Performance and N2O Formation of the Deammonification Process by Suspended Sludge and Biofilm Systems—A Pilot-Scale Study
Abstract
:1. Introduction
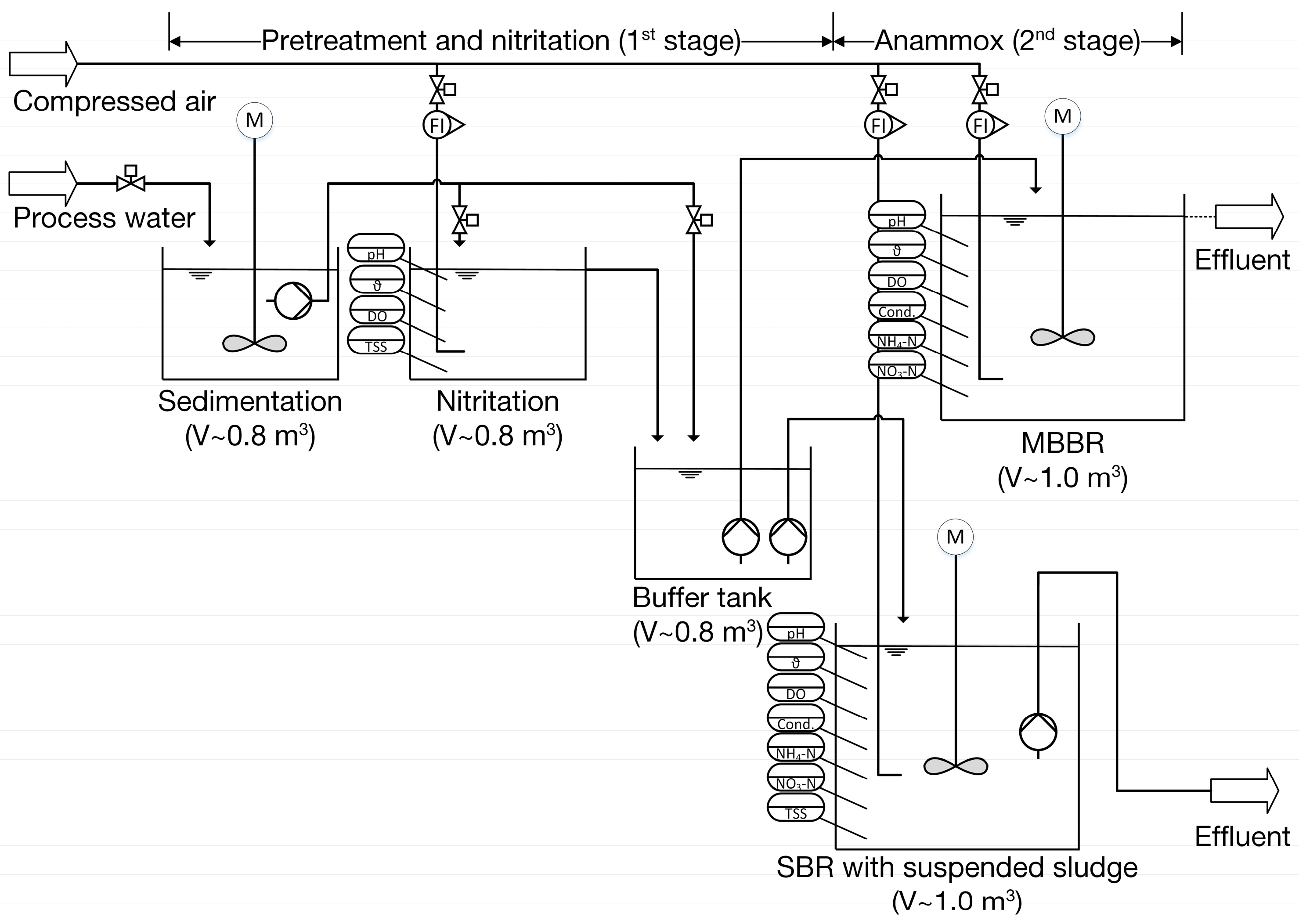
2. Materials and Methods
3. Results and Discussion
3.1. Reactor Performance
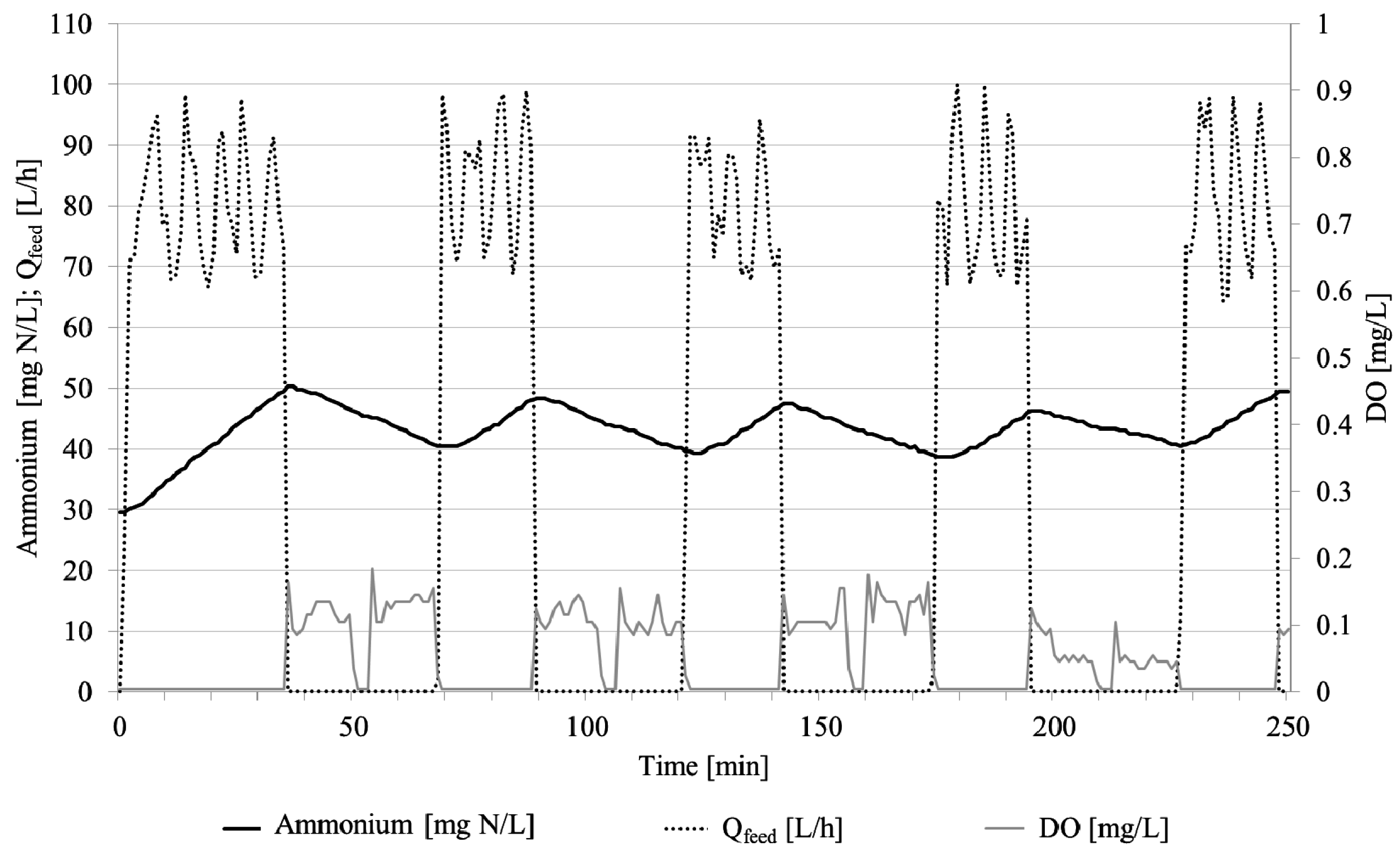
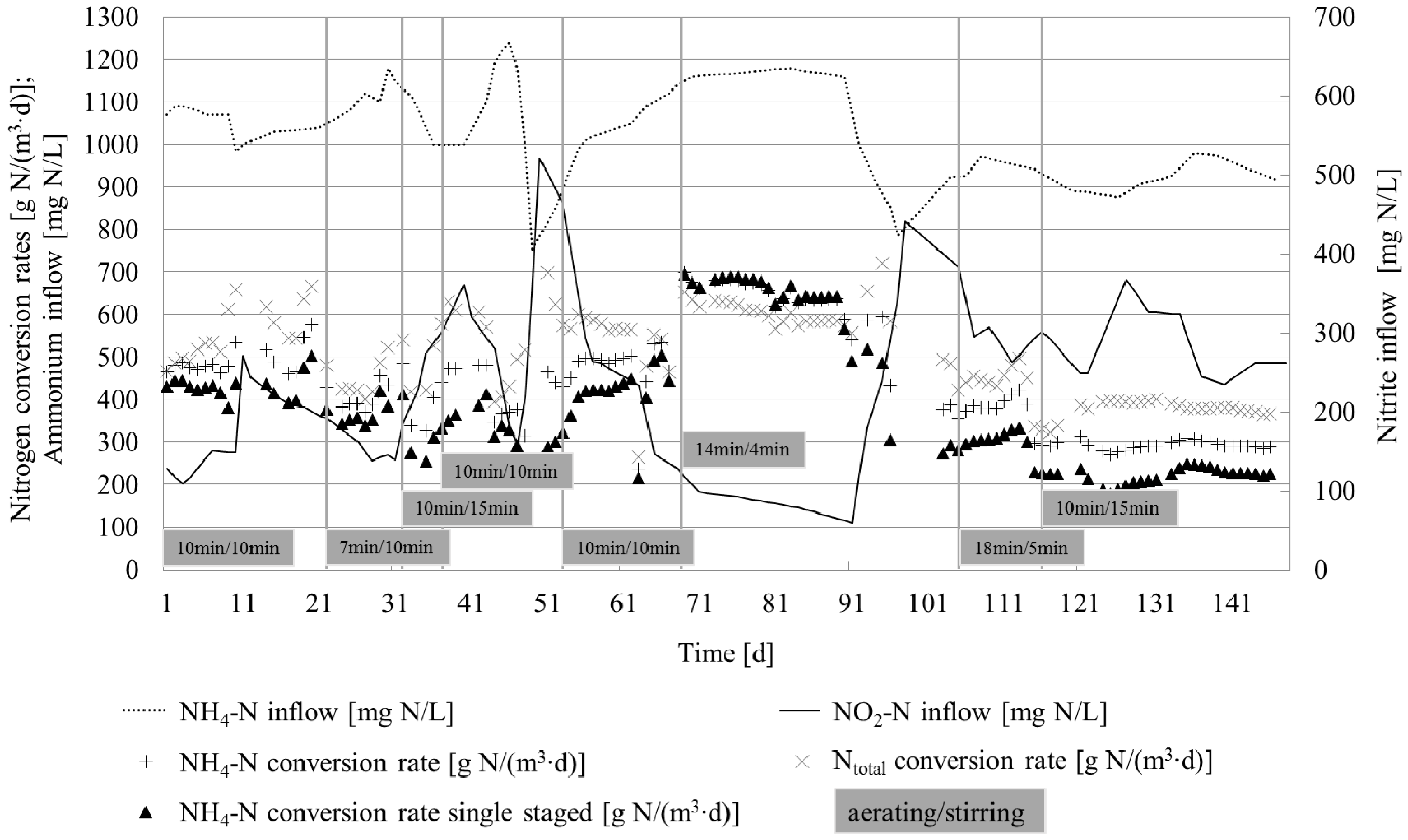
3.1.1. Performance of Nitritation Reactor
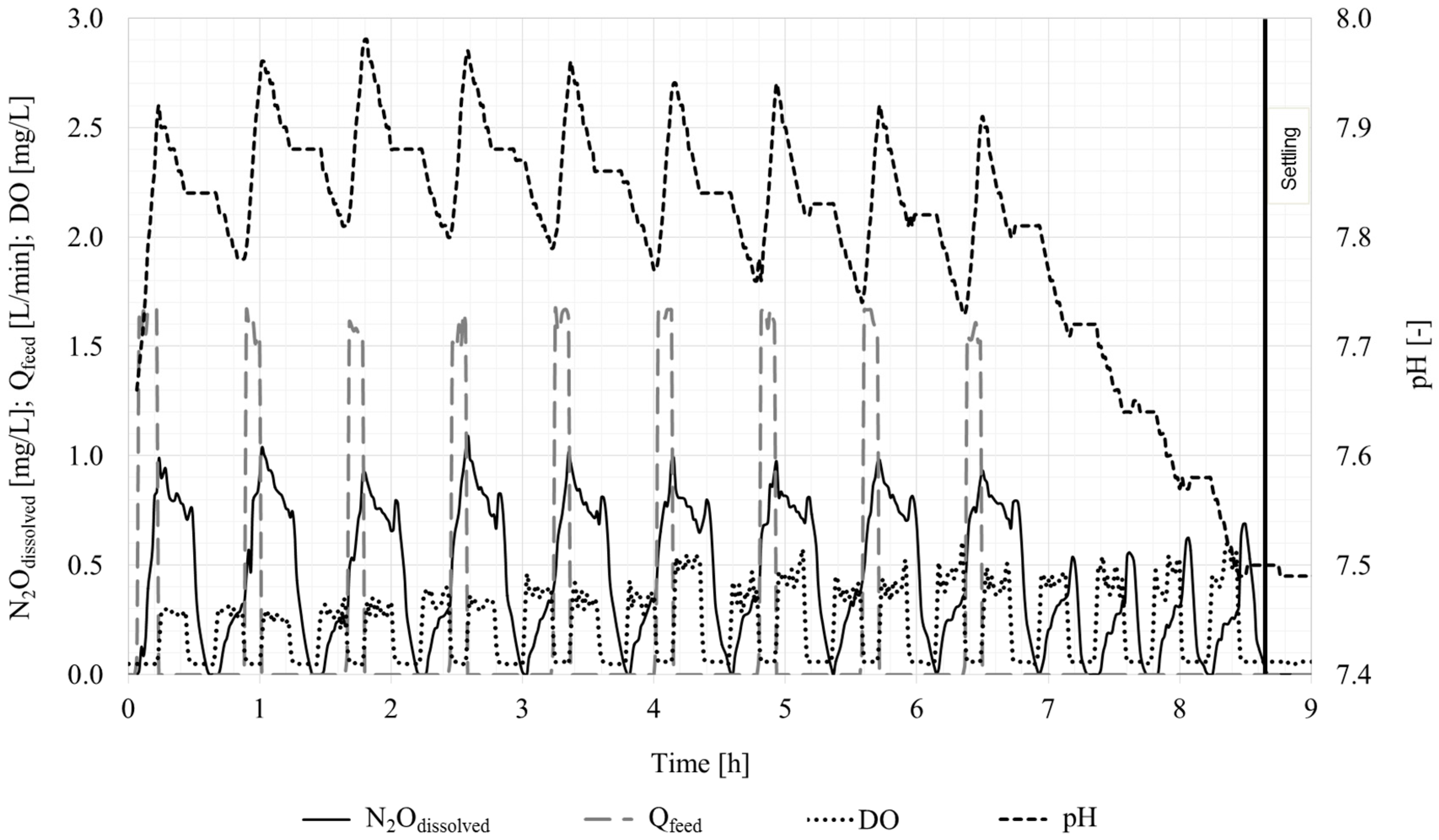
3.1.2. SBR Performance
3.1.3. MBBR Performance
3.1.4. Operational Assessment
3.2. Nitrous Oxide Formation
3.2.1. Measurements and Possible Production Pathways
3.2.2. Mitigation Strategies
4. Conclusions
- A high nitritation performance in the first stage providing an effluent ratio of as close as 1 mol of ammonium to 1.32 mol of nitrite is crucial for a fully anoxic ammonium oxidation in the second stage and high overall degradation rates. If this ratio cannot be achieved, a prolonged HRT, elevation of the temperature above 30 °C, and increased aeration intensity are suggested to improve the nitritation under the countermeasures necessary to suppress NOB growth.
- Although the nitritation effluent did not meet the required ammonium-to-nitrite ratio during the study periods, both the SBR and MBBR in the second stage were able to achieve nitrogen turnover rates and degradation rates above 500 g N/(m3∙day) and 80%, respectively. This was only possible because both second stages acted partially as single-stage deammonification processes due to supplementary aeration, which is highly uncommon for the second stage of the deammonification process.
- Comparing MBBR and SBR performances, the latter achieved higher nitrogen removal and degradation, attributed to the higher inflow NO2-N/NH4-N ratio and longer HRT in the SBR, respectively. In contrast, the calculated single-stage ammonium conversion rate of the MBBR was found to be higher, enabling moderate compensation of the smaller nitrite inflow concentrations.
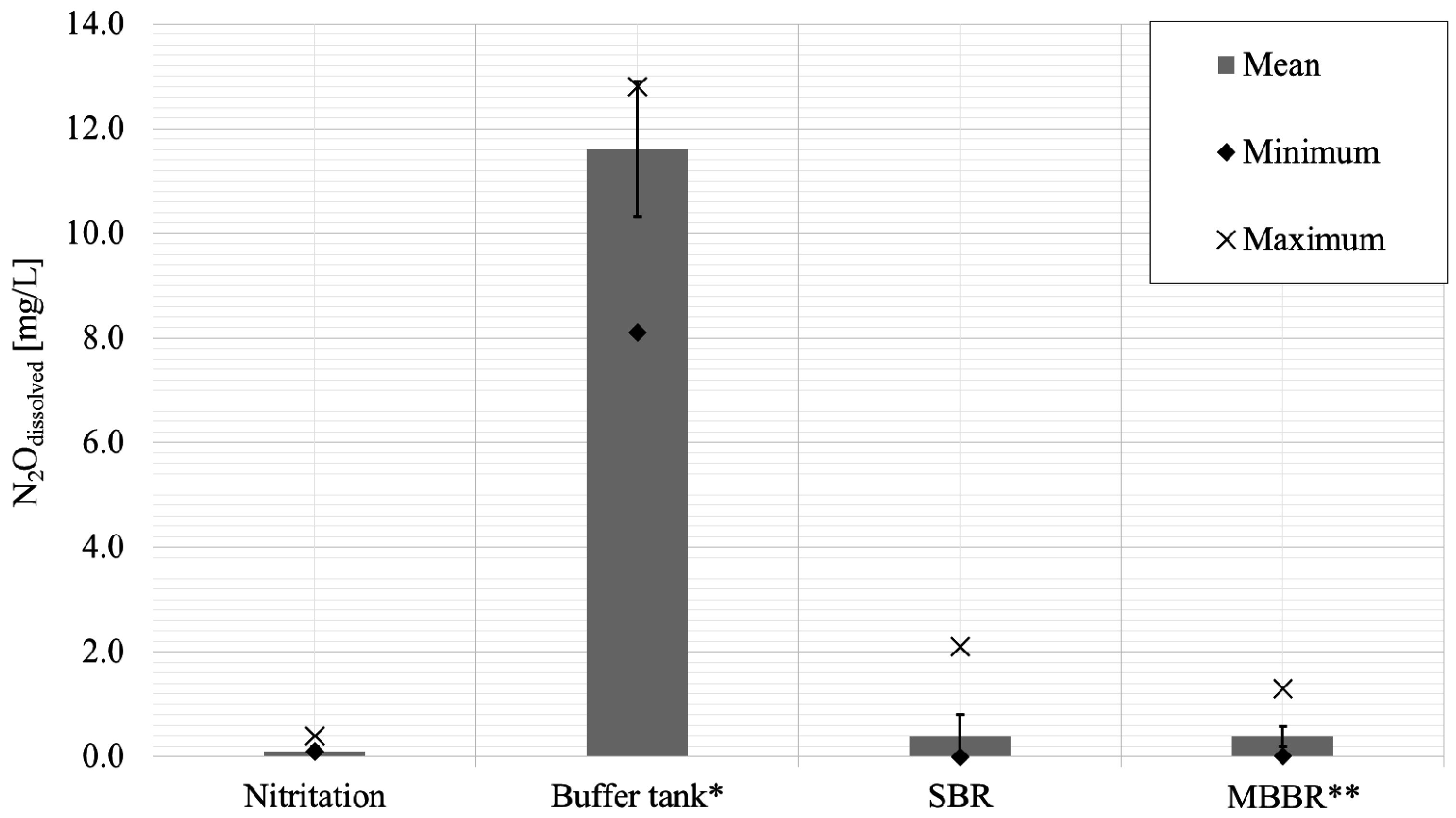
- The intermediate buffer tank exhibited the highest dissolved N2O concentrations of all reactors. Prevalent anoxic conditions, high nitrite and ammonium concentrations, an HRT of approximately one day, and abundant biomass introduced from the nitritation reactor into the non-mixed and non-aerated buffer tank with an infinite SRT has been identified to provide favourable conditions for N2O production.
- Nitritation with continuous aeration and the SBR in the second stage had lower dissolved N2O concentrations in comparison to the buffer tank. A transfer of dissolved N2O from the buffer tank into the SBR could be observed. The nitritation step and the SBR contributed to the emission of N2O due to the aeration; however, the specific amount could not be quantified due to missing off-gas measurements.
- N2O mitigation strategies were identified, including the use of a decreased HRT in the buffer tank to shorten the reaction time for N2O production and frequent removal of settled biomass. In the second stage, stripping effects could be limited by a rearrangement of the aeration phases with anoxic periods after feeding the SBR to allow for a reduction of N2O or implementation of a complete anammox process without aeration.
- To avoid uncontrolled N2O emissions from deammonification processes, the total off-gas could be collected by use of gastight reactors and then be applied as oxidant during the combustion of biogas, representing an end-of-pipe solution. Even if the proportion of N2O to the total amount of the off-gas would be low, this approach would not only ensure an environmentally friendly solution as the N2O is converted to inert N2 when incinerated, but would also make use of the 37% higher energy yield of the oxidant N2O in comparison to oxygen.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.M. Innovative nitrogen removal. In Biological Wastewater Treatment: Principles, Modelling and Design; Henze, M., van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovoic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 139–154. [Google Scholar]
- Weißbach, M.; Criddle, C.S.; Drewes, J.E.; Koch, K. A proposed nomenclature for biological processes that remove nitrogen. Environ. Sci. Water Res. Technol. 2016. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Ahn, K.-H.; Lee, H.-J.; Lee, K.-H.; Kwak, Y.-J.; Song, K.-G. Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res. 1999, 33, 145–154. [Google Scholar] [CrossRef]
- Ciudad, G.; Werner, A.; Bornhardt, C.; Muñoz, C.; Antileo, C. Differential kinetics of ammonia- and nitrite-oxidizing bacteria: A simple kinetic study based on oxygen affinity and proton release during nitrification. Process Biochem. 2006, 41, 1764–1772. [Google Scholar] [CrossRef]
- Van Hulle, S.W.; Vandeweyer, H.J.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Hellinga, C.; Schellen, A.A.J.C.; Mulder, A.J.; van Loosdrecht, M.C.M.; Heijnen, J.J. The SHARON process: An innovative method for nitrogen removal from ammonium-rich waste water. Water Sci. Technol. 1998, 37, 135–142. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar] [PubMed]
- Strous, M.; van Gerven, E.; Kuenen, G.J.; Jetten, M. Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (anammox) sludge. Appl. Environ. Microbiol. 1997, 63, 2446–2448. [Google Scholar] [PubMed]
- Lotti, T.; van der Star, W.R.L.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C.M. The effect of nitrite inhibition on the anammox process. Water Res. 2012, 46, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Wett, B.; Murthy, S.; Takács, I.; Hell, M.; Bowden, G.; Deur, A.; O’Shaughnessy, M. Key Parameters for Control of DEMON Deammonification Process. Water Pract. 2007, 1, 1–11. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K.; Kazama, F.; Sumino, T. Effects of nitrite inhibition on anaerobic ammonium oxidation. Appl. Microbiol. Biotechnol. 2010, 86, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Trigo, C.; Campos, J.L.; Garrido, J.M.; Mendez, R. Start-up of the Anammox process in a membrane bioreactor. J. Biotechnol. 2006, 126, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- United Nations Framework Convention on Climate Change. Outcomes of the U.N. Climate change conference in Paris. In Proceedings of the 21st Session of the Conference of the Parties to the United Nations Framework Convention on Climate Change, Paris, France, 30 November–12 December 2015.
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Firestone, M.K.; Firestone, R.B.; Tiedje, J.M. Nitric oxide as an intermediate in denitrification: Evidence from nitrogen-13 isotope exchange. Biochem. Biophys. Res. Commun. 1979, 91, 10–16. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; van der Star, W.R.; Wielders, H.A.; Mulder, J.W.; Jetten, M.S.; van Loosdrecht, M.C.M. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 2008, 42, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Kuypers, M.M.M.; Lavik, G.; Schalk, J.; Op den Camp, H.J.M.; Jetten, M.S.; Strous, M. Anammox bacteria disguised as denitrifiers: Nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ. Microbiol. 2007, 9, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Leix, C.; Drewes, J.E.; Koch, K. The role of residual quantities of suspended sludge on nitrogen removal efficiency in a deammonifying moving bed biofilm reactor. Bioresour. Technol. 2016, 219, 212–218. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association (APHA): Washington, DC, USA, 2012. [Google Scholar]
- Rodriguez-Sanchez, A.; Gonzalez-Martinez, A.; Martinez-Toledo, M.; Garcia-Ruiz, M.; Osorio, F.; Gonzalez-Lopez, J. The Effect of Influent Characteristics and Operational Conditions over the Performance and Microbial Community Structure of Partial Nitritation Reactors. Water 2014, 6, 1905–1924. [Google Scholar] [CrossRef]
- Gustavsson, D.J.I. Biological sludge liquor treatment at municipal wastewater treatment plants—A review. Vatten 2010, 66, 179–192. [Google Scholar]
- Goode, C.; Allen, D.G. Effect of Calcium on Moving-Bed Biofilm Reactor Biofilms. Water Environ. Res. 2011, 83, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.M.; Agrawal, S.; Schwartz, T.; Horn, H.; Lackner, S. Comparing different reactor configurations for Partial Nitritation/Anammox at low temperatures. Water Res. 2015, 81, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Gabarró, J.; González-Cárcamo, P.; Ruscalleda, M.; Ganigué, R.; Gich, F.; Balaguer, M.D.; Colprim, J. Anoxic phases are the main N2O contributor in partial nitritation reactors treating high nitrogen loads with alternate aeration. Bioresour. Technol. 2014, 163, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ni, B.-J.; Bond, P.L.; Ye, L.; Yuan, Z. Electron competition among nitrogen oxides reduction during methanol-utilizing denitrification in wastewater treatment. Water Res. 2013, 47, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.; Wang, L.; Lv, Y.; Zhang, X.; Miao, R.; Wang, X. Nitrous oxide emission in autotrophic partial nitritation system: Macro- and microanalyses. J. Biosci. Bioeng. 2015, 120, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Félez, C.; Mutlu, A.G.; Jensen, M.M.; Smets, B.F. Aeration strategies to mitigate nitrous oxide emissions from single-stage nitritation/anammox reactors. Environ. Sci. Technol. 2014, 48, 8679–8687. [Google Scholar] [CrossRef] [PubMed]
- Scherson, Y.D.; Wells, G.F.; Woo, S.-G.; Lee, J.; Park, J.; Cantwell, B.J.; Criddle, C.S. Nitrogen removal with energy recovery through N2O decomposition. Energy Environ. Sci. 2013, 6, 241–248. [Google Scholar] [CrossRef]

| Parameter | Unit | Nitritation | SBR | Nitritation | MBBR |
|---|---|---|---|---|---|
| (2014) | (2015) | ||||
| HRT 1 | [day] | ~1.3 | 2.3 ± 1.1 | ~1.3 | 2.2 ± 0.5 |
| Temperature | [°C] | 31.0 ± 0.3 | 31.2 ± 0.7 | 32.4 ± 0.4 | 32.0 ± 0.9 |
| pH | - | Invalid 2 | 7.84 ± 0.15 | Invalid 2 | 7.60 ± 0.26 |
| DO | [mg/L] | Invalid 2 | 0.10 ± 0.02 | Invalid 2 | 1.5 ± 1.3 |
| TSS | [g/L] | 0.2 ± 0.1 | 2.2 ± 0.7 | n/a | n/a |
| NO2-N/NH4-N 3 | - | 0.32 ± 0.15 | 0.32 ± 0.11 | 0.25 ± 0.14 | 0.23 ± 0.12 |
| NH4-N conversion rate | [g N/(m3∙day)] | 447.5 ± 154.9 | 448.9 ± 134.4 | 234.3 ± 82.7 | 445.8 ± 126.2 |
| 332.5 ± 109.6 4 | 388.5 ± 150.8 4 | ||||
| Ntotal conversion rate | [g N/(m3∙day)] | 95.9 ± 135.8 | 598.2 ± 200.8 | 12.8 ± 43.6 | 501.2 ± 100.0 |
| Ntotal effluent | [mg N/L] | 1321 ± 118 | 142 ± 33 | 1327 ± 61 | 234 ± 66 |
| Degradation 5 | [%] | 31.6 ± 6.5 | 90.1 ± 2.6 | 23.4 ± 9.2 | 81.9 ± 5.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leix, C.; Hartl, R.; Zeh, C.; Beer, F.; Drewes, J.E.; Koch, K. Performance and N2O Formation of the Deammonification Process by Suspended Sludge and Biofilm Systems—A Pilot-Scale Study. Water 2016, 8, 578. https://doi.org/10.3390/w8120578
Leix C, Hartl R, Zeh C, Beer F, Drewes JE, Koch K. Performance and N2O Formation of the Deammonification Process by Suspended Sludge and Biofilm Systems—A Pilot-Scale Study. Water. 2016; 8(12):578. https://doi.org/10.3390/w8120578
Chicago/Turabian StyleLeix, Carmen, Rebecca Hartl, Christian Zeh, Franz Beer, Jörg E. Drewes, and Konrad Koch. 2016. "Performance and N2O Formation of the Deammonification Process by Suspended Sludge and Biofilm Systems—A Pilot-Scale Study" Water 8, no. 12: 578. https://doi.org/10.3390/w8120578







