Testing Metallic Iron Filtration Systems for Decentralized Water Treatment at Pilot Scale
Abstract
:1. Introduction
1.1. Background
1.2. Membrane Technology Can Be a Bridging Solution
1.3. The Suitability of Fe0 Filters for Safe Water Provision in the Developing World
1.4. Fe0 Filters for Self-Reliance in Water Supply
2. Water Supply Systems
2.1. Centralized Water Supply
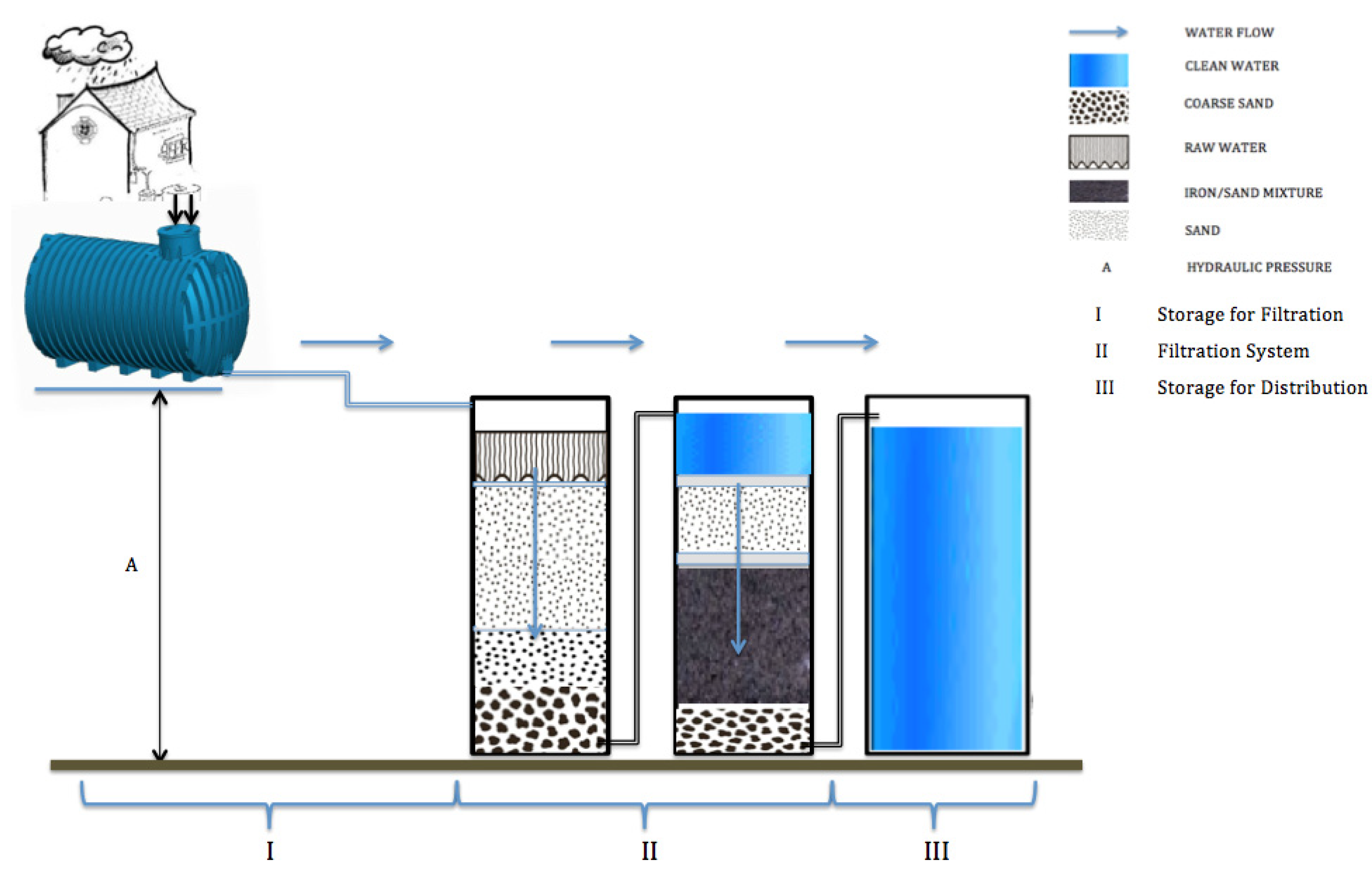
2.2. Disadvantages of the Centralized Water Supply
2.3. Decentralized Water Supply
2.4. Appropriateness of Decentralized Water Treatment Systems
3. Decentralized Water Treatment with Metallic Iron
3.1. An Overview of the Fe0/H2O System for Contaminant Mitigation
3.2. The Nature of the Fe0/H2O System
4. Rationale for Fe0 Filter Design
4.1. A Non-Exploited Pioneering Work

4.2. Lessons from the Pioneering Work
4.3. Disregarding Lessons from the Pioneering Work

4.4. Evaluation
| Fe0/Solid Ratio | Column Dimensions | Flow Rate | Duration | X | Reference | |
|---|---|---|---|---|---|---|
| D | L | |||||
| (vol:vol) | (cm) | (cm) | (mL/min) | (Days) | ||
| Fe0 (100%) | 5.0 | 30 | variable | weeks | NO3− | [124] |
| Fe0/sand (3:1) | 7.5 | 91 | n.s. | 365 | NO3− | [124] |
| Fe0 (100%) | 7.5 | 91 | n.s. | 365 | NO3− | [124] |
| Fe0/sand (3:1) | 16 | 107 | 4.2 to 201 | 0.21 | As | [54] |
| Fe0/sand (1:1) | 16 | 107 | 4.2 to 202 | 0.21 | As | [54] |
| Fe0 (100%) | 16 | 107 | 4.2 to 203 | 0.21 | As | [54] |
| Fe0/anthracite (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0/gravel (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0/pumice (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0/sand (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0 (100%) | 5.1 | 15.0 | n.s. | 14 | PO43− | [8] |
| Fe0 (100%) | 5.1 | 15.0 | 0.8 to 1.0 | 14 | As, Cr, Se | [9] |
| Fe0 (100%) | 5.1 | 15.0 | 0.8 to 1.0 | 14 | Cd, Cu, Pb | [9] |
| Material | Availability | Origin | Mass | d | Reference |
|---|---|---|---|---|---|
| (g) | (mm) | ||||
| Iron filings | scrap iron | Masterbuilders Inc. | 1636 | 0.05–0.6 | [124] |
| Iron chips | commercial | Baker Iron | 2271 | 0.5–5.0 | [124] |
| Iron fillings | commercial | Connelly-GPM Inc. | n.s. | 0.08–2.4 | [54] |
| Iron fillings | commercial | Gotthart Maier | n.s. | 1.0–2.0 | [54] |
| Granulated cast iron | commercial | Gotthart Maier | 100 | 0.3–2.0 | [125] |
| Zero-valent iron (ZVI) | commercial | Connelly-GPM | n.s. | 0.1–2.0 | [8,9] |
| Porous iron composite (PIC) | commercial | NA Höganäs Inc. | n.s. | 0.1–2.0 | [8,9] |
| Sulfur modified iron (SMI) | commercial | SMI_PS, Inc. | n.s. | 0.1–2.0 | [8,9] |
4.5. Rationally Designing Fe0 Filters
5. Designing Fe0 Filters for On-Site Water Treatment
5.1. Modular Fe0 Filter Design
5.2. Appropriateness of Fe0 Filters
5.3. Improving Available Designs

5.4. Ways for Efficient Fe0 On-Site Water Treatment Plants
5.5. Comparing Fe0 Filters to Other Technologies
5.6. Economic Considerations
5.7. Implementation of Fe0-Based Water Treatment Plants
5.7.1. Siting
5.7.2. Containment
5.7.3. Plumbing
5.7.4. Filter Materials
5.7.5. Implementation Plan
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Gujer, W.; Pronk, W. Intermittent operation of ultra-low pressure ultrafiltration for decentralized drinking water treatment. Water Res. 2012, 46, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, S. Improving the sustainability of water treatment systems: Opportunities for innovation. Solutions 2010, 1, 42–49. [Google Scholar]
- Tellen, V.; Nkeng, G.; Dentel, S. Improved filtration technology for pathogen reduction in rural water supplies. Water 2010, 2, 285–306. [Google Scholar] [CrossRef]
- Grady, C.; Younos, T. Bottled water technology and its global ramifications: An overview. Int. Water Technol. J. 2012, 2, 185–194. [Google Scholar]
- Sima, L.C.; Elimelech, M. More than a drop in the bucket: Decentralized membrane-based drinking water refill stations in Southeast Asia. Environ. Sci. Technol. 2013, 47, 7580–7588. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Racharaks, R. Laboratory comparison of four iron-based filter materials for drainage water phosphate treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Tost, B.C. Laboratory comparison of four iron-based filter materials for water treatment of trace element contaminants. Water Environ. Res. 2014, 86, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Zhang, B.; Li, F.; Toure, B.; Omosa, I.B.; Chiramba, T.; Abdel-Monem, M.; Pradhan, M. Water and wastewater treatment in Africa—Current practices and challenges. Clean Soil Air Water 2014, 42, 1029–1035. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Obi, C.L.; Thompson, P. Survey of disinfection efficiency of small drinking water treatment plants: Challenges facing small water treatment plants in South Africa. Water SA 2009, 35, 485–494. [Google Scholar]
- Frechen, F.-B.; Exler, H.; Romaker, J.; Schier, W. Long-term behaviour of a gravity-driven dead end membrane filtration unit for potable water supply in cases of disasters. Water Sci. Technol. Water Supply 2011, 11, 39–44. [Google Scholar] [CrossRef]
- Ali, I. Water treatment by adsorption columns: Evaluation at ground level. Sep. Purif. Rev. 2014, 43, 175–205. [Google Scholar] [CrossRef]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 2007, 42, 1879–1888. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water worldwide. Chem. Eng. J. 2010, 165, 740–749. [Google Scholar] [CrossRef]
- Chiu, P.C. Applications of zero-valent iron (ZVI) and nanoscale ZVI to municipal and decentralized drinking water systems—A review. In Novel Solutions to Water Pollution; Ahuja, S., Hristovski, K., Eds.; ACS Symposium Series; Volume 1123, American Chemical Society: Washington, DC, USA, 2013; pp. 237–249. [Google Scholar]
- Schäfer, A.I.; Broeckmann, A.; Richards, B.S. Renewable energy powered mem-brane technology. 1. Development and characterization of a photovoltaic hybrid membrane system. Environ. Sci. Technol. 2007, 41, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.I.; Hughes, G.; Richards, B.S. Renewable energy powered membrane technology: A leapfrog approach to rural water treatment in developing countries? Renew. Sustain. Energy Rev. 2014, 40, 542–556. [Google Scholar] [CrossRef]
- Johnson, D.M.; Hokanson, D.R.; Zhang, Q.; Czupinski, K.D.; Tang, J. Feasibility of water purification technology in rural areas of developing countries. J. Environ. Manag. 2008, 88, 416–427. [Google Scholar] [CrossRef]
- Litter, M.I.; Morgada, M.E.; Bundschuh, J. Possible treatments for arsenic removal in Latin American waters for human consumption. Environ. Pollut. 2010, 158, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Gottinger, A.M.; McMartin, D.W.; Wild, D.J.; Moldovan, B. Integration of zero valent iron sand beds into biological treatment systems for uranium removal from drinking water wells in rural Canada. Can. J. Civ. Eng. 2013, 40, 945–950. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.-B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Kowalski, K. New Method for Arsenic Compounds Elimination from Naturally Contaminated Drinking Water Systems. Ph.D. Thesis, Aalborg Universitet, Esbjerg, Denmark, January 2014. [Google Scholar]
- Kowalski, K.P.; Søgaard, E.G. Implementation of zero-valent iron (ZVI) into drinking water supply—Role of the ZVI and biological processes. Chemosphere 2014, 117, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Schöner, A. Metallic iron for environmental remediation: Learning from electrocoagulation. J. Hazard. Mater. 2010, 175, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Bojic, A.; Purenovic, M.; Kocic, B.; Perovic, J.; Ursic-Jankovic, J.; Bojic, D. The inactivation of Escherichia coli by microalloyed aluminium based composite. Facta Univ. 2001, 2, 115–124. [Google Scholar]
- Bojic, A.; Purenovic, M.; Bojic, D. Removal of chromium(VI) from water by micro-alloyed aluminium based composite in flow conditions. Water SA 2004, 30, 353–359. [Google Scholar] [CrossRef]
- Bojic, A.L.; Purenovic, M.; Bojic, D.; Andjelkovic, T. Dehalogenation of trihalomethanes by a micro-alloyed aluminium composite under flow conditions. Water SA 2007, 33, 297–304. [Google Scholar]
- Bojic, A.L.; Bojic, D.; Andjelkovic, T. Removal of Cu2+ and Zn2+ from model wastewaters by spontaneous reduction–coagulation process in flow conditions. J. Hazard. Mater. 2009, 168, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Lackovic, J.A.; Nikolaidis, N.P.; Dobbs, G.M. Inorganic arsenic removal by zero-valent iron. Environ. Eng. Sci. 2000, 17, 29–39. [Google Scholar] [CrossRef]
- Scherer, M.M.; Richter, S.; Valentine, R.L.; Alvarez, P.J.J. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Rev. Environ. Sci. Technol. 2000, 30, 363–411. [Google Scholar] [CrossRef]
- Mantha, R.; Taylor, K.E.; Biswas, N.; Bewtra, J.K. A continuous system for Fe0 reduction of nitrobenzene in synthetic wastewater. Environ. Sci. Technol. 2001, 35, 3231–3236. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. On the mechanism microbe inactivation by metallic iron. J. Hazard. Mater. 2011, 198, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wie, J.; Jin, Y.; Kniel, K.E.; Chiu, P.C. Removal of viruses and bacteriophages from drinking water using zero-valent iron. Sep. Purif. Technol. 2012, 84, 72–78. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Cheng, C.; Liu, P.; Shi, L.; Ma, Z.; Zheng, X. Removal of bacteriophage f2 in water by nanoscale zero-valent iron and parameters optimization using response surface methodology. Chem. Eng. J. 2014, 252, 150–158. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Yang, M.; Hashimoto, T.; Hoshi, N.; Myoga, H. Fluoride removal in a fixed bed packed with granular calcite. Water Res. 1999, 33, 3395–3402. [Google Scholar] [CrossRef]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef] [PubMed]
- García, M.G.; Borgnino, L.; Bia, G.; Depetris, P.J. Mechanisms of arsenic and fluoride release from Chacopampean sediments (Argentina). Int. J. Environ. Health 2014, 7, 41–57. [Google Scholar] [CrossRef]
- Trikha, R.; Sharma, B.K. Studies on factors affecting fluoride removal from water using passive system. J. Environ. Chem. Eng. 2014, 2, 172–176. [Google Scholar] [CrossRef]
- Omenka, E. Improvement of Decentralised Wastewater Treatment in Asaba, Nigeria. Master’s Thesis, Lund University, Lund, Sweden, July 2010. [Google Scholar]
- Younos, T. Paradigm shift: Holistic approach for water management in urban environments. Front. Earth Sci. 2011, 5, 421–427. [Google Scholar]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Noubactep, C. Affordable safe drinking water for victims of natural disasters. In Natural Disasters and Sustainable Development, Proceedings of the International Seminar Held in Göttingen, Germany, 17–19 April 2013; Kätsch, C., Meliczek, H., Eds.; Cuvillier Verlag: Göttingen, Germany, 2014; pp. 57–75. [Google Scholar]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for groundwater of Bangladesh. J. Environ. Sci. Health A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- Antia, D.D.J. Modification of aquifer pore-water by static diffusion using nano-zero-valent metals. Water 2011, 3, 79–112. [Google Scholar] [CrossRef]
- Gottinger, A.M.; Wild, D.J.; McMartin, D.; Moldovan, B.; Wang, D. Development of an iron-amended biofilter for removal of arsenic from rural Canadian prairie potable water. In Water Pollution X; Marinov, A.M., Brebbia, C.A., Eds.; WIT Press: Southampton, UK, 2010; pp. 333–344. [Google Scholar]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Trois, C.; Cibati, A. South African sands as a low cost alternative solution for arsenic removal from industrial effluents in permeable reactive barriers: Column tests. Chem. Eng. J. 2015, 259, 981–989. [Google Scholar] [CrossRef]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J. Iron-mediated reductive transformations: Investigation of reaction mechanism. Environ. Sci. Technol. 1996, 30, 716–719. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Sarr, D. Zero-valent-iron permeable reactive barriers—How long will they last? Remediation 2001, 11, 1–18. [Google Scholar]
- Murugan, S.; Paulpandian, P. Synergistic antibacterial evaluation of commercial antibiotics combined with nanoiron against human pathogens. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 183–190. [Google Scholar]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Comba, S.; Di Molfetta, A.; Sethi, R. A Comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut. 2011, 215, 595–607. [Google Scholar] [CrossRef]
- Crane, R.A.; Dickinson, M.; Popescu, I.C.; Scott, T.B. Magnetite and zero-valent iron nanoparticles for the remediation of uranium contaminated environmental water. Water Res. 2011, 45, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Scott, T.B.; Popescu, I.C.; Crane, R.A.; Noubactep, C. Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J. Hazard. Mater. 2011, 186, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J. Laboratory evaluation of porous iron composite for agricultural drainage water filter treatment. Trans. ASABE 2012, 55, 1683–1697. [Google Scholar] [CrossRef]
- Allred, B.J. Laboratory evaluation of zero valent iron and sulfur-modified iron for agricultural drainage water treatment. Ground Water Monit. Remediat. 2012, 32, 81–95. [Google Scholar] [CrossRef]
- Crane, R.; Noubactep, C. Elemental metals for environmental remediation: Lessons from hydrometallurgy. Fresenius Environ. Bull. 2012, 21, 1192–1196. [Google Scholar]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S.; Crane, R.A. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, H.; Kawase, Y. Kinetic modeling and simulation of zero-valent iron wastewater treatment process: Simultaneous reduction of nitrate, hydrogen peroxide, and phosphate in semiconductor acidic wastewater. Ind. Eng. Chem. Res. 2013, 52, 17829–17840. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. The removal of uranium onto nanoscale zero-valent iron particles in anoxic batch systems. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Vodyanitskii, Y.N. Effect of reduced iron on the degradation of chlorinated hydrocarbons in contaminated soil and ground water: A review of publications. Eurasian Soil Sci. 2014, 47, 119–133. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Artificial permeable redox barriers for purification of soil and ground water: A review of publications. Eurasian Soil Sci. 2014, 47, 1058–1068. [Google Scholar] [CrossRef]
- Crane, R.A.; Dickinson, M.; Scott, T.B. Nanoscale zero-valent iron particles for the remediation of plutonium and uranium contaminated solutions. Chem. Eng. J. 2015, 262, 319–325. [Google Scholar] [CrossRef]
- Naidu, R.; Birke, V. Permeable Reactive Barrier: Sustainable Groundwater Remediation; CRC Press: Taylor & Francis Group, London, UK, 2015; p. 333. ISBN ISBN 9781482224474. [Google Scholar]
- Ghauch, A.; Abou Assi, H.; Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: Bimetallic systems. J. Hazard. Mater. 2010, 182, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Abou Assi, H.; Baydoun, H.; Tuqan, A.M.; Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem. Eng. J. 2011, 172, 1033–1044. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-Based Metallic Systems: An Excellent Choice for Sustainable Water Treatment. Ph.D. Thesis, University of Grenoble, Grenoble, France, November 2013. [Google Scholar]
- Lai, K.C.K.; Lo, I.M.C.; Birkelund, V.; Kjeldsen, P. Field monitoring of a permeable reactive barrier for removal of chlorinated organics. J. Environ. Eng. 2006, 132, 199–210. [Google Scholar] [CrossRef]
- Jia, Y.; Aagaard, P.; Breedveld, G.D. Sorption of triazoles to soil and iron minerals. Chemosphere 2007, 67, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Characterizing the discoloration of methylene blue in Fe0/H2O systems. J. Hazard. Mater. 2009, 166, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Nanoscale iron particles for environmental remediation: An overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Korte, N.E.; Zutman, J.L.; Schlosser, R.M.; Liang, L.; Gu, B.; Fernando, Q. Field application of palladized iron for the dechlorination of trichloroethene. Waste Manag. 2000, 20, 687–694. [Google Scholar] [CrossRef]
- Huang, Y.H.; Tang, C.L.; Zeng, H. Removing molybdate from water using a hybridized zero-valent iron/magnetite/Fe(II) treatment system. Chem. Eng. J. 2012, 200, 205–263. [Google Scholar]
- Huang, Y.H.; Peddi, P.K.; Zeng, H.; Tang, C.L.; Teng, X.J. Pilot-scale demonstration of the hybrid zero-valent iron process for treating flue-gas-desulfurization wastewater: Part I. Water Sci. Technol. 2013, 67, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Peddi, P.K.; Zeng, H.; Tang, C.L.; Teng, X.J. Pilot-scale demonstration of the hybrid zero-valent iron process for treating flue-gas-desulfurization wastewater: Part II. Water Sci. Technol. 2013, 67, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.A.; Roberts, A.L. Pathways of chlorinated ethylene and chlorinated acetylene reaction with Zn(0). Environ. Sci. Technol. 1998, 32, 3017–3025. [Google Scholar] [CrossRef]
- Arnold, W.A.; Ball, W.P.; Roberts, A.L. Polychlorinated ethane reaction with zero-valent zinc: Pathways and rate control. J. Contam. Hydrol. 1999, 40, 183–200. [Google Scholar] [CrossRef]
- Suresh, S. Reductive remediation of pollutants using metals. Open Waste Manag. J. 2009, 2, 6–16. [Google Scholar] [CrossRef]
- Salter-Blanc, A.J.; Tratnyek, P.G. Effects of solution chemistry on the dechlorination of 1,2,3-trichloropropane by zero-valent zinc. Environ. Sci. Technol. 2011, 45, 4073–4079. [Google Scholar] [CrossRef] [PubMed]
- Han, V.; Chen, Z.; Tong, L.N.; Yang, L.; Shen, J.M.; Wang, B.Y.; Liu, Y.; Liu, Y.; Chen, Q. Reduction of N-Nitrosodimethylamine with zero-valent zinc. Water Res. 2013, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.E.; Gray, H.B.; Haight, G.P., Jr. Chemical Principles, 3rd ed.; Benjamin/Cummings Inc.: San Francisco, CA, USA, 1979; p. 944. [Google Scholar]
- Noubactep, C. The suitability of metallic iron for environmental remediation. Environ. Progr. Sust. En. 2010, 29, 286–291. [Google Scholar] [CrossRef]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu-K., B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solut. Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Gillham, R.W. Discussion of Papers/Discussion of nano-scale iron for dehalogenation. Ground Water Monit. Remediat. 2003, 23, 6–8. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabro, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modelling the permeability loss of metallic iron water filtration systems. Clean Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Sato, N. 1989 Whitney award lecture: Toward a more fundamental understanding of corrosion processes. Corrosion 1989, 45, 354–368. [Google Scholar] [CrossRef]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Sato, N. Surface oxides affecting metallic corrosion. Corros. Rev. 2001, 19, 253–272. [Google Scholar]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Dickinson, M.; Scott, T.B.; Crane, R.A.; Riba, O.; Barnes, R.J.; Hughes, G.M. The effects of vacuum annealing on the structure and surface chemistry of iron: nickel alloy nanoparticles. J. Nanoparticle Res. 2010, 12, 2081–2092. [Google Scholar] [CrossRef]
- Scott, T.B.; Dickinson, M.; Crane, R.A.; Riba, O.; Hughes, G.M.; Allen, G.C. The effects of vacuum annealing on the structure and surface chemistry of iron nanoparticles. J. Nanoparticle Res. 2010, 12, 1765–1775. [Google Scholar] [CrossRef]
- Caule, E.J.; Cohen, M. An electron-micrograph study of oxide films on electropolished surfaces of iron. Can. J. Chem. 1953, 31, 237–241. [Google Scholar] [CrossRef]
- Cohen, M. The formation and properties of passive films on iron. Can. J. Chem. 1959, 37, 286–291. [Google Scholar] [CrossRef]
- Sikora, E.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid I. General electrochemical behavior. J. Electrochem. Soc. 2000, 147, 4087–4092. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0-H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Metallic iron for water treatment: A critical review. Clean Soil Air Water 2013, 41, 702–710. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Sauter, M. Significance of oxide-film in discussing the mechanism of contaminant removal by elemental iron materials. In Photo-Electrochemistry & Photo-Biology for the Sustainablity; Kaneco, S., Viswanathan, B., Katsumata, H., Eds.; Union Press: Osaka, Japan, 2012; pp. 97–122. [Google Scholar]
- Noubactep, C. Processes of contaminant removal in “Fe0-H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. An analysis of the evolution of reactive species in Fe0/H2O systems. J. Hazard. Mater. 2009, 168, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 2010, 36, 663–670. [Google Scholar] [CrossRef]
- Noubactep, C. Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 2011, 37, 419–426. [Google Scholar] [CrossRef]
- Noubactep, C. Relevant reducing agents in remediation Fe0/H2O systems. Clean Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, C.; Huang, L.; Wu, K.; Ma, H.; Chen, S.; Ma, L.; Wu, L. Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2009, 91, 434–440. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water production. Freib. Online Geosci. 2011, 27, 38. [Google Scholar]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef]
- Noubactep, C. Flaws in the design of Fe(0)-based filtration systems? Chemosphere 2014, 117, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Designing metallic iron packed-beds for water treatment: A critical review. Clean Soil Air Water 2015. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, A.S.; Ünal, N.; Jekel, M. Evaluation of two-component Fe(0) fixed bed filters with porous materials for reductive dechlorination. Chem. Eng. J. 2012, 209, 401–406. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Martin, J. Impacts of Fe(0) grain sizes and grain size distributions in permeable reactive barriers. Chem. Eng. J. 2012, 213, 245–250. [Google Scholar] [CrossRef]
- Bi, E.; Devlin, J.F.; Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Ground Water Monit. Remediat. 2009, 29, 56–62. [Google Scholar] [CrossRef]
- Ulsamer, S. A Model to Characterize the Kinetics of Dechlorination of Tetrachloroethylene and Trichloroethylene by a Zero Valent Iron Permeable Reactive Barrier. Master’s Thesis, Worcester Polytechnic Institute, Worcester, UK, 2011; p. 73. [Google Scholar]
- Firdous, R.; Devlin, J.F. Consideration of grain packing in granular iron treatability studies. J. Contam. Hydrol. 2014, 164, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S. Dimensioning metallic iron beds for efficient contaminant removal. Chem. Eng. J. 2010, 163, 454–460. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Designing laboratory metallic iron columns for better result comparability. J. Hazard. Mater. 2011, 189, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Btatkeu-K., B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 60. [Google Scholar]
- Phukan, M. Characterizing the Fe0/Sand System by the Extent of Dye Discoloration. Master’s Thesis, University of Göttingen, Göttingen, Germany, January 2015. [Google Scholar]
- Caré, S.; Nguyen, Q.T.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Gillham, R.W. Development of the granular iron permeable reactive barrier technology (good science or good fortune). In Advances in Environmental Geotechnics: Proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China; Chen, Y., Tang, X., Zhan, L., Eds.; Springer: Berlin, Germany, 2010; pp. 5–15. [Google Scholar]
- Phillips, D.H.; van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468–469, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Mushovic, P.S.; Niesen, P.L. Early breakthrough of molybdenum and uranium in a permeable reactive barrier. Environ. Sci. Technol. 2006, 40, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.L.; Gillham, R.W.; Reardon, E.J.; Fagan, R.; Focht, R.M.; Vogan, J.L. An examination of zero-valent iron sources used in permeable reactive barriers. In Proceedings of the 3rd International Containment Technology Conference, Tallahassee, Orlando, FL, USA, 10–13 June 2001; Florida State University; p. 5.
- Miehr, R.; Tratnyek, G.P.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Scherer, M.M.; Tratnyek, P.G. Kinetics of halogenated organic compound degradation by iron metal. Environ. Sci. Technol. 1996, 30, 2634–2640. [Google Scholar] [CrossRef]
- McGeough, K.L.; Kalin, R.M.; Myles, P. Carbon disulfide removal by zero valent iron. Environ. Sci. Technol. 2007, 41, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.-M.; Habibian, M.T.; O’melia, C.R. Water and waste water filtration: Concepts and applications. Environ. Sci. Technol. 1971, 5, 1105–1112. [Google Scholar] [CrossRef]
- Bedrikovetsky, P.; Siqueira, F.D.; Furtado, C.A.; Souza, A.L.S. Modified particle detachment model for colloidal transport in porous media. Transp. Porous Med. 2011, 86, 353–383. [Google Scholar] [CrossRef]
- You, Z.; Osipov, Y.; Bedrikovetsky, P.; Kuzmina, L. Asymptotic model for deep bed filtration. Chem. Eng. J. 2014, 258, 374–385. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in ground water: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Meinrath, G.; Merkel, J.B. Investigating the mechanism of uranium removal by zerovalent iron materials. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium (VI) fixation by elemental iron. J. Hazard. Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, N.P.; Dobbs, G.M.; Lackovic, J.A. Arsenic removal by zero-valent iron: field, laboratory and modeling studies. Water Res. 2003, 37, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.; Wang, J.; O’Day, P.; Conklin, M. Electrochemical and spectroscopic study of arsenate removal from water using zero-valent iron media. Environ. Sci. Technol. 2001, 35, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Burris, D.R.; Delcomyn, C.A.; Smith, M.H.; Roberts, A.L. Reductive dechlorination of tetrachloroethylene catalyzed by vitamin B12 in homogeneous and heterogeneous systems. Environ. Sci. Technol. 1996, 30, 3047–3052. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kim, J.-W.; Watkins, J.; Wilkin, R.T. Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ. Sci. Technol. 2002, 36, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for water treatment. Chem. Eng. J. 2015, 262, 891–896. [Google Scholar] [CrossRef]
- Btatkeu-K., B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Btatkeu-K., B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P. The application of rapid small-scale column tests in iron-based packed bed arsenic treatment systems. In Advances in Arsenic Research, ACS Symp. Ser.; Oxford University Press: Oxford, UK, 2005; Volume 915, pp. 268–283. [Google Scholar]
- Gu, B.; Phelps, T.J.; Liang, L.; Dickey, M.J.; Roh, Y.; Kinsall, B.L.; Palumbo, A.V.; Jacobs, G.K. Biogeochemical dynamics in zero-valent iron columns: Implications for permeable reactive barriers. Environ. Sci. Technol. 1999, 33, 2170–2177. [Google Scholar] [CrossRef]
- Farrell, J.; Kason, M.; Melitas, N.; Li, T. Investigation of the long-term performance of zero-valent iron for reductive dechlorination of trichloroethylene. Environ. Sci. Technol. 2000, 34, 514–521. [Google Scholar] [CrossRef]
- Melitas, N.; Wang, J.P.; Conklin, M.; O’Day, P.; Farrell, J. Understanding soluble arsenate removal kinetics by zerovalent iron media. Environ. Sci. Technol. 2002, 36, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Bilardi, S.; Calabrò, P.S.; Moraci, N. Are accelerated column tests used in permeable reactive barriers design sufficiently reliable? In Proceedings of the 3rd International Conference “Hazardous and Industrial Waste Management”, Create, Greece, 12–14 September 2012.
- Moraci, N.; Bilardi, S.; Calabrò, P. Critical aspects related to Fe0 and Fe0/pumice PRB design. Environ. Geotech. 2014. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Matis, K.A. The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials 2013, 6, 5131–5158. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Review on dye removal from its aqueous solution into alternative cost effective and non-conventional adsorbents. J. Chem. Proc. Eng. 2014, 1, 1–11. [Google Scholar]
- Chapman, A.C.; Siebold, A. On the application of adsorption to the detection and separation of certain dyes. Analyst 1912, 37, 339–345. [Google Scholar] [CrossRef]
- Ewing, W.W.; Liu, F.W.J. Adsorption of dyes from aqueous solutions on pigments. J. Colloid Sci. 1953, 8, 204–213. [Google Scholar] [CrossRef]
- Whetstone, J. The adsorption of dyes by crystals. Discuss. Faraday Soc. 1954, 16, 132–140. [Google Scholar] [CrossRef]
- Haldeman, R.G.; Emmett, P.H. Specific adsorption of alkyl orange dyes on silica gel. J. Phys. Chem. 1955, 59, 1039–1043. [Google Scholar] [CrossRef]
- Mitchell, G.; Poole, P.; Segrove, H.D. Adsorption of methylene blue by high-silica sands. Nature 1955, 176, 1025–1026. [Google Scholar] [CrossRef]
- Prasad, R.; Dey, A.K. Adsorption of dyestuffs by hydrous thorium oxide: Heat of adsorption of the dyes by various samples of the hydroxide. Kolloid Z. Z. Polym. 1962, 183, 153–155. [Google Scholar] [CrossRef]
- Brooks, C.S. Mechanism of methylene blue dye adsorption on siliceous minerals. Kolloid Z. Z. Polym. 1964, 199, 31–36. [Google Scholar] [CrossRef]
- Padday, J.F. Adsorption of cyanine dyes at silver-halide surfaces. Trans. Faraday Soc. 1964, 60, 1325–1334. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Luft, C.P.; Hand, D.W. Prediction of multicomponent adsorption equilibria in background mixtures of unknown composition. Water Res. 1985, 19, 1537–1548. [Google Scholar] [CrossRef]
- Worch, E. Fixed-bed adsorption in drinking water treatment: A critical review on models and parameter estimation. J. Water Supply Res. Technol. AQUA 2008, 57, 171–183. [Google Scholar] [CrossRef]
- Xu, Z.; Cai, J.; Pan, B. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zhejiang Univers. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef]
- Nitzsche, K.S.; Lan, V.M.; Trang, P.T.K.; Viet, P.H.; Berg, M.; Voegelin, A.; Planer-Friedrich, B.; Zahoransky, J.; Müller, S.-K.; Byrne, J.M.; et al. Arsenic removal from drinking water by a household sand filter in Vietnam—Effect of filter usage practices on arsenic removal efficiency and microbiological water quality. Sci. Total Environ. 2015, 502, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura Mbenguela, B. Water treatment with Fe0/H2O systems: Learning from internal electrolysis. Fresenius Environ. Bull. 2014, 23, 2663–2669. [Google Scholar]
- Burghardt, D.; Kassahun, A. Development of a reactive zone technology for simultaneous in situ immobilisation of radium and uranium. Environ. Geol. 2005, 49, 314–320. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–740. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J. Sustainable decentralized water treatment for rural and developing communities using locally generated biochar adsorbents. Water Cond. Purif. Int. 2012, 54, 7–12. [Google Scholar]
- Tillman, D.E. Combination of Zero-Valent Iron and Granular Activated for the Treatment of Groundwater Contaminated with Chlorinated Solvents. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, June 1996. [Google Scholar]
- Indelicato, B.M. Comparision of Zero-Valent Iron and Activated Carbon for Treating Chlorinated Contaminants in Groundwater. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, June 1998. [Google Scholar]
- Bayer, P.; Finkel, M. Modelling of sequential groundwater treatment with zero valent iron and granular activated carbon. J. Contam. Hydrol. 2005, 78, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Van Nooten, T.; Diels, L.; Bastiaens, L. Design of a multifunctional permeable reactive barrier for the treatment of landfill leachate contamination: Laboratory column evaluation. Environ. Sci. Technol. 2008, 42, 8890–8895. [Google Scholar] [CrossRef] [PubMed]
- Doula, M.K. Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Res. 2009, 43, 3659–3672. [Google Scholar] [CrossRef] [PubMed]
- Westholm, L.J.; Repo, E.; Sillanpää, M. Filter materials for metal removal from mine drainage—A review. Environ. Sci. Pollut. Res. 2014, 21, 9109–9128. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and points of zero charge: V. Update. J. Colloid Interf. Sci. 2011, 353, 1–15. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Gottinger, A.M. Chemical-Free Arsenic Removal from Potable Water with a ZVI-Amended Biofilter. Master’s Thesis, University of Regina, Saskatchewan, Canada, January 2010; p. 90. [Google Scholar]
- Clark, P.A.; Pinedo, C.A.; Fadus, M.; Capuzzi, S. Slow-sand water filter: Design, implementation, accessibility and sustainability in developing countries. Med. Sci. Monit. 2012, 18, RA105–RA117. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J. Sustainable Decentralized Water Treatment for Rural and Developing Communities Using Gasifier Biochar Version 1.0, March 2012. Available online: http://www.aqsolutions.org/ (accessed on 30 January 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing Metallic Iron Filtration Systems for Decentralized Water Treatment at Pilot Scale. Water 2015, 7, 868-897. https://doi.org/10.3390/w7030868
Tepong-Tsindé R, Crane R, Noubactep C, Nassi A, Ruppert H. Testing Metallic Iron Filtration Systems for Decentralized Water Treatment at Pilot Scale. Water. 2015; 7(3):868-897. https://doi.org/10.3390/w7030868
Chicago/Turabian StyleTepong-Tsindé, Raoul, Richard Crane, Chicgoua Noubactep, Achille Nassi, and Hans Ruppert. 2015. "Testing Metallic Iron Filtration Systems for Decentralized Water Treatment at Pilot Scale" Water 7, no. 3: 868-897. https://doi.org/10.3390/w7030868





