Potential of Constructed Wetlands for Removal of Antibiotics from Saline Aquaculture Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
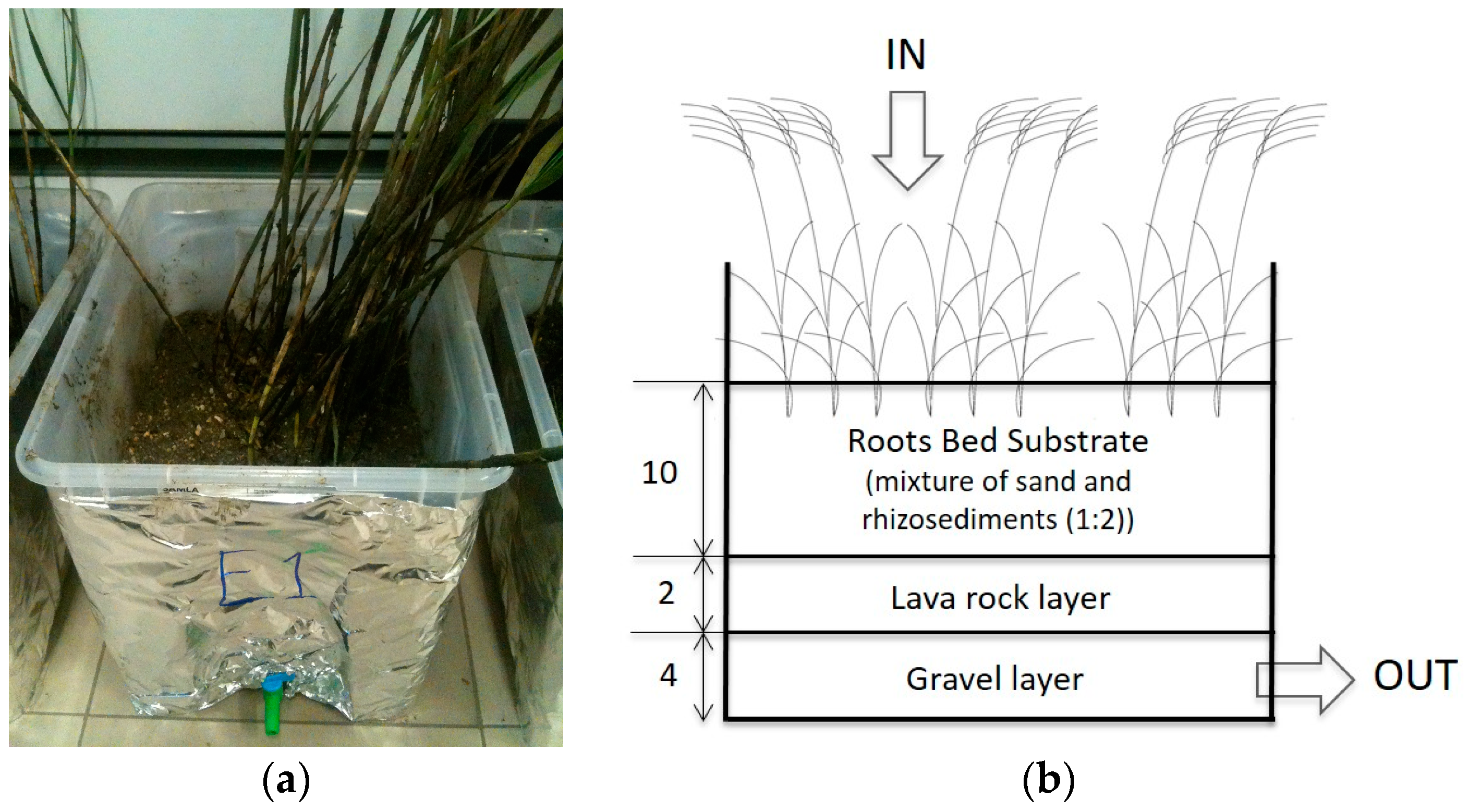
2.2. Microcosms Assembly and CWs Experiment
2.3. Sample Collection
2.4. Antibiotics Analysis
2.5. Enumeration of Cultivable Bacteria
2.6. Wastewater Characterization
2.7. Statistical Analysis
3. Results
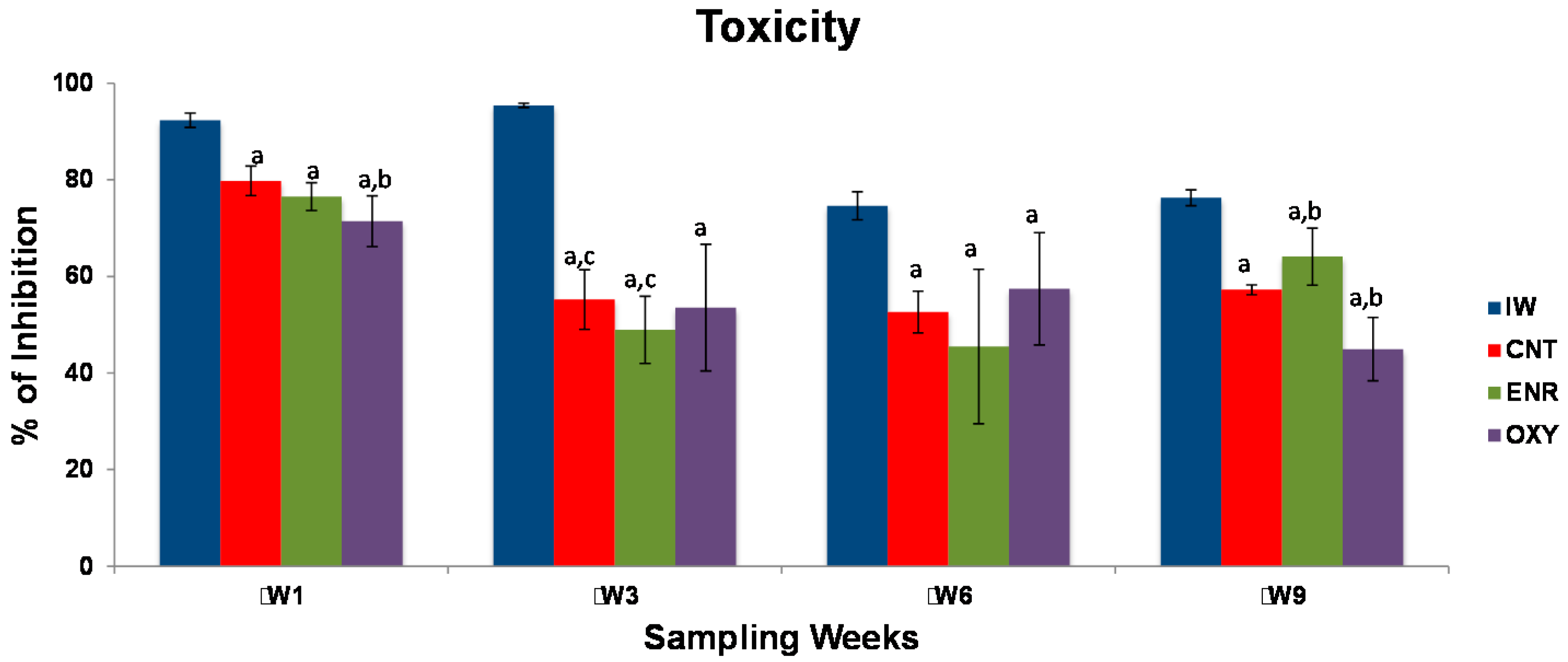
3.1. Antibiotics Concentrations
3.2. Enumeration of Cultivable Bacteria
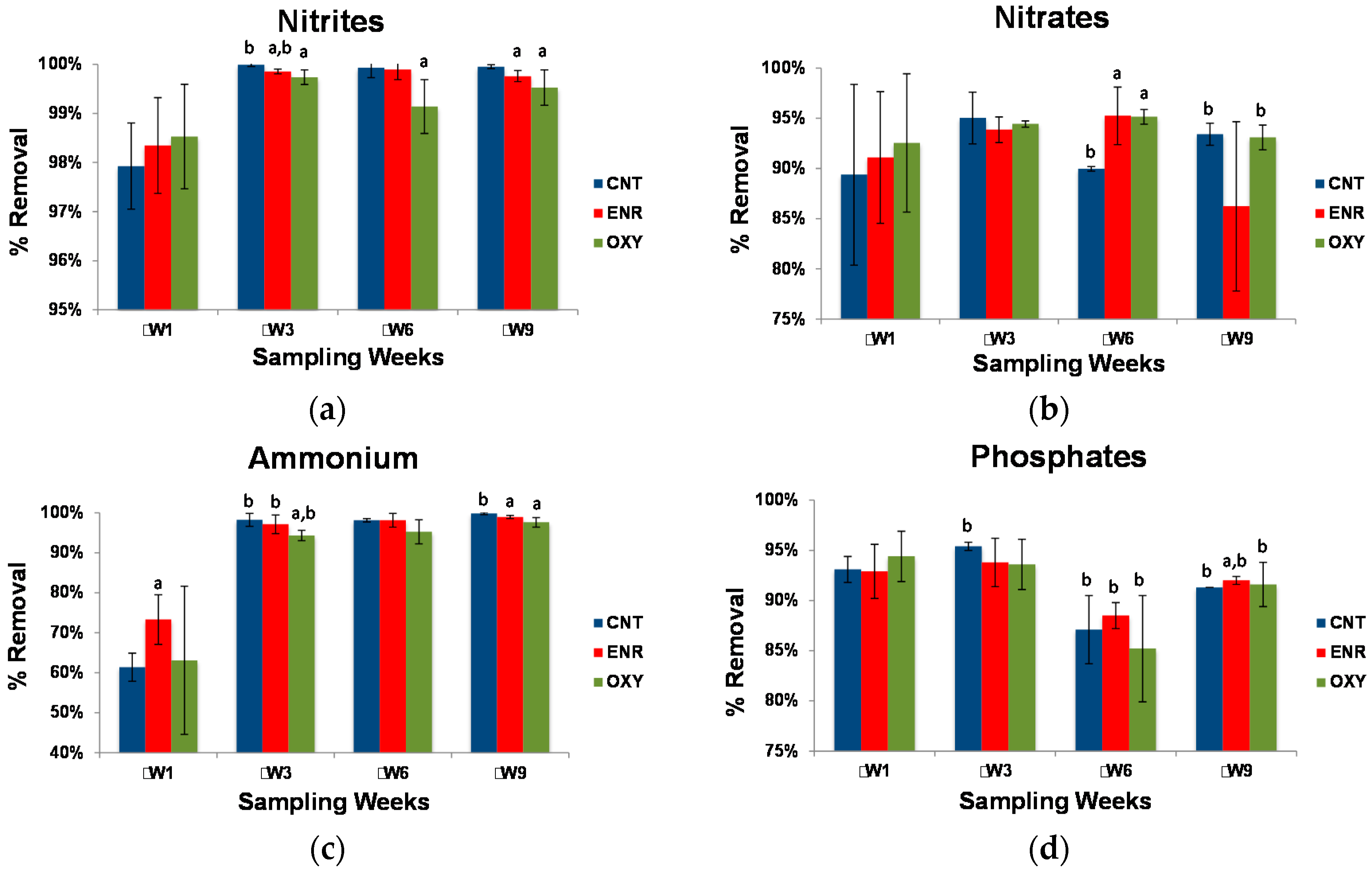
3.3. Wastewater Characterization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2014; ISBN 978-92-5-108275-1 (print). E-ISBN 978-92-5-108276-8 (PDF). [Google Scholar]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.J.; Grant, A.; Davies, I.M.; Cecchini, S.; Papoutsoglou, S.; Quigley, D.; Saroglia, M. The control of chemicals used in aquaculture in Europe. J. Appl. Ichthyol. 2001, 17, 173–180. [Google Scholar] [CrossRef]
- Romero, J.; Feijoó, C.G.; Navarrete, P. Antibiotics in Aquaculture–Use, Abuse and Alternatives. In Health and Environment in Aquaculture; Carvalho, E.D., David, G.S., Silva, R.J., Eds.; InTech: Rijeka, Croatia, 2012; pp. 160–198. [Google Scholar]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Pereira, A.C.; Ribeiro, I.L.; Reis, I.; Carvalho, P.; Basto, M.C.P.; Mucha, A.P. Microbial community dynamics associated with veterinary antibiotics removal in constructed wetlands microcosms. Bioresour. Technol. 2015, 182, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Ministério do Ambiente. Decreto-Lei n° 236/98 de 1 Agosto. Diário da República—I Série-A (Annex XVIII); 1998. [Google Scholar]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, 327, 1–72. [Google Scholar]
- Deng, Y.; Zhang, Y.; Gao, Y.; Li, D.; Liu, R.; Liu, M.; Zhang, H.; Hu, B.; Yu, T.; Yang, M. Microbial community compositional analysis for series reactors treating high level antibiotic wastewater. Environ. Sci. Technol. 2012, 46, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R. Potential of Phragmites australis for the removal of veterinary pharmaceuticals from aquatic media. Bioresour. Technol. 2012, 116, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontés, C.; Salvad, V. The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—A review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Constructed wetlands for wastewater treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Webb, J.M.; Quintã, R.; Papadimitriou, S.; Norman, L.; Rigby, M.; Thomas, D.N.; Le Vay, L. Halophyte filter beds for treatment of saline wastewater from aquaculture. Water Res. 2012, 46, 5102–5114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Keffala, C.; Ghrabi, A. Nitrogen and bacterial removal in constructed wetlands treating domestic waste water. Desalination 2005, 185, 383–389. [Google Scholar] [CrossRef]
- Sleytr, K.; Tietz, A.; Langergraber, G.; Haberl, R. Investigation of bacterial removal during the filtration process in constructed wetlands. Sci. Total Environ. 2007, 380, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Jing, S.R.; Lee, D.Y.; Wang, T.W. Nutrient removal from aquaculture wastewater using a constructed wetlands system. Aquaculture 2002, 209, 169–184. [Google Scholar] [CrossRef]
- Quintã, R.; Hill, P.W.; Jones, D.L.; Santos, R.; Thomas, D.N.; LeVay, L. Uptake of an amino acid (alanine) and its peptide (trialanine) by the saltmarsh halophytes Salicornia europaea and Aster tripolium and its potential role in ecosystem N cycling and marine aquaculture wastewater treatment. Ecol. Eng. 2015, 75, 145–154. [Google Scholar] [CrossRef]
- Quintã, R.; Santos, R.; Thomas, D.; Vay, L.L. Growth and nitrogen uptake by Salicornia europaea and Aster tripolium in nutrient conditions typical of aquaculture wastewater. Chemosphere 2015, 120, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.M.; Quintã, R.; Papadimitriou, S.; Norman, L.; Rigby, M.; Thomas, D.N.; Le Vay, L. The effect of halophyte planting density on the efficiency of constructed wetlands for the treatment of wastewater from marine aquaculture. Ecol. Eng. 2013, 61, 145–153. [Google Scholar] [CrossRef]
- Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Wong, C.S.; Knapp, C.W.; Hanson, M.L. Performance of a constructed wetland in Grand Marais, Manitoba, Canada: Removal of nutrients, pharmaceuticals, and antibiotic resistance genes from municipal wastewater. Chem. Cent. J. 2013, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Araújo, J.L.; Mucha, A.P.; Basto, M.C.P.; Almeida, C.M.R. Potential of constructed wetlands microcosms for the removal of veterinary pharmaceuticals from livestock wastewater. Bioresour. Technol. 2013, 134, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Liaw, E.T.; Fan, K.M. Removal of veterinary antibiotics, alkylphenolic compounds, and estrogens from the Wuluo constructed wetland in southern Taiwan. J. Environ. Sci. Health A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Khan, G.A.; Weisner, S.E.B.; Ehde, P.M.; Fick, J.; Lindgren, P.E. Efficient removal of antibiotics in surface-flow constructed wetlands, with no observed impact on antibiotic resistance genes. Sci. Total Environ. 2014, 476–477, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gastalho, S.; Silva, G.J.; Ramos, F. Uso de antibióticos em aquacultura e resistência bacteriana: Impacto em saúde pública Antibiotics in aquaculture and bacterial resistance: Health care impact. Acta Farmacêutica Portuguesa 2014, 3, 29–45. [Google Scholar]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A review of plant-pharmaceutical interactions: From uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Pavlović, D.M.; Ašperger, D.; Periša, M.; Zrnčić, M.; Horvat, A.J.M.; Kaštelan-Macan, M. Determination of multi-class pharmaceuticals in wastewater by liquid chromatography-tandem mass spectrometry (LC–MS–MS). Anal. Bioanal. Chem. 2010, 398, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Cavenati, S.; Carvalho, P.N.; Almeida, C.M.R.; Basto, M.C.P.; Vasconcelos, M.T.S.D. Simultaneous determination of several veterinary pharmaceuticals in effluents from urban, livestock and slaughterhouse wastewater treatment plants using a simple chromatographic method. Water Sci. Technol. 2012, 66, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Novo, A.; Manaia, C.M. Factors influencing antibiotic resistance burden in municipal wastewater treatment plants. Appl. Microbiol. Biotechnol. 2010, 87, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Micalizzi, G.R.; Bates, J.R.; Costanzo, S.D. Novel method for rapid assessment of antibiotic resistance in Escherichia coli isolates from environmental waters by use of a modified chromogenic agar. Appl. Environ. Microbiol. 2007, 73, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Ulitzur, S.; Lahav, T.; Ulitzur, N. A novel and sensitive test for rapid determination of water toxicity. Environ. Toxicol. 2002, 17, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.R.; Mucha, A.P.; Vasconcelos, M.T.S.D. Influence of the sea rush Juncus maritimus on metal concentration and speciation in estuarine sediment colonized by the plant. Environ. Sci. Technol. 2004, 38, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis, 2nd ed.; Verlag Chemie: Weinheim, Germany, 1983. [Google Scholar]
- Jones, M.N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 1984, 18, 643–646. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Knight, R.L. Treatment Wetlands; Lewis Publishers: Boca Raton, FL, USA, 1996; p. 348. [Google Scholar]
- Brix, H. Functions of macrophytes in constructed wetlands. Water Sci. Technol. 1994, 29, 71–78. [Google Scholar]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Ottová, V.; Balcarová, J.; Vymazal, J. Microbial characteristics of constructed wetlands. Water Sci. Technol. 1997, 35, 117–123. [Google Scholar] [CrossRef]
- Dordio, A.V.; Duarte, C.; Barreiros, M.; Carvalho, A.P.; Pinto, A.; Costa, C.T. Toxicity and removal efficiency of pharmaceutical metabolite clofibric acid by Typha spp.–Potential use for phytoremediation? Bioresour. Technol. 2009, 100, 1156–1161. [Google Scholar] [CrossRef] [PubMed]


| Bacterial Taxonomic | Initial Wastewater | Treated Wastewater CWs Microcosms | |||
|---|---|---|---|---|---|
| CNT | ENR | OXY | |||
| CFU/mL | % Resistance | % Removal | % Removal | % Removal | |
| HT | 3.4 × 102–1.54 × 104 | - | 47–98 | 0–100 | 17–100 |
| HE | 1.92 × 101–2.3 × 102 | 0–9 | 73–100 | 90–100 | - |
| HO | 2.1 × 101–3.38 × 102 | 1–89 | 98–99 | - | 99–100 |
| CT | 6.0 × 100–7.0 × 101 | - | 0–100 | 50–100 | 0–100 |
| CE | 6.0 × 10−1–5.0 × 100 | 3–11 | 0–100 | 72–100 | - |
| CO | 1.0 × 100–9.0 × 100 | 3–20 | 7–100 | - | 0–100 |
| ET | 1.05 × 101–4.7 × 101 | - | 99–100 | 99–100 | 99–100 |
| EE | 1.0 × 100–2.3 × 100 | 2–11 | 99 | 99–100 | - |
| EO | 3.1 × 100–1.2 × 101 | 7–57 | 99–100 | - | 97–100 |
| Metal | Treatment | W0–W1 | W2–W3 | W5–W6 | W8–W9 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | σ | Mean | σ | Mean | σ | Mean | σ | ||
| Fe | CNT | 41% | 5% | −120% b | 47% | 31% | 0% | 54% | 2% |
| ENR | 36% | 28% | −42% | 55% | 25% | 11% | 65% a,b | 7% | |
| OXY | 31% | 64% | −71% | 93% | 31% | 16% | 70% a,b | 2% | |
| Mn | CNT | −105% | 40% | −20% | 10% | 32% | 0% | - | - |
| ENR | 36% a | 37% | −50% | 78% | 27% | 22% | - | - | |
| OXY | −47% | 93% | 27% | 29% | 22% | 25% | - | - | |
| Zn | CNT | - | - | 9% | 33% | 40% | 23% | 66% b | 3% |
| ENR | - | - | −41% | 79% | 32% b | 9% | 25% a | 15% | |
| OXY | - | - | 13% | 13% | 54% b | 3% | 22% a,b | 9% | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bôto, M.; Almeida, C.M.R.; Mucha, A.P. Potential of Constructed Wetlands for Removal of Antibiotics from Saline Aquaculture Effluents. Water 2016, 8, 465. https://doi.org/10.3390/w8100465
Bôto M, Almeida CMR, Mucha AP. Potential of Constructed Wetlands for Removal of Antibiotics from Saline Aquaculture Effluents. Water. 2016; 8(10):465. https://doi.org/10.3390/w8100465
Chicago/Turabian StyleBôto, Maria, C. Marisa R. Almeida, and Ana P. Mucha. 2016. "Potential of Constructed Wetlands for Removal of Antibiotics from Saline Aquaculture Effluents" Water 8, no. 10: 465. https://doi.org/10.3390/w8100465






