Indicator and Pathogen Removal by Low Impact Development Best Management Practices
Abstract
:1. Introduction
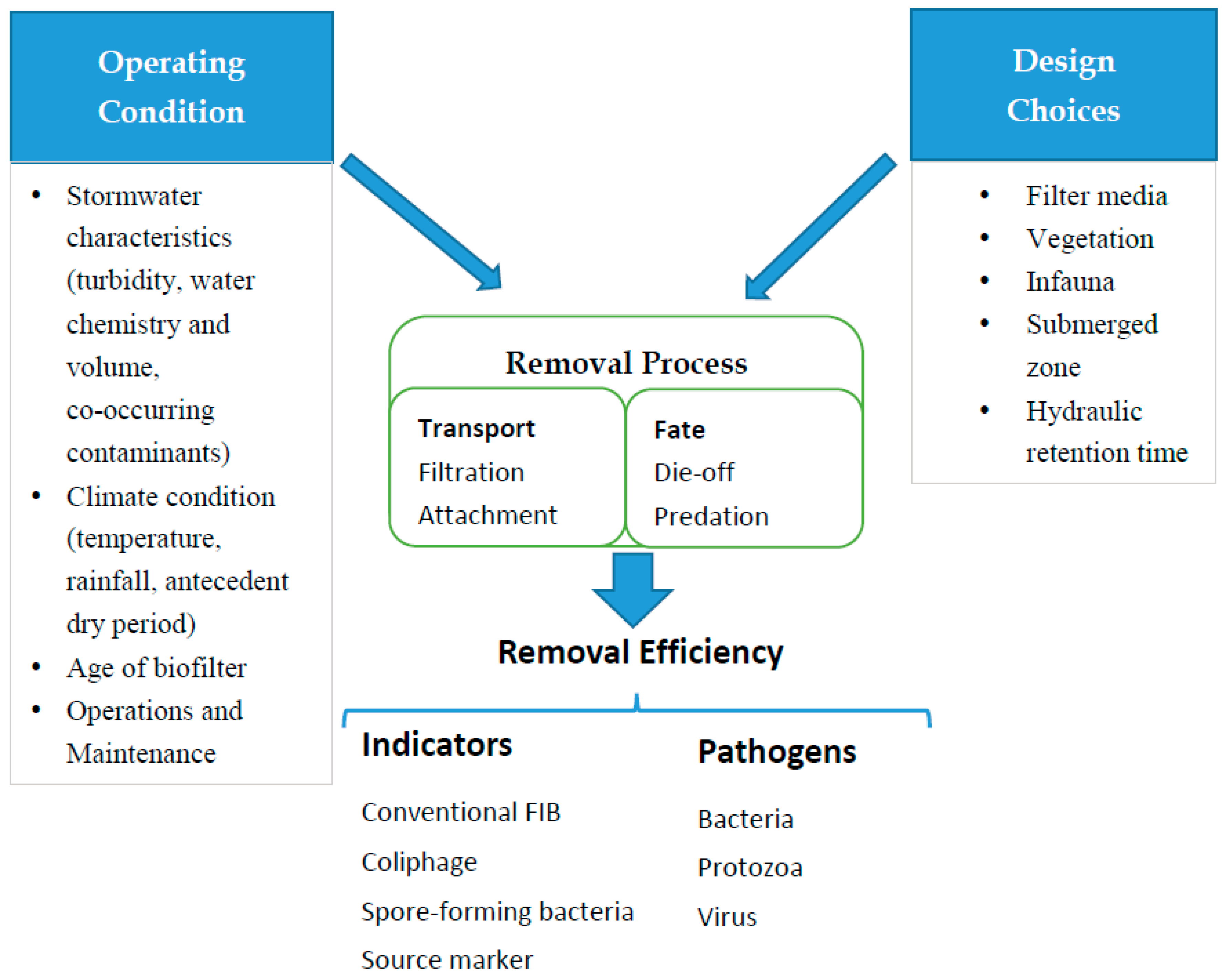
2. Conceptual Model for Removal of Microbial Contaminants
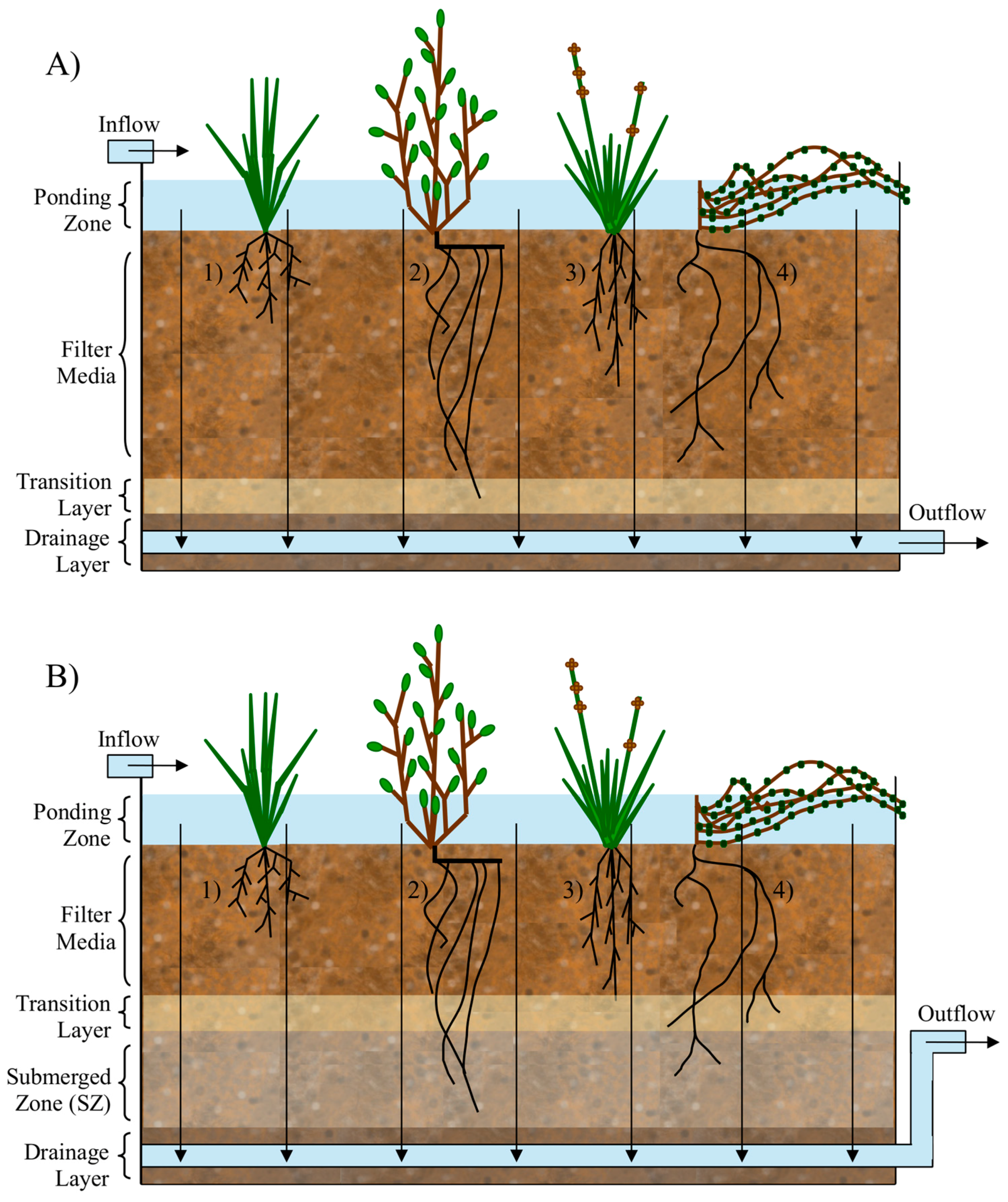
3. Biofilter Design Consideration for Removal of Microbial Contaminants
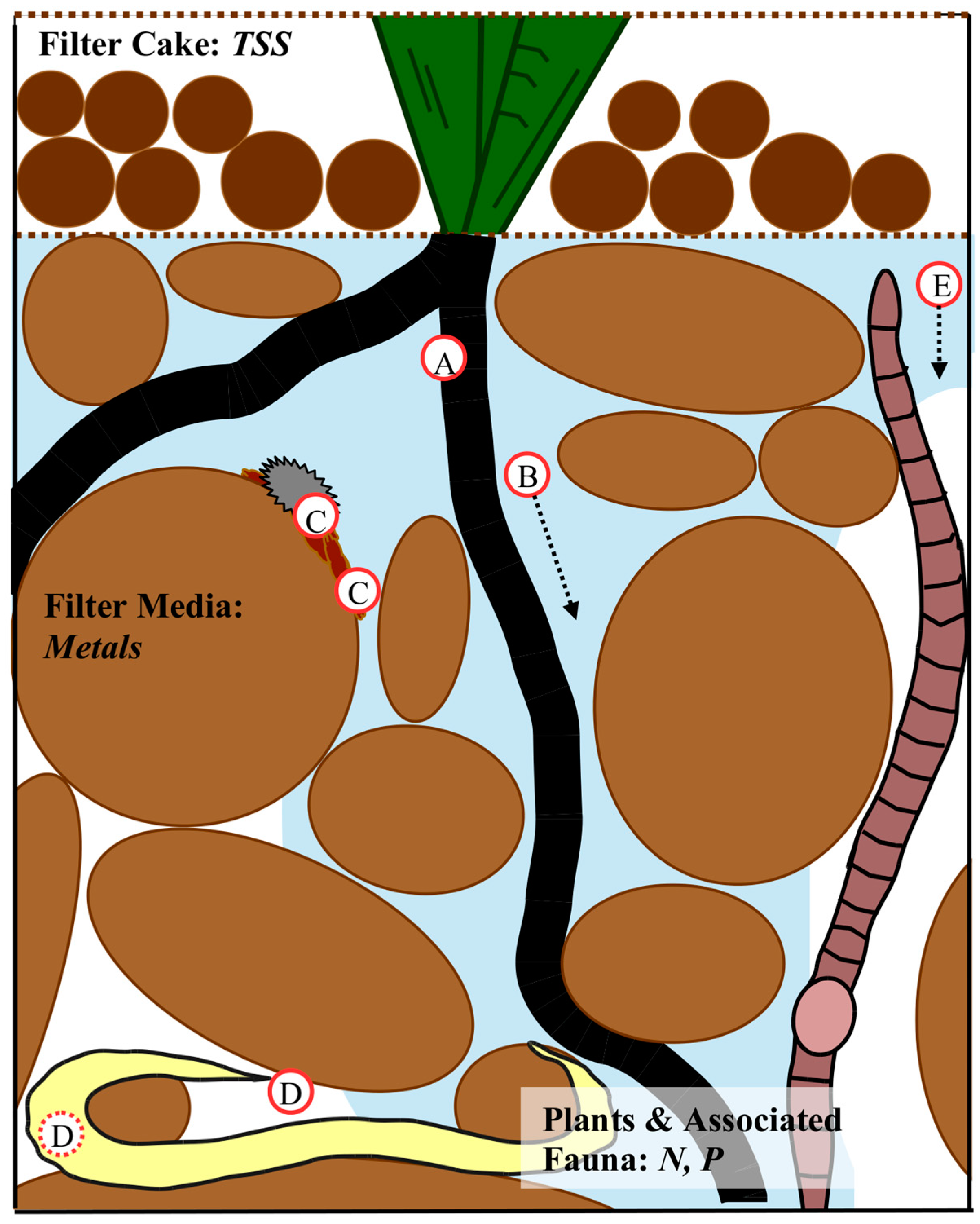
3.1. Filter Media
3.1.1. Amendments to Sand Biofilters
3.1.2. Surface Modification of the Filter Media and Amendments
3.1.3. Biofilm
3.2. Vegetation
3.3. Infauna
3.4. Submerged Zone
3.5. Hydraulic Retention Time
4. Summary and Future Research Needs
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Askarizadeh, A.; Rippy, M.A.; Fletcher, T.D.; Feldman, D.L.; Peng, J.; Bowler, P.; Mehring, A.S.; Winfrey, B.K.; Vrugt, J.A.; AghaKouchak, A.; et al. From Rain Tanks to Catchments: Use of Low-Impact Development to Address Hydrologic Symptoms of the Urban Stream Syndrome. Environ. Sci. Technol. 2015, 49, 11264–11280. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, L. The Economics of Using Ocean Observing Systems to Improve Beach Closure Policy. Coast. Manag. 2008, 36, 165–178. [Google Scholar] [CrossRef]
- U.S. EPA. National Summary of Impaired Water and TMDL Information. Available online: https://iaspub.epa.gov/waters10/attains_nation_cy.control?p_report_type=T (accessed on 18 October 2016).
- Gaffield, S.J.; Goo, R.L.; Richards, L.A.; Jackson, R.J. Public Health Effects of Inadequately Managed Stormwater Runoff. Am. J. Public Health 2003, 93, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.B.; Saphores, J.D.; Feldman, D.L.; Hamilton, A.J.; Fletcher, T.D.; Cook, P.L.; Stewardson, M.; Sanders, B.F.; Levin, L.A.; Ambrose, R.F.; et al. Taking the “waste” out of “wastewater” for human water security and ecosystem sustainability. Science 2012, 337, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Pitt, R.; Field, R.; Lalor, M.; Brown, M. Urban Stormwater Toxic Pollutants: Assessment, Sources, and Treatability. Water Environ. Res. 1995, 67, 260–275. [Google Scholar] [CrossRef]
- Chandrasena, G.; Deletic, A.; McCarthy, D. Evaluating Escherichia coli removal performance in stormwater biofilters: A preliminary modelling approach. Water Sci. Technol. 2013, 67, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.A.; Simunek, J.; Walker, S.L. Transport of Giardia and manure suspensions in saturated porous media. Water Resour. Res. 2006, 35, 749–757. [Google Scholar]
- Li, Y.L.; Deletic, A.; Alcazar, L.; Bratieres, K.; Fletcher, T.D.; McCarthy, D.T. Removal of Clostridium perfringens, Escherichia coli and F-RNA coliphages by stormwater biofilters. Ecol. Eng. 2012, 49, 137–145. [Google Scholar] [CrossRef]
- Bratières, K.; Schang, C.; Deletić, A.; McCarthy, D.T. Performance of enviss™ stormwater filters: Results of a laboratory trial. Water Sci. Technol. 2012, 66, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Grebel, J.E.; Mohanty, S.K.; Torkelson, A.A.; Boehm, A.B.; Higgins, C.P.; Maxwell, R.M.; Nelson, K.L.; Sedlak, D.L. Engineered infiltration systems for urban stormwater reclamation. Environ. Eng. Sci. 2013, 30, 437–454. [Google Scholar] [CrossRef]
- Rippy, M.A. Meeting the criteria: Linking biofilter design to fecal indicator bacteria removal. Wiley Interdiscip. Rev. Water 2015, 2, 577–592. [Google Scholar] [CrossRef]
- Jiang, S.C.; Lim, K.-Y.; Huang, X.; McCarthy, D.; Hamilton, A.J. Human and environmental health risks and benefits associated with use of urban stormwater. Wiley Interdiscip. Rev. Water 2015, 2, 683–699. [Google Scholar] [CrossRef]
- Elimelech, M.; O’Melia, C.R. Kinetics of deposition of colloidal particles in porous media. Environ. Sci. Technol. 1990, 24, 1528–1536. [Google Scholar] [CrossRef]
- Yao, K.-M.; Habibian, M.T.; O’Melia, C.R. Water and waste water filtration. Concepts and applications. Environ. Sci. Technol. 1971, 5, 1105–1112. [Google Scholar] [CrossRef]
- Chen, G.; Walker, S.L. Fecal indicator bacteria transport and deposition in saturated and unsaturated porous media. Environ. Sci. Technol. 2012, 46, 8782–8790. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Flury, M.; Deng, Y. Force measurements between particles and the air-water interface: Implications for particle mobilization in unsaturated porous media. Water Resour. Res. 2009, 45, W06420. [Google Scholar] [CrossRef]
- Surbeck, C.Q.; Jiang, S.C.; Grant, S.B. Ecological control of fecal indicator bacteria in an urban stream. Environ. Sci. Technol. 2010, 44, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Rippy, M.A.; Franks, P.J.; Feddersen, F.; Guza, R.T.; Moore, D.F. Factors controlling variability in nearshore fecal pollution: The effects of mortality. Mar. Pollut. Bull. 2013, 66, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, G.I.; Deletic, A.; McCarthy, D.T. Survival of Escherichia coli in stormwater biofilters. Environ. Sci. Poll. Res. Int. 2014, 21, 5391–5401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Seagren, E.A.; Davis, A.P. Column studies on the capture and destruction of E. coli from simulated urban stormwater runoff using conventional bioretention media and iron oxide-coated sand. In Proceedings of the World Environmental and Water Resources Congress, Honolulu, HI, USA, 12–16 May 2008; pp. 1–7.
- Zhang, L.; Seagren, E.A.; Davis, A.P.; Karns, J.S. The capture and destruction of Escherichia coli from simulated urban runoff using conventional bioretention media and iron oxide-coated sand. Water Environ. Res. 2010, 82, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, C.H.; Andersen, G.L.; Holden, P.A. Community analysis-based methods. In Microbial Source Tracking: Methods, Applications, and Case Studies; Hagedorn, C., Blanch, A., Harwood, V., Eds.; Springer: New York, NY, USA, 2011; pp. 251–282. [Google Scholar]
- Soller, J.A.; Bartrand, T.; Ashbolt, N.J.; Ravenscroft, J.; Wade, T.J. Estimating the primary etiologic agents in recreational freshwaters impacted by human sources of faecal contamination. Water Res. 2010, 44, 4736–4747. [Google Scholar] [CrossRef] [PubMed]
- Cabelli, V.J. Health Effects Criteria for Marine Recreational Waters; EPA-600/1-80-031; Health Effects Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency: Washington, DC, USA, 1983.
- Fujioka, R.S.; Solo-Gabriele, H.M.; Byappanahalli, M.N.; Kirs, M.U.S. Recreational Water Quality Criteria: A vision for the future. Int. J. Environ. Res. Public Health 2015, 12, 7752–7776. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.P.; Yamahara, K.M.; Boehm, A.B. Persistence of nucleic acid markers of health-relevant organisms in seawater microcosms: Implications for their use in assessing risk in recreational waters. Water Res. 2009, 43, 4929–4939. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Recreational Water Quality Criteria; Office of Water 820-F-12-058: Washington, DC, USA, 2012.
- Jofre, J.; Lucena, F.; Blanch, A.; Muniesa, M. Coliphages as Model Organisms in the Characterization and Management of Water Resources. Water 2016, 8, 199. [Google Scholar] [CrossRef]
- Layton, B.A.; Cao, Y.; Ebentier, D.L.; Hanley, K.; Balleste, E.; Brandao, J.; Byappanahalli, M.; Converse, R.; Farnleitner, A.H.; Gentry-Shields, J.; et al. Performance of human fecal anaerobe-associated PCR-based assays in a multi-laboratory method evaluation study. Water Res. 2013, 47, 6897–6908. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Weisberg, S.B.; Arnold, B.F.; Cao, Y.; Schiff, K.C.; Colford, J.M., Jr. Epidemiologic evaluation of multiple alternate microbial water quality monitoring indicators at three California beaches. Water Res. 2016, 94, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hagedorn, C.; Shanks, O.C.; Wang, D.; Ervin, J.; Griffith, J.F.; Layton, B.A.; McGee, C.D.; Riedel, T.E.; Weisberg, S.B. Towards establishing a human fecal contamination index in microbial source tracking. Int. J. Environ. Sci. Eng. Res. 2013, 4, 46–58. [Google Scholar]
- Gannon, J.; Manilal, V.; Alexander, M. Relationship between cell surface properties and transport of bacteria through soil. Appl. Environ. Microbiol. 1991, 57, 190–193. [Google Scholar] [PubMed]
- Camper, A.K.; Hayes, J.T.; Sturman, P.J.; Jones, W.L.; Cunningham, A.B. Effects of motility and adsorption rate coefficient on transport of bacteria through saturated porous media. Appl. Environ. Microbiol. 1993, 59, 3455–3462. [Google Scholar] [PubMed]
- Templeton, M.R.; Andrews, R.C.; Hofmann, R. Particle-Associated Viruses in Water: Impacts on Disinfection Processes. Crit. Rev. Environ. Sci. Technol. 2008, 38, 137–164. [Google Scholar] [CrossRef]
- Schillinger, J.E.; Gannon, J.J. Bacterial Adsorption and Suspended Particles in Urban Stormwater. J. Water Pollut. Control Fed. 1985, 57, 384–389. [Google Scholar]
- Walters, E.; Graml, M.; Behle, C.; Müller, E.; Horn, H. Influence of Particle Association and Suspended Solids on UV Inactivation of Fecal Indicator Bacteria in an Urban River. Water Air Soil Pollut. 2013, 225, 1–9. [Google Scholar] [CrossRef]
- Bauman, W.J.; Nocker, A.; Jones, W.L.; Camper, A.K. Retention of a model pathogen in a porous media biofilm. Biofouling 2009, 25, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.A.; Fierer, N. Microbial processes in the vadose zone. Vadose Zone J. 2005, 4, 1–21. [Google Scholar] [CrossRef]
- International Stormwater BMP Database. International Stormwater Best Management Practices (BMP) Database Pollutant Category Summary: TSS, Bacteria, Nutrients, and Metals; Geosyntec Consultants: Portland, OR, USA; Wright Water Engineer: Denver, CO, USA, July 2012. [Google Scholar]
- Rusciano, G.; Obropta, C. Bioretention column study: Fecal coliform and total suspended solids reductions. Trans. ASABE 2007, 50, 1261–1269. [Google Scholar] [CrossRef]
- Strecker, E.W.; Quigley, M.M.; Urbonas, B.R.; Jones, J.E.; Clary, J.K. Determining urban storm water BMP effectiveness. J. Water Resour. Plan. Manag. 2001, 127, 144–149. [Google Scholar] [CrossRef]
- Chandrasena, G.; Pham, T.; Payne, E.; Deletic, A.; McCarthy, D. E. coli removal in laboratory scale stormwater biofilters: Influence of vegetation and submerged zone. J. Hydrol. 2014, 519, 814–822. [Google Scholar] [CrossRef]
- Kandasamy, J.; Beecham, S.; Dunphy, A. Stormwater sand filters in water sensitive urban design. Water Manag. 2008, 161, 55–64. [Google Scholar] [CrossRef]
- Martin, M.J.; Logan, B.E.; Johnson, W.P.; Jewett, D.G.; Arnold, R.G. Scaling bacterial filtration rates in different sized porous media. J. Environ. Eng. 1996, 122, 407–415. [Google Scholar] [CrossRef]
- Rollinger, Y.; Dott, W. Survival of selected bacterial species in sterilized activated carbon filters and biological activated carbon filters. Appl. Environ. Microbiol. 1987, 53, 777–781. [Google Scholar] [PubMed]
- Bichai, F.; Barbeau, B.; Dullemont, Y.; Hijnen, W. Role of predation by zooplankton in transport and fate of protozoan (oo) cysts in granular activated carbon filtration. Water Res. 2010, 44, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Deletic, A.; McCarthy, D.T. Removal of E. coli from urban stormwater using antimicrobial-modified filter media. J. Hazard. Mater. 2014, 271, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Guest, R.; Schang, C.; Deletic, A.; McCarthy, D. Zinc-sulphate-heptahydrate coated activated carbon for microbe removal from stormwater. Water Sci. Technol. 2012, 66, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, K.; Brownstein, J.; Hepner, B. Method and Apparatus for Combined Filtration and Anti-Microbial Treatment of Storm Water Resident in Storm Water Systems. Available online: https://www.google.com/patents/US20060260996 (accessed on 18 October 2016).
- Kerr, G.T. Chemistry of crystalline aluminosilicates. II. The synthesis and properties of zeolite ZK-4. Inorg. Chem. 1966, 5, 1537–1539. [Google Scholar] [CrossRef]
- Langmi, H.; Book, D.; Walton, A.; Johnson, S.; Al-Mamouri, M.; Speight, J.; Edwards, P.; Harris, I.; Anderson, P. Hydrogen storage in ion-exchanged zeolites. J. Alloys Compd. 2005, 404, 637–642. [Google Scholar] [CrossRef]
- Du, X.; Wu, E. Porosity of microporous zeolites A, X and ZSM-5 studied by small angle X-ray scattering and nitrogen adsorption. J. Phys. Chem. Solids 2007, 68, 1692–1699. [Google Scholar] [CrossRef]
- Abbaszadegan, M.; Monteiro, P.; Ouwens, R.N.; Ryu, H.; Alum, A. Removal and inactivation of Cryptosporidium and microbial indicators by a quaternary ammonium chloride (QAC)-treated zeolite in pilot filters. J. Environ. Sci. Health A 2006, 41, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Makuch, D.; Pillai, S.D.; Guan, H.; Bowman, R.; Couroux, E.; Hielscher, F.; Totten, J.; Espinosa, I.Y.; Kretzschmar, T. Surfactant-modified zeolite can protect drinking water wells from viruses and bacteria. EOS Trans. Am. Geophys. Union 2002, 83, 193–201. [Google Scholar] [CrossRef]
- Li, Y.; McCarthy, D.; Deletic, A. WSUD 2012: Water sensitve urban design; Building the water sensitve community. In Removal and Inactivation of E. coli from Water Using Copper Modified Natural Zeolite, Proceedings of the 7th International Conference on Water Sensitive Urban Design, Melbourne, Australia, 21–23 February 2012; Engineers Australia: Barton, Australia, 2012; p. 517. [Google Scholar]
- Li, Y.L.; McCarthy, D.T.; Deletic, A. Stable copper-zeolite filter media for bacteria removal in stormwater. J. Hazard. Mater. 2014, 273, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon-on-Thames, UK, 2015. [Google Scholar]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Boehm, A.B. Escherichia coli removal in biochar-augmented biofilter: Effect of infiltration rate, initial bacterial concentration, biochar particle size, and presence of compost. Environ. Sci. Technol. 2014, 48, 11535–11542. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Cantrell, K.B.; Nelson, K.L.; Boehm, A.B. Efficacy of biochar to remove Escherichia coli from stormwater under steady and intermittent flow. Water Res. 2014, 61, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Boehm, A.B. Effect of weathering on mobilization of biochar particles and bacterial removal in a stormwater biofilter. Water Res. 2015, 85, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Torkelson, A.A.; Dodd, H.; Nelson, K.L.; Boehm, A.B. Engineering solutions to improve the removal of fecal indicator bacteria by bioinfiltration systems during intermittent flow of stormwater. Environ. Sci. Technol. 2013, 47, 10791–10798. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J. Environ. Eng. 2014, 140, 04014043. [Google Scholar] [CrossRef]
- Schifman, L.A.; Kasaraneni, V.K.; Sullivan, R.K.; Oyanedel-Craver, V.; Boving, T.B. New Antimicrobially Amended Media for Improved Nonpoint Source Bacterial Pollution Treatment. Environ. Sci. Technol. 2015, 49, 14383–14391. [Google Scholar] [CrossRef] [PubMed]
- Schifman, L.A.; Kasaraneni, V.K.; Sullivan, R.K.; Oyanedel-Craver, V.; Boving, T.B. Bacteria Removal from Stormwater Runoff Using Tree Filters: A Comparison of a Conventional and an Innovative System. Water 2016, 8, 76. [Google Scholar] [CrossRef]
- Kasaraneni, V.K.; Schifman, L.A.; Boving, T.B.; Oyanedel-Craver, V. Enhancement of Surface Runoff Quality Using Modified Sorbents. ACS Sustain. Chem. Eng. 2014, 2, 1609–1615. [Google Scholar] [CrossRef]
- Torkelson, A.A.; da Silva, A.K.; Love, D.C.; Kim, J.Y.; Alper, J.P.; Coox, B.; Dahm, J.; Kozodoy, P.; Maboudian, R.; Nelson, K.L. Investigation of quaternary ammonium silane-coated sand filter for the removal of bacteria and viruses from drinking water. J. Appl. Microbiol. 2012, 113, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.M.; Onnis-Hayden, A.; Lewis, K. Antimicrobial Polycationic Sand Filter for Water Disinfection. Available online: https://www.google.com/patents/US20140202964#backward-citations (accessed on 18 October 2016).
- Ahammed, M.M.; Davra, K. Performance evaluation of biosand filter modified with iron oxide-coated sand for household treatment of drinking water. Desalination 2011, 276, 287–293. [Google Scholar] [CrossRef]
- Ahammed, M.M.; Meera, V. Iron hydroxide-coated sand filter for household drinking water from roof-harvested rainwater. J. Water Supply Res. Technol. AQUA 2006, 55, 493–498. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; De Beer, D.; Lewandowski, Z. Liquid Flow in Biofilm Systems. Appl. Environ. Microbiol. 1994, 60, 2711–2716. [Google Scholar] [PubMed]
- Clement, T.; Hooker, B.; Skeen, R. Macroscopic models for predicting changes in saturated porous media properties caused by microbial growth. Ground Water 1996, 34, 934–942. [Google Scholar] [CrossRef]
- Cunningham, A.; Warwood, B.; Sturman, P.; Horrigan, K.; James, G.; Costerton, J.W.; Hiebert, R. Biofilm processes in porous media-practical applications. In The Microbiology of the Terrestrial Deep Subsurface; Amy, P.S., Halderman, D.L., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 325–344. [Google Scholar]
- Taylor, S.W.; Jaffé, P.R. Biofilm growth and the related changes in the physical properties of a porous medium: 1. Experimental investigation. Water Resour. Res. 1990, 26, 2153–2159. [Google Scholar] [CrossRef]
- Dai, X.; Hozalski, R.M. Effect of NOM and biofilm on the removal of Cryptosporidium parvum oocysts in rapid filters. Water Res. 2002, 36, 3523–3532. [Google Scholar] [CrossRef]
- Wang, A.; Lin, B.; Sleep, B.E.; Liss, S.N. The impact of biofilm growth on transport of Escherichia coli O157: H7 in sand. Ground Water 2011, 49, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Papineau, I.; Tufenkji, N.; Servais, P.; Barbeau, B. Impact of media aging on the removal of Cryptosporidium in granular media filters. J. Environ. Eng. 2012, 139, 603–611. [Google Scholar] [CrossRef]
- Bozorg, A.; Gates, I.D.; Sen, A. Impact of biofilm on bacterial transport and deposition in porous media. J. Contam. Hydrol. 2015, 183, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J. Role of Pseudomonas aeruginosa biofilm in the initial adhesion, growth and detachment of Escherichia coli in porous media. Environ. Sci. Technol. 2007, 42, 443–449. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Sung, C.Y.; Li, M.-H.; Chu, K.-H. Bioretention for stormwater quality improvement in Texas: Removal effectiveness of Escherichia coli. Sep. Purif. Technol. 2012, 84, 120–124. [Google Scholar] [CrossRef]
- Barrett, M.E.; Limouzin, M.; Lawler, D.F. Effects of media and plant selection on biofiltration performance. J. Environ. Eng. 2012, 139, 462–470. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Read, J.; Fletcher, T.D.; Wevill, T.; Deletic, A. Plant traits that enhance pollutant removal from stormwater in biofiltration systems. Int. J. Phytoremediat. 2009, 12, 34–53. [Google Scholar] [CrossRef]
- Le Coustumer, S.; Fletcher, T.D.; Deletic, A.; Barraud, S. Hydraulic performance of biofilters: First lessons from both laboratory and field studies. Water Sci. Technol. 2007, 56, 93–100. [Google Scholar] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Mehring, A.S.; Levin, L.A. Potential roles of soil fauna in improving the efficiency of rain gardens used as natural stormwater treatment systems. J. Appl. Ecol. 2015, 52, 1445–1454. [Google Scholar] [CrossRef]
- Mehring, A.S.; Hatt, B.E.; Kraikittikun, D.; Orelo, B.D.; Rippy, M.A.; Grant, S.B.; Gonzalez, J.P.; Jiang, S.C.; Ambrose, R.F.; Levin, L.A. Soil invertebrates in Australian rain gardens and their potential roles in storage and processing of nitrogen. Ecol. Eng. 2016, 97, 138–143. [Google Scholar] [CrossRef]
- Ayers, E.M. Pedogenesis in Rain Gardens: The Role of Earthworms and Other Organisms in Long-Term Soil Development; ProQuest: Ann Arbor, MI, USA, 2009. [Google Scholar]
- Zhao, L.; Wang, Y.; Yang, J.; Xing, M.; Li, X.; Yi, D.; Deng, D. Earthworm–microorganism interactions: A strategy to stabilize domestic wastewater sludge. Water Res. 2010, 44, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.; Parry, J.D.; Hill, P.J.; Laybourn-Parry, J. The filter-feeding ciliates Colpidium striatum and Tetrahymena pyriformis display selective feeding behaviours in the presence of mixed, equally-sized, bacterial prey. Protist 2010, 161, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Rønn, R.; Vestergård, M.; Ekelund, F. Interactions between bacteria, protozoa and nematodes in soil. Acta Protozool. 2015, 51, 223–235. [Google Scholar]
- Avery, L.; Shtonda, B.B. Food transport in the C. elegans pharynx. J. Exp. Biol. 2003, 206, 2441–2457. [Google Scholar] [CrossRef] [PubMed]
- Ronn, R.; Thomsen, I.K.; Jensen, B. Naked amoebae, flagellates, and nematodes in soils of different texture. Eur. J. Soil Biol. 1995, 31, 135–141. [Google Scholar]
- Adl, S. Motility and migration rate of protozoa in soil columns. Soil Biol. Biochem. 2007, 39, 700–703. [Google Scholar] [CrossRef]
- Zhang, L.; Seagren, E.A.; Davis, A.P.; Karns, J.S. Long-term sustainability of Escherichia coli removal in conventional bioretention media. J. Environ. Eng. 2011, 137, 669–677. [Google Scholar] [CrossRef]
- Monash University. Facility for Advancing Water Biofiltration. In Adoption Guidelines for Stormwater Bioinfiltration Systems: Facility for Advancing Water Biofiltration; Monash University: Clayton, Australia, 2009. [Google Scholar]
- Dietz, M.E.; Clausen, J.C. Saturation to improve pollutant retention in a rain garden. Environ. Sci. Technol. 2006, 40, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Payne, E.G.; Fletcher, T.D.; Russell, D.G.; Grace, M.R.; Cavagnaro, T.R.; Evrard, V.; Deletic, A.; Hatt, B.E.; Cook, P.L. Temporary storage or permanent removal? The division of nitrogen between biotic assimilation and denitrification in stormwater biofiltration systems. PLoS ONE 2014, 9, e90890. [Google Scholar] [CrossRef] [PubMed]
- Levenspiel, O. Chemical Reaction Engineering; Wiley: Hoboken, NJ, USA, 1972. [Google Scholar]
- Monash University. Guidelines for Filter Media in Biofiltration Systems; Version 3.01; Monash University: Clayton, Australia, 2009. [Google Scholar]
- Wehner, J.F.; Wilhelm, R.H. Boundary conditions of flow reactor. Chem. Eng. Sci. 1956, 6, 89–93. [Google Scholar] [CrossRef]
- Schulze-Makuch, D. Longitudinal dispersivity data and implications for scaling behavior. Ground Water 2005, 43, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.A.; Grant, S.B.; Rippy, M.A.; Mehring, A.; Winfrey, B.; Vrugt, J.A.; Hatt, B.E.; Azizan, M.; Gomez, E.; Patel, C. Residence time matters: The impact of plants on the removal of fecal indicator bacteria in biofilters. In press.
- Briggs, M.A.; Day-Lewis, F.D.; Zarnetske, J.P.; Harvey, J.W. A physical explanation for the development of redox micrzones in hyporheic flow. Geophys. Res. Lett. 2015, 42, 4402–4410. [Google Scholar] [CrossRef]
- Sidhu, J.; Toze, S.; Hodgers, L.; Shackelton, M.; Barry, K.; Page, D.; Dillon, P. Pathogen inactivation during passage of stormwater through a constructed reedbed and aquifer transfer, storage and recovery. Water Sci. Technol. 2010, 62, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, G.I.; Filip, S.; Zhang, K.; Osborne, C.A.; Deletic, A.; McCarthy, D.T. Pathogen and Indicator Microorganism Removal in Feield Scale Stormwater Biofilters. In WSUD 2012: Water Sensitive Urban Design; Building the Water Sensiitve Community, Proceedings of the 7th International Conference on Water Sensitive Urban Design, Melbourne, Australia, 21–23 February 2012; Wang, T., McCarthy, D., Eds.; Centre for Water Sensitive Cities: Melbourne, Australia, 2012; pp. 1–9. [Google Scholar]
- Chandrasena, G.I.; Deletic, A.; McCarthy, D.T. Biofiltration for stormwater harvesting: Comparison of Campylobacter spp. and Escherichia coli removal under normal and challenging operational conditions. J. Hydrol. 2016, 537, 248–259. [Google Scholar] [CrossRef]
- Zimmerman, B.D.; Korajkic, A.; Brinkman, N.E.; Grimm, A.C.; Ashbolt, N.J.; Garland, J.L. A spike cocktail approach to improve microbial performance monitoring for water reuse. Water Environ. Res. 2016, 88, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Griffith, J.F.; Weisberg, S.B. The next-generation PCR-based quantification method for ambient waters: Digital PCR. Methods Mol. Biol. 2016, 1452, 113–130. [Google Scholar] [PubMed]
- Boehm, A.B.; Van De Werfhorst, L.C.; Griffith, J.F.; Holden, P.A.; Jay, J.A.; Shanks, O.C.; Wang, D.; Weisberg, S.B. Performance of forty-one microbial source tracking methods: A twenty-seven lab evaluation study. Water Res. 2013, 47, 6812–6828. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Weilenmann, H.U.; Ihssen, J.; Bassin, C.; Egli, T. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl. Environ. Microbiol. 2006, 72, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Randelovic, A.; Deletic, A.; Page, D.; McCarthy, D.T. Stormwater biofilters: A new validation modelling tool. Ecol. Eng. 2016, 87, 53–61. [Google Scholar] [CrossRef]
- Harper, H.H. Stormwater Chemistry and Water Quality; Environmental Research & Design, Inc.: Orlando, FL, USA, 1998. [Google Scholar]
- Zhang, K.; Valognes, V.; Page, D.; Deletic, A.; McCarthy, D. Validation of stormwater biofilters using in-situ columns. Sci. Total Environ. 2016, 544, 48–55. [Google Scholar] [CrossRef] [PubMed]

| Dimension (mm) | Particle Size | Microbe Type |
|---|---|---|
| 100 | cobble | macrofauna |
| 10 | pebble | meso- to macrofauna |
| 1 | coarse sand | mesofauna |
| 0.1 | fine sand | protozoa |
| 0.01 | fine silt | protozoa/bacteria |
| 0.001 | clay/colloid | bacteria |
| 0.0001 | colloid/macromolecule | virus |
| Sand Type | % Sand | Amendment, Modification a | Particle Size (mm) | Log10 Removal | Column Size (cm) | Reference | |
|---|---|---|---|---|---|---|---|
| E. coli | Enterococci | ||||||
| Fine Sand | 100% | - | d10 = 0.33 | 0.69 | - | 2.5 × 23 | [22] |
| d50 = 0.46 | |||||||
| Ottawa Sand | 100% | - | 0.6-0.8 | 0.52 | 0.36 | 2.5 × 15 | [65] |
| Coarse Sand | 100% | Iron oxide | d10 = 0.61 | 0.86 | - | 2.5 × 23 | [21,22] |
| d50 = 0.85 | |||||||
| Fine Sand | 100% | Iron oxide | d10 = 0.33 | 1.92 | - | ||
| d50 = 0.46 | |||||||
| Ottawa Sand | 100% | Iron oxide | 0.6–0.8 | 2 | 1.52 | 2.5 × 15 | [66] |
| Ottawa Sand | 50% | Iron oxide | 0.6–0.8 | 2 | 1.52 | ||
| Fine Sand | 53% | GAC, - | 0.3–0.6 | 0.58 | - | 2.5 × 15 | [48] |
| Fine Sand | 20% | GAC, Cu | 0.3–0.6 | 1.13 | - | 2.5 × 15 | |
| Fine Sand | 53% | GAC, TiO2 | 0.3–0.6 | 0.42 | - | 2.5 × 15 | |
| Fine Sand | 53% | GAC, Zn(OH)2 | 0.3–0.6 | 1.93 | - | 2.5 × 15 | |
| Fine Sand | 53% | GAC, Cu(OH)2 | 0.3–0.6 | 0.4 | - | 2.5 × 15 | |
| Fine Sand | 53% | GAC, Si-QAC | 0.3–0.6 | 0.47 | - | 2.5 × 15 | |
| Sand | 50% | GAC, ZnSO4.7H2O | 0.3–0.6 | 1.70 | - | 2.8 × 10 | [49] |
| Fine Sand | 53% | Zeolite, - | 0.3–0.6 | 0.40 | - | 2.5 × 15 | [57] |
| Fine Sand | 20% | Zeolite, - | 0.1–0.3 | 0.20 | - | 1.8 × 20 | |
| Fine Sand | 20% | Zeolite, - | 0.1–0.3 | 0.64 | - | 1.8 × 20 | |
| Fine Sand | 53% | Zeolite, Cu | 0.3–0.6 | 3.44 | - | 2.5 × 15 | |
| Fine Sand | 20% | Zeolite, Cu | 0.3–0.6 | 2.13 | - | 1.8 × 20 | |
| Fine Sand | 53% | Zeolite, Zn | 0.3–0.6 | 0.92 | - | 2.5 × 15 | |
| Fine Sand | 53% | Zeolite, TiO2 | 0.3–0.6 | 0.42 | - | 2.5 × 15 | |
| Fine Sand | 53% | Zeolite, Zn (OH)2 | 0.3–0.6 | 0.41 | - | 2.5 × 15 | |
| Fine Sand | 53% | Zeolite, TPA | 0.3–0.6 | 0.81 | - | 2.5 × 15 | |
| Fine Sand | 20% | Zeolite, CuO | 0.3–0.6 | 0.20–2.04 | - | 1.8 × 20 | |
| Biochar Source and Properties | Log10 Removal | Reference | |||||
|---|---|---|---|---|---|---|---|
| Feedstock | Production Process, Temperature | % Sand | Particle Size (mm) | Surface Area (m2/gm) | Column Size (cm × cm) | ||
| Waste Wood Pellets | Gasification, 520 °C | 0% | 0.45–4.75 | - | 7 × 23 | 0.14 | [67] |
| Wood Chips | 350 °C | 78% | 0.6–0.8 | 65.9 ± 1.2 | 2.5 × 15 | 1.18 ± 0.22 | [64] |
| 700 °C | 64.9 ± 6.5 | 0.83 ± 0.05 | |||||
| Sonoma | 326.2 ± 5.9 | 1.16 ± 0.20 | |||||
| Softwood + Bark | Pyrolysis, 815–1315 °C | 70% | <1 | - | 1.3 ± 0.01 | [63] | |
| 70% | 0.125–1 | - | 0.42 ± 0.02 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Cao, Y.; Rippy, M.A.; Afrooz, A.R.M.N.; Grant, S.B. Indicator and Pathogen Removal by Low Impact Development Best Management Practices. Water 2016, 8, 600. https://doi.org/10.3390/w8120600
Peng J, Cao Y, Rippy MA, Afrooz ARMN, Grant SB. Indicator and Pathogen Removal by Low Impact Development Best Management Practices. Water. 2016; 8(12):600. https://doi.org/10.3390/w8120600
Chicago/Turabian StylePeng, Jian, Yiping Cao, Megan A. Rippy, A. R. M. Nabiul Afrooz, and Stanley B. Grant. 2016. "Indicator and Pathogen Removal by Low Impact Development Best Management Practices" Water 8, no. 12: 600. https://doi.org/10.3390/w8120600





