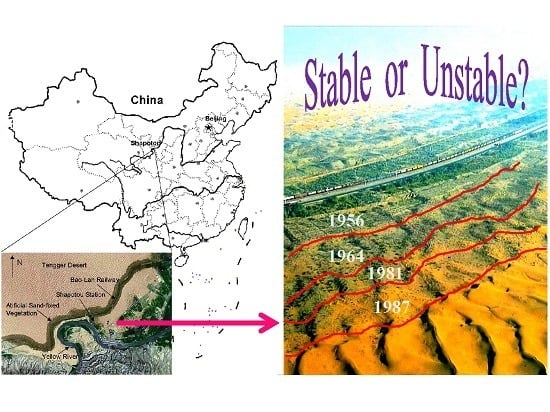
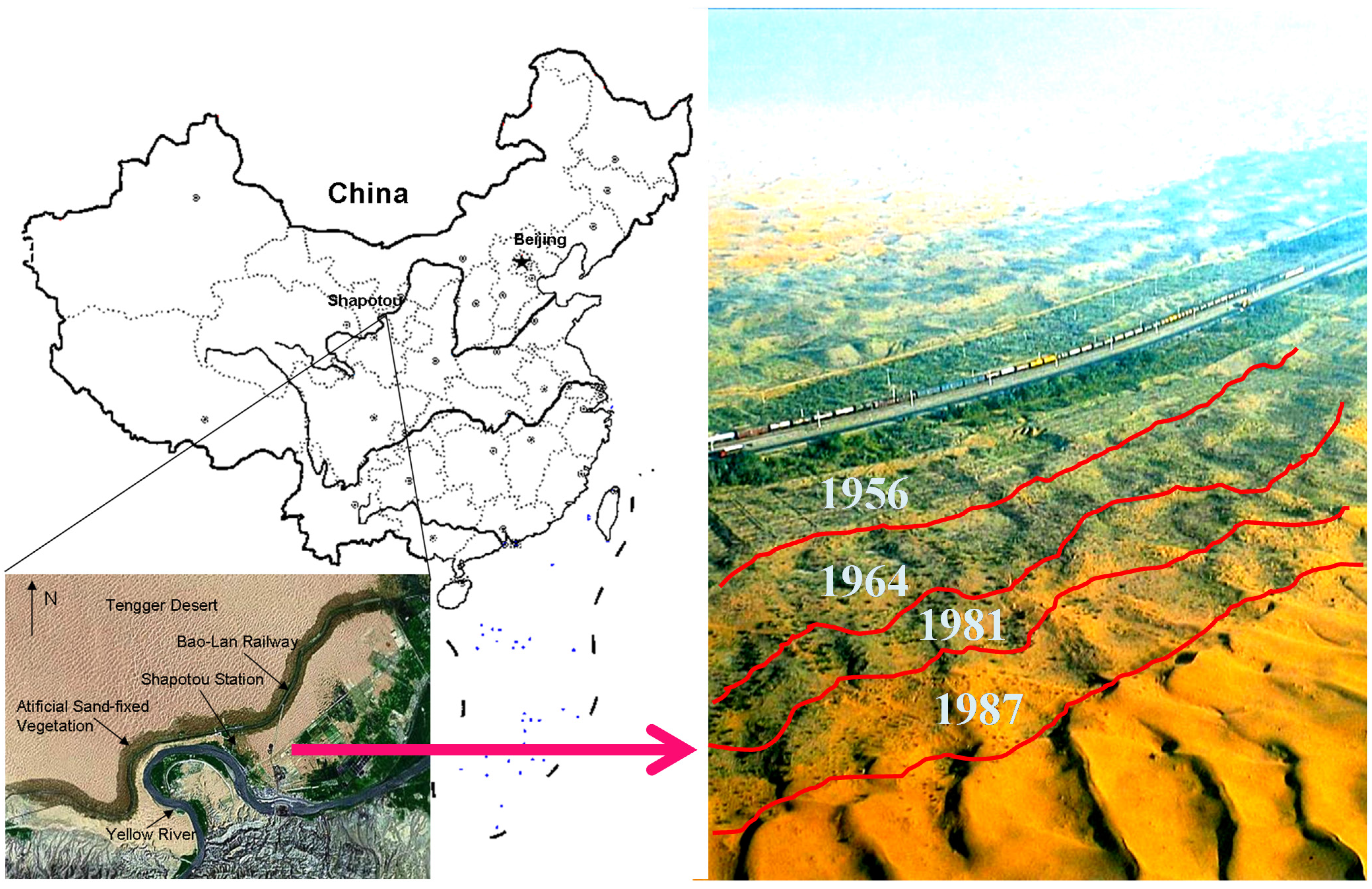
The Stability of Revegetated Ecosystems in Sandy Areas: An Assessment and Prediction Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
| Year of Revegetation | Approaches to Sand Stabilization and Revegetation | Remaining Shrub Species of Revegetation | Native/Invasion Dominant Plant Species |
|---|---|---|---|
| 1956 | Straw-checkerboard of 1 m2 planted with 10 xerophytic shrubs at a density of 16 individuals per 100 m2 | Artemisia ordosica, Caragana korshinskii, Hedysarum scoparium | Artemisia ordosica, Scorzonera mongolica, Sonchus arvensis, Chloris virgata, Aristida adscensionis, Setaria viridis, Bassia dasyphylla, Chenopodium aristatum |
| 1964 | Straw-checkerboard of 1 m2 planted with 10 xerophytic shrubs at a density of 16 individuals per 100 m2 | Artemisia ordosica, Caragana korshinskii, Hedysarum scoparium | Artemisia ordosica, Bassia dasyphylla, Eragrostis poaeoides, Sonchus arvensis, Scorzonera mongolica, Euphorbia humifusa |
| 1981 | Straw-checkerboard of 1 m2 planted with 10 xerophytic shrubs at a density of 16 individuals per 100 m2 | Artemisia ordosica, Caragana korshinskii, C. microphylla, Hedysarum scoparium | Artemisia ordosica, Hedysarum scoparium, Bassia dasyphylla, Eragrostis poaeoides, Corispermum patelliforme |
| 1987 | Straw-checkerboard of 1 m2 planted with 10 xerophytic shrubs at a density of 16 individuals per 100 m2 | Amorpha fruticosa, Artemisia ordosica, A. sphaerocephala, Caragana korshinskii, C. microphylla, Calligonum arborescens, Hedysarum scoparium | Hedysarum scoparium, Agriophyllum squarrosum, Bassia dasyphylla, Echinos gmelinii, Eragrostis poaeoides |
| Natural | No | No | Artemisia ordosica, Caragana korshinskii, Lespedeza davurica, Ceratoides latens, Oxytropis aciphylla, Stipa breviflora, Carex stenophylloides, Cleistogenes sogorica, Allium mongolicum, Oxytropis myriophylla, Enneapogon brachystachyus, Asparagus gobicus |
2.2. Methods
2.2.1. Sampling Method and Data Collection
2.2.2. Analytic Hierarchy Process Methodology

2.2.3. Coupled Dynamics of Soil Moisture and Vegetation
3. Results
3.1. Results of the Analytic Hierarchy Process (AHP) Application
| A | B1 | B2 | B3 | B4 | Priorities | AHP Criteria |
|---|---|---|---|---|---|---|
| B1 | 1.00 | 3.00 | 5.00 | 4.00 | 0.91 | = 4.23; CI = 0.078; RI = 0.900; CR = 0.086 |
| B2 | 0.33 | 1.00 | 0.50 | 1.00 | 0.22 | |
| B3 | 0.20 | 2.00 | 1.00 | 0.50 | 0.23 | |
| B4 | 0.25 | 1.00 | 2.00 | 1.00 | 0.28 |
| B1 | C1 | C2 | C3 | Priorities | AHP Criteria |
|---|---|---|---|---|---|
| C1 | 1.00 | 5.00 | 4.00 | 0.95 | = 3.09; CI = 0.047; RI = 0.58; CR = 0.081. |
| C2 | 0.20 | 1.00 | 2.00 | 0.26 | |
| C3 | 0.25 | 0.50 | 1.00 | 0.18 |
| B2 | C4 | C5 | C6 | C7 | C8 | C9 | Priorities | AHP Criteria |
|---|---|---|---|---|---|---|---|---|
| C4 | 1.00 | 0.33 | 2.00 | 1.00 | 5.00 | 0.50 | 0.27 | = 6.04; CI = 0.008; RI = 1.24; CR = 0.006. |
| C5 | 3.00 | 1.00 | 5.00 | 3.00 | 9.00 | 2.00 | 0.78 | |
| C6 | 0.50 | 0.20 | 1.00 | 0.50 | 3.00 | 0.33 | 0.15 | |
| C7 | 1.00 | 0.33 | 2.00 | 1.00 | 5.00 | 0.50 | 0.27 | |
| C8 | 0.20 | 0.11 | 0.33 | 0.20 | 1.00 | 0.14 | 0.06 | |
| C9 | 2.00 | 0.50 | 3.00 | 2.00 | 7.00 | 1.00 | 0.47 |
| B3 | C10 | C11 | Priorities | AHP Criteria |
|---|---|---|---|---|
| C10 | 1.00 | 5.00 | 0.98 | = 2; CI = 0; RI = 0.00; CR = 0. |
| C11 | 0.20 | 1.00 | 0.20 |
| B4 | C12 | C13 | C14 | Priorities | AHP Criteria |
|---|---|---|---|---|---|
| C12 | 1.00 | 1.00 | 2 | 0.63254 | = 3.02; CI = 0.008; RI = 0.58; CR = 0.013. |
| C13 | 1.00 | 1.00 | 3 | 0.72389 | |
| C14 | 0.5 | 0.33 | 1 | 0.27546 |
| Indices | B1 | B2 | B3 | B4 | Overall Priorities |
|---|---|---|---|---|---|
| 0.91 | 0.22 | 0.23 | 0.28 | ||
| C1 | 0.95 | – | – | – | 0.86 |
| C2 | 0.26 | – | – | – | 0.23 |
| C3 | 0.18 | – | – | – | 0.16 |
| C4 | – | 0.27 | – | – | 0.06 |
| C5 | – | 0.78 | – | – | 0.18 |
| C6 | – | 0.15 | – | – | 0.03 |
| C7 | – | 0.27 | – | – | 0.06 |
| C8 | – | 0.06 | – | – | 0.01 |
| C9 | – | 0.47 | – | – | 0.11 |
| C10 | – | – | 0.98 | – | 0.25 |
| C11 | – | – | 0.20 | – | 0.05 |
| C12 | – | – | – | 0.63 | 0.18 |
| C13 | – | – | – | 0.72 | 0.20 |
| C14 | – | – | – | 0.28 | 0.08 |
| CI = 0.052; RI = 0.955; CR = 0.055<0.1 | |||||
3.2. Stability Index Definition, Measurement and Prediction


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Appendix
A.1. Analytic process hierarchy (AHP) methodology
| Intensity of Importance | Definition | Explanation |
|---|---|---|
| 1 | Equal Importance | Two elements have equal importance regarding the element in higher level |
| 3 | Moderate Importance | Experience or judgement slightly favours one element |
| 5 | Strong Importance | Experience or judgement strongly favours one element |
| 7 | Very Strong Importance | Dominance of one element proved in practise |
| 9 | Extreme Importance | The highest order dominance of one element over another |
| 2,4,6,8 | Compromises between the Above | When compromise is needed |
| Adverse | Adverse Comparisions | The adverse evaluation of the same criteria, adverse of the same point under multiplication |
| n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| RI | 0 | 0 | 0.58 | 0.90 | 1.12 | 1.24 | 1.32 | 1.41 | 1.45 | 1.49 |
A.2. The Coupled Soil-Vegetation Model

| Parameters | Symbol (Unit) | Value |
|---|---|---|
| Soil porosity | n | 0.43 |
| Active soil depth | Zr (cm) | 40 |
| Critical soil moisture below which plant undergoes water stress | s* | 0.11 |
| Field capacity | s1 | 0.56 |
| Pore size distribution parameter | β | 12.7 |
| Saturated hydraulic conductivity | Ks (cm/d) | 800 |
| Pure soil evaporation | (mm/d) | 0.1 |
| Maximum evapotranspiration rate | (mm/d) | 3.67 |
| Average rainfall frequency | λ (/d) | 0.15 |
| Average precipitation depth | h (mm/d) | 0.61 |
Conflicts of Interest
References
- McCann, K.S. The diversity-stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Allesina, S.; Tang, S. Stability criteria for complex ecosystems. Nature 2012, 483, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Donohue, I.; Petchey, O.L.; Montoya, J.M.; Jackson, A.L.; McNally, L.; Viana, M.; Healy, K.; Lurgi, M.; O’Connor, N.E.; Emmerson, M.C. On the dimensionality of ecological stability. Ecol. Lett. 2013, 16, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.R. Ecosystem stability, some recent perspectives. Prog. Phys. Geogr. 1987, 11, 315–333. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, Z.S.; Huang, L.; Wang, X.P. Review of the ecohydrological processes and feedback mechanisms controlling sand-binding vegetation systems in sandy desert regions of China. Chin. Sci. Bull. 2013, 58, 1483–1496. [Google Scholar] [CrossRef]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions, an inventory and analysis of terminology and guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef]
- Cottingham, K.L.; Brown, B.L.; Lennon, J.T. Biodiversity may regulate the temporal variability of ecological systems. Ecol. Lett. 2001, 4, 72–85. [Google Scholar] [CrossRef]
- Deimeke, E.; Cohena, M.J.; Reissb, K.C. Temporal stability of vegetation indicators of wetland condition. Ecol. Indic. 2013, 34, 69–75. [Google Scholar] [CrossRef]
- Ives, A.R.; Carpenter, S.R. Stability and diversity of ecosystems. Science 2007, 317, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Haydon, D.T. Pivotal assumptions determining the relationship between stability and complexity: An analytical synthesis of the stability complexity debate. Am. Nat. 1994, 144, 14–29. [Google Scholar] [CrossRef]
- Townsend, S.E.; Haydon, D.T.; Matthews, L. On the generality of stability complexity relationships in Lotka-Volterra ecosystems. J. Theor. Biol. 2010, 267, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mougi, A.; Kondoh, M. Diversity of interaction types and ecological community stability. Science 2012, 337, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E. Biodiversity and ecosystem stability. Nat. Education Knowl. 2012, 3, 14. [Google Scholar]
- Sterk, M.; Gort, G.; Klimkowska, A.; van Ruijven, J.; van Teeffelen, A.J.A.; Wamelink, G.W.W. Assess ecosystem resilience: Linking response and effect traits to environmental variability. Ecol. Indic. 2013, 30, 21–27. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Auld, M.H.D.; Le Duc, M.G.; Marrs, R.H. Ecosystem stability and resilience, a review of their relevance for the conservation management of lowland heaths. Perspect. Plant Ecol. 2000, 3, 142–160. [Google Scholar] [CrossRef]
- Ives, A.R.; Dennis, B.; Cottingham, K.L.; Carpenter, S.R. Estimating community stability and ecological interactions from time series data. Ecol. Monogr. 2003, 73, 301–330. [Google Scholar] [CrossRef]
- Loreau, M.; de Mazancourt, C. Biodiversity and ecosystem stability, a synthesis of underlying mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef] [PubMed]
- May, R.M. Qualitative stability in model ecosystems. Ecology 1973, 54, 638–641. [Google Scholar] [CrossRef]
- Grimm, V.; Schmidt, E.; Wissel, C. On the application of stability concepts in ecology. Ecol. Model. 1992, 63, 143–161. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Barker, G.M. Stability of ecosystem properties in response to above-ground functional group richness and composition. Oikos 2000, 89, 11–23. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.I.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning, a consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef] [Green Version]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Saaty, T.L. A scaling method for priorities in hierarchical structures. J. Math. Psychol. 1977, 15, 234–281. [Google Scholar] [CrossRef]
- Locantore, N.W.; Tran, L.T.; O’Neill, R.V.; McKinnis, P.W.; Smith, E.R.; O’Connell, M. An overview of data integration methods for regional assessment. Environ. Monit. Assess. 2004, 94, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Zhang, X.D.; Yang, J.Y.; Yuan, H. Wetland ecosystem stability evaluation by using analytical hierarchy process AHP, approach in Yinchuan Plain: China. Math. Comput. Model. 2013, 57, 366–374. [Google Scholar] [CrossRef]
- Saaty, T.L. The Analytical Hierarchy Process: Planning, Priority Setting, Resource Allocation; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Vaidya, O.S.; Kumar, S. Analytic hierarchy process, An overview of applications. Eur. J. Oper. Res. 2006, 169, 1–29. [Google Scholar] [CrossRef]
- Banai, R. Anthropocentric problem solving in planning and design, with analytic hierarchy process. J. Archit. Plann. Res. 2005, 22, 107–120. [Google Scholar]
- Cheng, E.W.L.; Li, H.; Yu, L. The analytic network process (ANP), approach to location selection: A shopping mall illustration. Onstr. Innov. 2005, 5, 83–97. [Google Scholar] [CrossRef]
- Ho, W. Integrated analytic hierarchy process and its applications—A literature review. Eur. J. Oper. Res. 2008, 186, 211–228. [Google Scholar] [CrossRef]
- Barzekar, G.; Aziz, A.; Mariapan, M.; Ismail, M.H.; Hosseni, S.M. Using analytical hierarchy Pprocess (AHP), for prioritizing and ranking of ecological indicators for monitoring sustainability of ecotourism in northern forest: Iran. Ecologia Balkanica 2011, 3, 59–67. [Google Scholar]
- Convertino, M.; Baker, K.M.; Vogel, J.T.; Lu, C.; Suedel, B.; Linkov, I. Multi-criteria decision analysis to select metrics for design and monitoring of sustainable ecosystem restorations. Ecol. Indic. 2013, 26, 76–86. [Google Scholar] [CrossRef]
- Le Houerou, H.N. Restoration and rehabilitation of arid and semiarid Mediterranean ecosystems in North Africa and West Asia, a review. Arid Soil Res. Rehabil. 2000, 14, 3–14. [Google Scholar] [CrossRef]
- Li, X.R.; He, M.Z.; Duan, Z.H.; Xiao, H.L.; Jia, X.H. Recovery of topsoil physiochemical properties in revegetated sites in the sand-burial ecosystems of the Tengger Desert: Northern China. Geomorph 2007, 88, 254–265. [Google Scholar] [CrossRef]
- Li, X.R.; Xiao, H.L.; Zhang, J.G.; Wang, X.P. Long-term ecosystem effects of sand-binding vegetation in the Tengger Desert: Northern China. Restor. Ecol. 2004, 12, 376–390. [Google Scholar] [CrossRef]
- Li, X.R. Influence of variation of soil spatial heterogeneity on vegetation restoration. Sci. China Ser. D 2005, 35, 361–370. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Koen, T.B.; Harrison, L. Plant composition of three woodland communities of variable condition in the western Riverina, New South Wales, Australia. Cunninghamia 2007, 10, 189–198. [Google Scholar]
- Loveland, P.J.; Walley, W.R. Particle size analysis. In Soil and Environmental Analysis, Physical Methods, 2nd ed.; Simth, K.A., Mullins, C.E., Eds.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Nanjing Institute of Soil Research. Analysis of Soil Physic-Chemical Features; Shanghai Science and Technology Press: Shanghai, China, 1980. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–577. [Google Scholar]
- Cinelli, M.; Coles, S.R.; Kirwan, K. Analysis of the potentials of multi criteria decision analysis methods to conduct sustainability assessment. Ecol. Indic. 2014, 46, 138–148. [Google Scholar] [CrossRef]
- Baudena, M.; Boni, G.; Ferraris, L.; von Hardenberg, J.; Provenzale, A. Vegetation response to rainfall intermittency in drylands, results from a simple ecohydrological box model. Adv. Water Res. 2007, 30, 1320–1328. [Google Scholar] [CrossRef]
- Lee, J.K.L.; Chan, E.H.W. The analytic hierarchy process AHP, approach for assessment of Urban renewal proposals. Soc. Indicat. Res. 2008, 89, 155–168. [Google Scholar] [CrossRef]
- Altuzarra, A.; Moreno-Jiménez, J.M.; Salvador, M. Consensus building in AHP-group decision making: A Bayesian approach. Oper. Res. 2010, 58, 1755–1773. [Google Scholar] [CrossRef]
- Cagno, E.; Caron, F.; Mancini, M.; Ruggeri, F. Using AHP in determining the prior distributions on gas pipeline failures in a robust Bayesian approach. Reliab. Eng. Syst. Safe. 2000, 67, 275–284. [Google Scholar] [CrossRef]
- Hahn, E.D. Decision making with uncertain judgments: A stochastic formulation of the analytic hierarchy process. Decis. Sci. 2003, 34, 443–466. [Google Scholar] [CrossRef]
- Mirza, M.Q.; Warrick, R.A.; Ericksen, N.J.; Kenny, G.J. Trends and persistence in precipitation in Ganges, Brahmaputra and Meghna river basins. Hydrol. Sci. J. 1998, 43, 845–858. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhang, J.G.; Li, X.R.; Li, J.G. Distribution, trends and variability of precipitation in Shapotou region. J. Desert Res. 2001, 4, 260–264. [Google Scholar]
- Lehman, C.L.; Tilman, D. Biodiversity, stability and productivity in competitive communities. Am. Nat. 2000, 156, 534–552. [Google Scholar] [CrossRef]
- Shen, W.S. The status of Artemisia ordosica in vegetation succession at Shapotou area. J. Desert Res. 1986, 6, 13–22. [Google Scholar]
- Zhao, X.L. Research on Problem of Controlling Sand of Vegetable; Ningxia People’s Publishing House: Yinchuan, China, 1988. [Google Scholar]
- Baudena, M.; Provenzale, A. Rainfall intermittency and vegetation feedbacks in drylands. Hydrol. Earth Syst. Sci. 2008, 12, 679–689. [Google Scholar] [CrossRef]
- Baudena, M.; D’Andrea, F.; Provenzale, A. An idealized model for tree-grass coexistence in savannas. J. Ecol. 2010, 98, 74–80. [Google Scholar] [CrossRef]
- Borgogno, F.; D’Odorico, P.; Laio, F.; Ridolfi, L. Mathematical models of vegetation pattern formation in ecohydrology. Rev. Geophys. 2009, 47, RG1005. [Google Scholar] [CrossRef]
- Walker, B.H. Biodiversity and ecological redundancy. Conserv. Biol. 1992, 6, 18–23. [Google Scholar] [CrossRef]
- Klausmeier, C.A. Regular and irregular patterns in semiarid vegetation. Science 1999, 284, 1826–1828. [Google Scholar] [CrossRef] [PubMed]
- Rietkerk, M.; Dekker, S.C.; de Ruiter, P.C.; van de Koppel, J. Self-organized patchiness and catastrophic shifts in ecosystems. Science 2004, 305, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Kefi, S.; RieterkI, M.; Alados, C.L.; Pueyo, Y.; Papanastasis, V.P.; ElAich, A.; de Ruiter, C. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature 2007, 449, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R. Eco-hydrology of biological soil crusts in desert regions of China; Higher Education Press: Beijing, China, 2012. [Google Scholar]
- Lotfi, S.; Habibi, K.; Koohsari, M.J. An analysis of urban land development using multi-criteria decision model and geographical information system (A case study of Babolsar city). Am. J. Environ. Sci. 2009, 5, 87–93. [Google Scholar] [CrossRef]
- Laio, F.; Porporato, A.; Ridolfi, L.; Rodriguez-Iturbe, I. Plants in water-controlled ecosystems: active role in hydrologic processes and response to water stress—II Probabilistic soil moisture dynamics. Adv. Water Resour. 2001, 24, 707–723. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, L.C.; Li, X.R.; Zheng, J.G.; He, M.Z.; Tan, H.J. Evaporation properties of a revegetated area of the Tengger Desert North China. J. Arid Environ. 2008, 72, 964–973. [Google Scholar]
- Huang, L.; Zhang, Z.S.; Li, X.R. The extrapolation of the leaf area-based transpiration of two xerophytic shrubs in a revegetated desert area in the Tengger Desert, China. Hydrol. Res. 2014. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zhang, Z. The Stability of Revegetated Ecosystems in Sandy Areas: An Assessment and Prediction Index. Water 2015, 7, 1969-1990. https://doi.org/10.3390/w7051969
Huang L, Zhang Z. The Stability of Revegetated Ecosystems in Sandy Areas: An Assessment and Prediction Index. Water. 2015; 7(5):1969-1990. https://doi.org/10.3390/w7051969
Chicago/Turabian StyleHuang, Lei, and Zhishan Zhang. 2015. "The Stability of Revegetated Ecosystems in Sandy Areas: An Assessment and Prediction Index" Water 7, no. 5: 1969-1990. https://doi.org/10.3390/w7051969