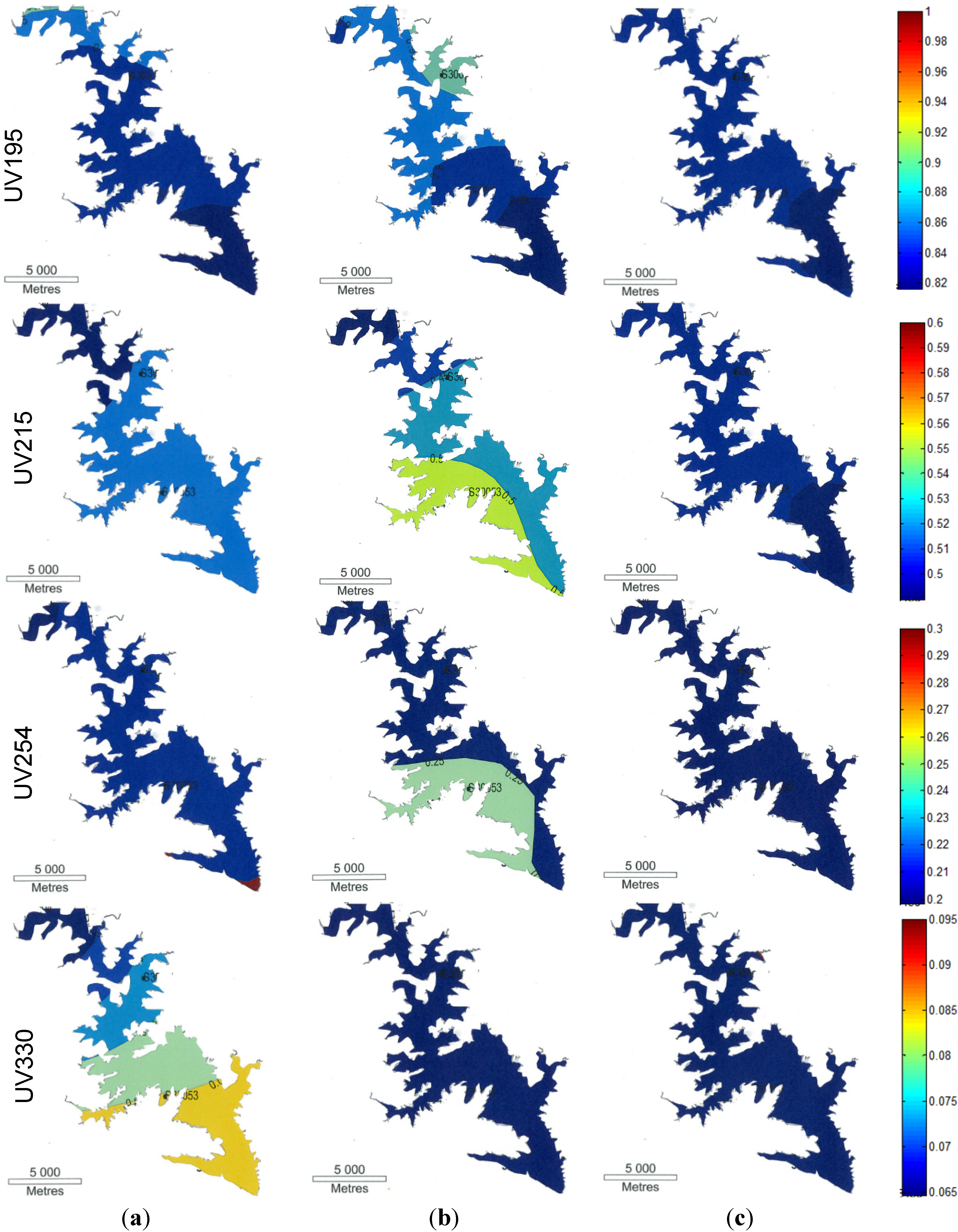
3.2.2. Fluorescence Spectra
4. Conclusions
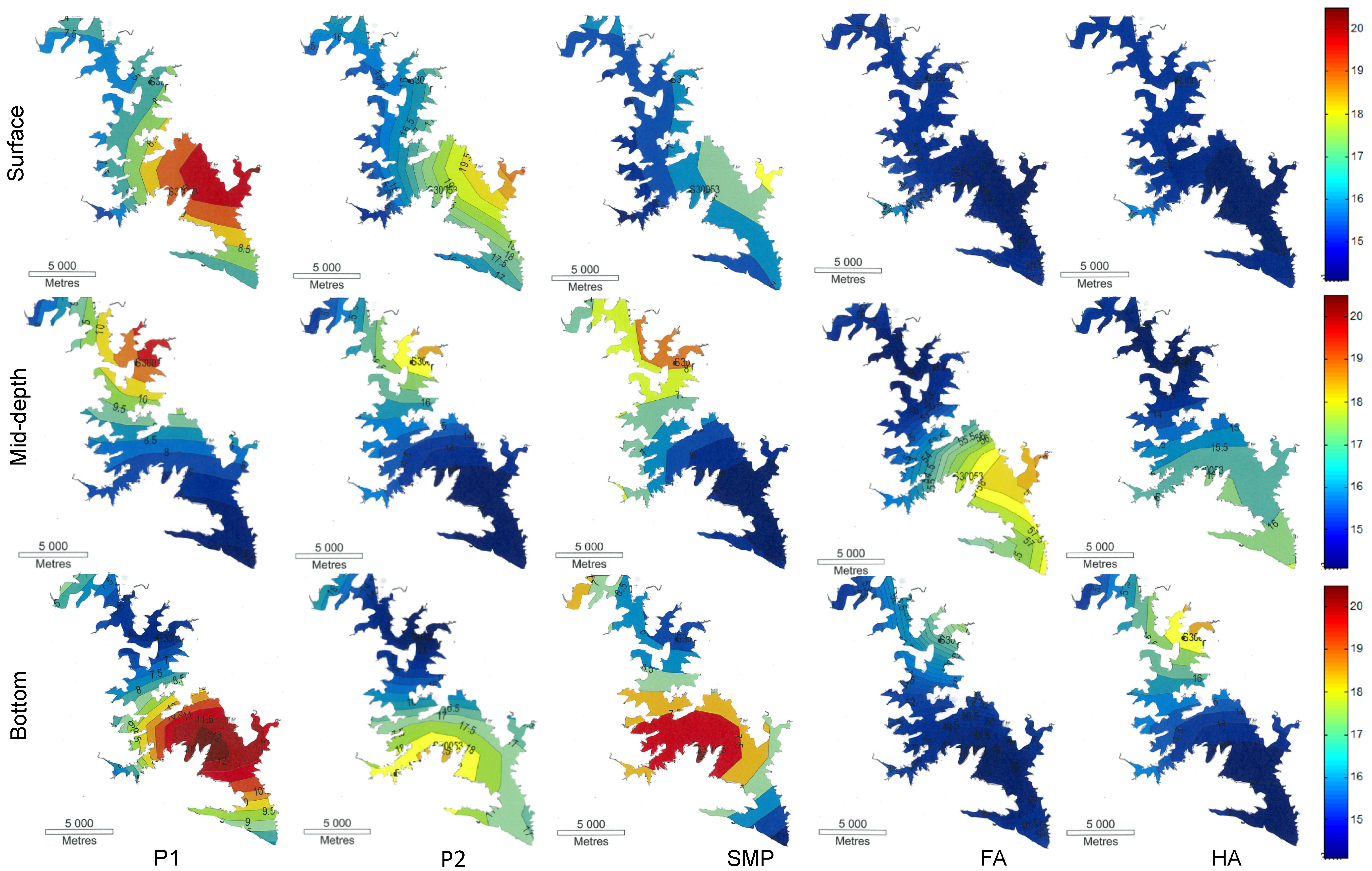
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grinham, A.; Gibbes, B.; Gale, D.; Watkinson, A.; Bartkow, M. Extreme rainfall and drinking water quality: A regional perspective. Proc. Water Pollut. 2012, 164, 183–194. [Google Scholar]
- Zafiriou, O.C.; Joussot-Dubien, J.; Zepp, R.G.; Zika, R.G. Photochemistry of natural waters. Environ. Sci. Technol. 1984, 18, 358A–371A. [Google Scholar]
- Mostofa, K.M.; Wu, F.; Liu, C.-Q.; Vione, D.; Yoshioka, T.; Sakugawa, H.; Tanoue, E. Photochemical, microbial and metal complexation behavior of fluorescent dissolved organic matter in the aquatic environments. Geochem. J. 2011, 45, 235–254. [Google Scholar] [CrossRef]
- Davis, J.A. Complexation of trace metals by adsorbed natural organic matter. Geochim. Cosmochim. Acta 1984, 48, 679–691. [Google Scholar] [CrossRef]
- Tranvik, L.; Kokalj, S. Decreased biodegradability of algal DOC due to interactive effects of UV radiation and humic matter. Aquat. Microb. Ecol. 1998, 14, 301–307. [Google Scholar] [CrossRef]
- Jansson, M.; Bergström, A.-K.; Blomqvist, P.; Drakare, S. Allochthonous organic carbon and phytoplankton/bacterioplankton production relationships in lakes. Ecology 2000, 81, 3250–3255. [Google Scholar] [CrossRef]
- McKnight, D.M.; Smith, R.L.; Harnish, R.A.; Miller, C.L.; Bencala, K.E. Seasonal relationships between planktonic microorganisms and dissolved organic material in an alpine stream. Biogeochemistry 1993, 21, 39–59. [Google Scholar] [CrossRef]
- Stone, L.; Berman, T. Positive feedback in aquatic ecosystems: The case of the microbial loop. Bull. Math. Biol. 1993, 55, 919–936. [Google Scholar] [CrossRef]
- Schindler, D.; Bayley, S.; Curtis, P.; Parker, B.; Stainton, M.; Kelly, C. Natural and man-caused factors affecting the abundance and cycling of dissolved organic substances in precambrian shield lakes. Hydrobiologia 1992, 229, 1–21. [Google Scholar] [CrossRef]
- McKnight, D.; Thurman, E.M.; Wershaw, R.L.; Hemond, H. Biogeochemistry of Aquatic Humic Substances in Thoreau’s Bog, Concord, Massachusetts. Ecology 1985, 66, 1339–1352. [Google Scholar] [CrossRef]
- Qualls, R.G.; Richardson, C.J. Factors controlling concentration, export, and decomposition of dissolved organic nutrients in the Everglades of Florida. Biogeochemistry 2003, 62, 197–229. [Google Scholar] [CrossRef]
- Mladenov, N.; McKnight, D.M.; Wolski, P.; Ramberg, L. Effects of annual flooding on dissolved organic carbon dynamics within a pristine wetland, the Okavango Delta, Botswana. Wetlands 2005, 25, 622–638. [Google Scholar] [CrossRef]
- GHOSH, K.; Schnitzer, M. UV and visible absorption spectroscopic investigations in relation to macromolecular characteristics of humic substances. J. Soil Sci. 1979, 30, 735–745. [Google Scholar] [CrossRef]
- Aitken, A.; Learmonth, M. The Protein Protocols Handbook 1996; Springer: New York, NY, USA, 1996; pp. 3–6. [Google Scholar]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- Dilling, J.; Kaiser, K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry. Water Res. 2002, 36, 5037–5044. [Google Scholar] [CrossRef]
- Roig, B.; Thomas, O. UV spectrophotometry: A powerful tool for environmental measurement. Manag. Environ. Qual. 2003, 14, 398–404. [Google Scholar]
- Aryal, R.; Kandel, D.; Acharya, D.; Chong, M.N.; Beecham, S. Unusual Sydney dust storm and its mineralogical and organic characteristics. Environ. Chem. 2012, 9, 537–546. [Google Scholar] [CrossRef]
- Hong, S.; Aryal, R.; Vigneswaran, S.; Johir, M.A.H.; Kandasamy, J. Influence of hydraulic retention time on the nature of foulant organics in a high rate membrane bioreactor. Desalination 2012, 287, 116–122. [Google Scholar] [CrossRef]
- Hussain, S.; van Leeuwen, J.; Chow, C.; Beecham, S.; Kamruzzaman, M.; Wang, D.; Drikas, M.; Aryal, R. Removal of organic contaminants from river and reservoir waters by three different aluminum-based metal salts: Coagulation adsorption and kinetics studies. Chem. Eng. J. 2013, 225, 394–405. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—Organic carbon detection—Organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Aryal, R.K.; Murakami, M.; Furumai, H.; Nakajima, F.; Jinadasa, H.K.P.K. Prolonged deposition of heavy metals in infiltration facilities and its possible threat to groundwater contamination. Water Sci. Technol. 2006, 54, 205–212. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Moran, M.A.; Sheldon, W.M., Jr.; Zepp, R.G. Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol. Oceanogr. 2000, 45, 1254–1264. [Google Scholar] [CrossRef]
- Salonen, K.; Vähätalo, A. Photochemical mineralisation of dissolved organic matter in lake Skjervatjern. Environ. Int. 1994, 20, 307–312. [Google Scholar] [CrossRef]
- Ma, H.; Allen, H.E.; Yin, Y. Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent. Water Res. 2001, 35, 985–996. [Google Scholar] [CrossRef]
- Kalbitz, K.; Geyer, S.; Geyer, W. A comparative characterization of dissolved organic matter by means of original aqueous samples and isolated humic substances. Chemosphere 2000, 40, 1305–1312. [Google Scholar] [CrossRef]
- Imai, A.; Fukushima, T.; Matsushige, K.; Kim, Y.H. Fractionation and characterization of dissolved organic matter in a shallow eutrophic lake, its inflowing rivers, and other organic matter sources. Water Res. 2001, 35, 4019–4028. [Google Scholar] [CrossRef]
- Yabushita, S.; Wada, K.; Inagaki, T.; Arakawa, E. UV and vacuum UV spectra of organic extract from Yamato carbonaceous chondrites. Mon. Not. R. Astron. Soc. 1987, 229, 45P–48P. [Google Scholar] [CrossRef]
- Aryal, R.; Vigneswaran, S.; Kandasamy, J. Application of Ultraviolet (UV) spectrophotometry in the assessment of membrane bioreactor performance for monitoring water and wastewater treatment. Appl. Spectrosc. 2011, 65, 227–232. [Google Scholar] [CrossRef]
- Kuipers, B.J.; Gruppen, H. Prediction of molar extinction coefficients of proteins and peptides using UV absorption of the constituent amino acids at 214 nm to enable quantitative reverse phase high-performance liquid chromatography-mass spectrometry analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef]
- Liu, P.-F.; Avramova, L.V.; Park, C. Revisiting absorbance at 230 nm as a protein unfolding probe. Anal. Biochem. 2009, 389, 165–170. [Google Scholar] [CrossRef]
- Hur, J.; Schlautman, M.A. Using selected operational descriptors to examine the heterogeneity within a bulk humic substance. Environ. Sci. Technol. 2003, 37, 880–887. [Google Scholar] [CrossRef]
- Chevakidagarn, P. Surrogate parameters for rapid monitoring of contaminant removal for activated sludge treatment plants for para rubber and seafood industries in Southern Thailand. J. Songklanakarin. 2005, 27, 417–424. [Google Scholar]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Traina, S.J.; Novak, J.; Smeck, N.E. An ultraviolet absorbance method of estimating the percent aromatic carbon content of humic acids. J. Environ. Qual. 1990, 19, 151–153. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Artinger, R.; Buckau, G.; Geyer, S.; Fritz, P.; Wolf, M.; Kim, J. Characterization of groundwater humic substances: Influence of sedimentary organic carbon. Appl. Geochem. 2000, 15, 97–116. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L. Aquatic microalgae as potential sources of UV-screening compounds. Philipp. J. Sci. 2010, 139, 5–16. [Google Scholar]
- Winter, A.R.; Fish, T.A.E.; Playle, R.C.; Smith, D.S.; Curtis, P.J. Photodegradation of natural organic matter from diverse freshwater sources. Aquat. Toxicol. 2007, 84, 215–222. [Google Scholar] [CrossRef]
- Whitehead, K.; Vernet, M. Influence of mycosporine-like amino acids (MAAs) on UV absorption by particulate and dissolved organic matter in La Jolla Bay. Limnol. Oceanogr. 2000, 45, 1788–1796. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Kim, B.; Choi, K.; Kim, C.; Lee, U.H.; Kim, Y.-H. Effects of the summer monsoon on the distribution and loading of organic carbon in a deep reservoir, Lake Soyang, Korea. Water Res. 2000, 34, 3495–3504. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Sinha, R.; Klisch, M.; Gröniger, A.; Häder, D.-P. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B 1998, 47, 83–94. [Google Scholar] [CrossRef]
- Vincent, W.F.; Roy, S. Solar ultraviolet-B radiation and aquatic primary production: Damage, protection, and recovery. Environ. Rev. 1993, 1, 1–12. [Google Scholar] [CrossRef]
- Chong, M.N.; Sidhu, J.; Aryal, R.; Tang, J.; Gernjak, W.; Escher, B.; Toze, S. Urban stormwater harvesting and reuse: A probe into the chemical, toxicology and microbiological contaminants in water quality. Environ. Monit. Assess. 2012, 1–8. [Google Scholar]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aryal, R.; Grinham, A.; Beecham, S. Tracking Inflows in Lake Wivenhoe during a Major Flood Using Optical Spectroscopy. Water 2014, 6, 2339-2352. https://doi.org/10.3390/w6082339
Aryal R, Grinham A, Beecham S. Tracking Inflows in Lake Wivenhoe during a Major Flood Using Optical Spectroscopy. Water. 2014; 6(8):2339-2352. https://doi.org/10.3390/w6082339
Chicago/Turabian StyleAryal, Rupak, Alistair Grinham, and Simon Beecham. 2014. "Tracking Inflows in Lake Wivenhoe during a Major Flood Using Optical Spectroscopy" Water 6, no. 8: 2339-2352. https://doi.org/10.3390/w6082339





