The Effect of Membrane Material and Surface Pore Size on the Fouling Properties of Submerged Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hollow Fiber Membrane Preparation
2.3. Air Bubble Contact Angle Measurement
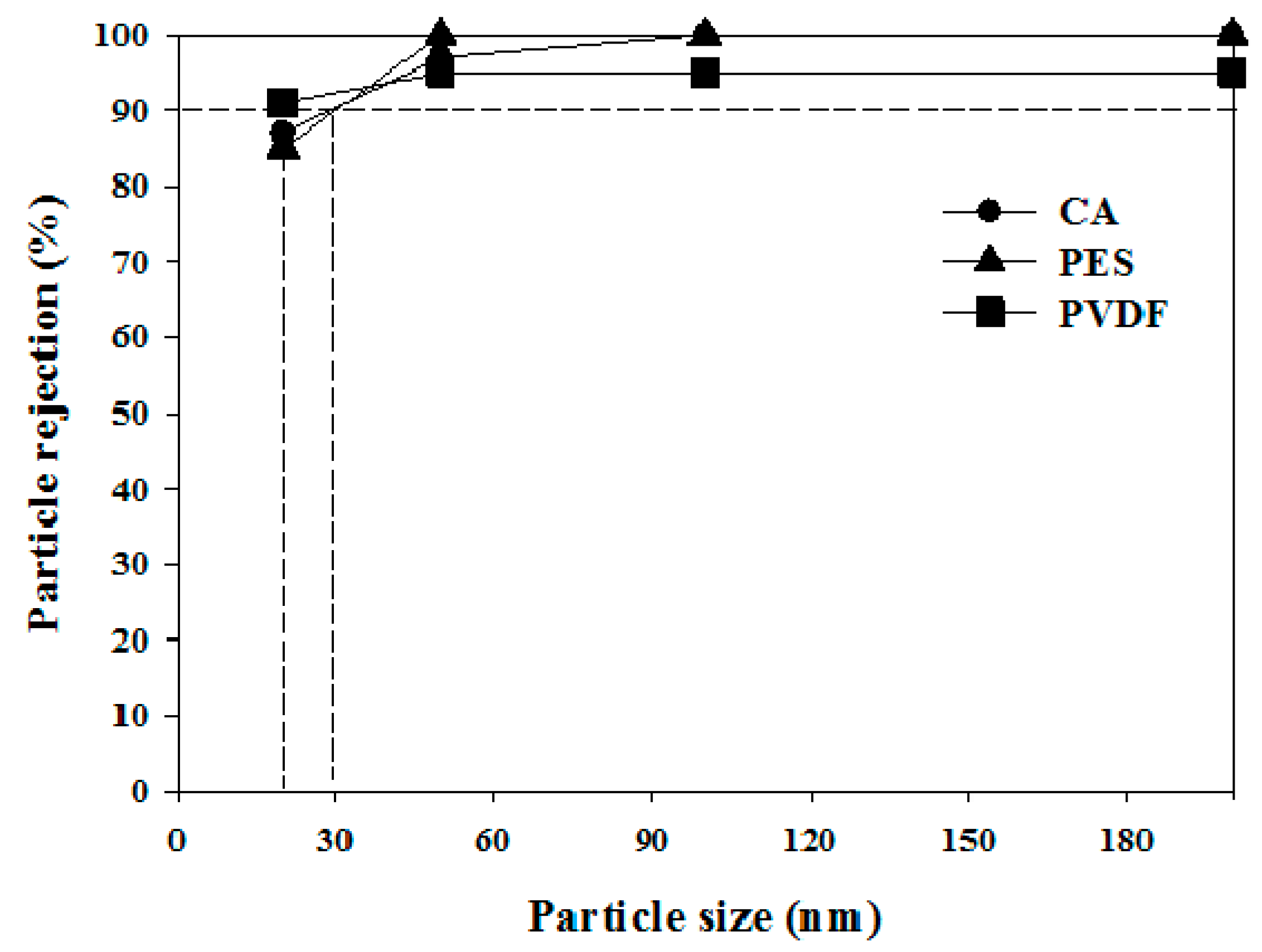
2.4. Polystyrene Particle Rejection Test
2.5. Batch Filtration Test Using Single Membrane Module
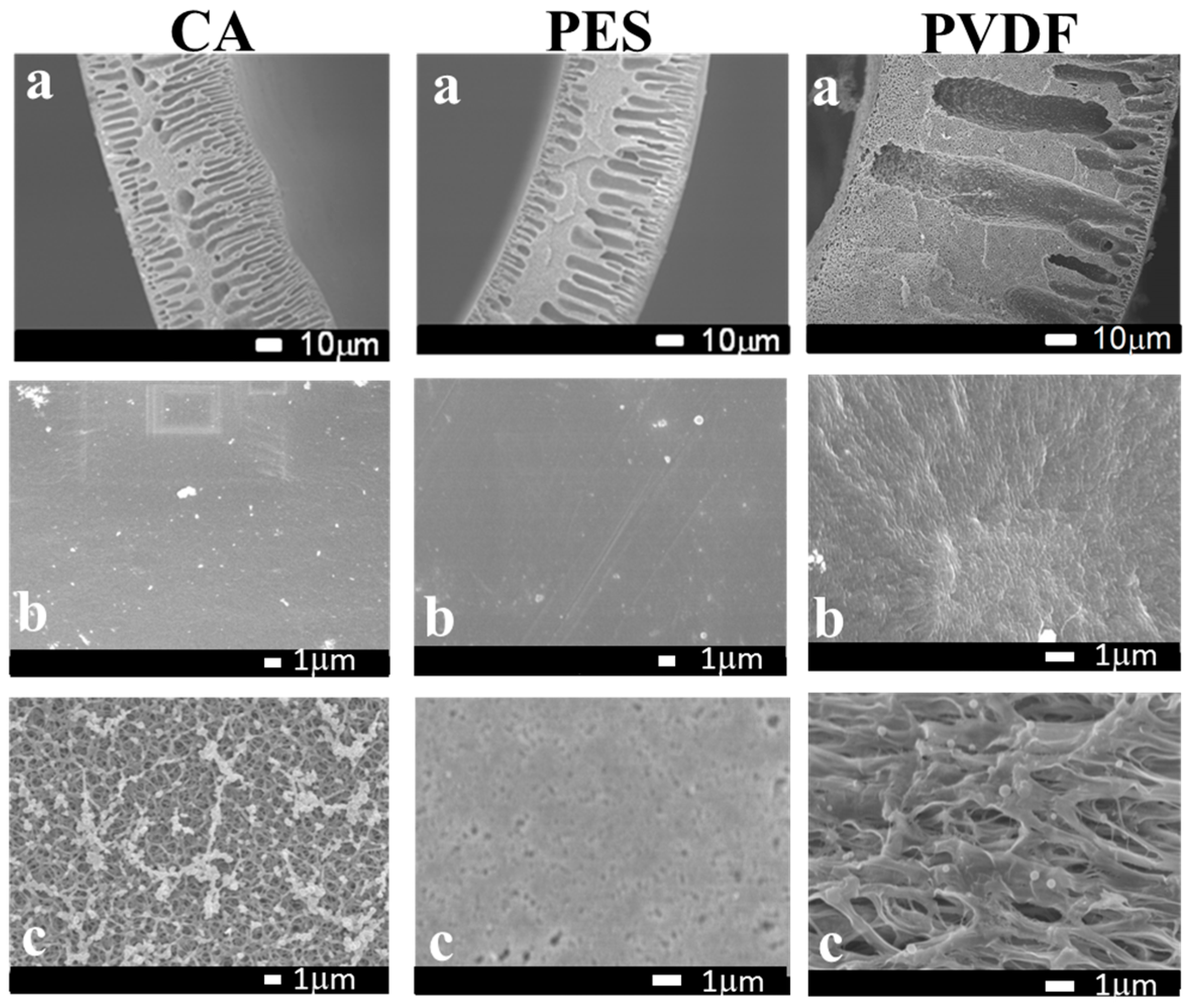
2.6. Membrane Morphology Observations
2.7. Microbial Floc Attachment Test
3. Results and Discussion
3.1. Properties of Hollow Fiber Membranes
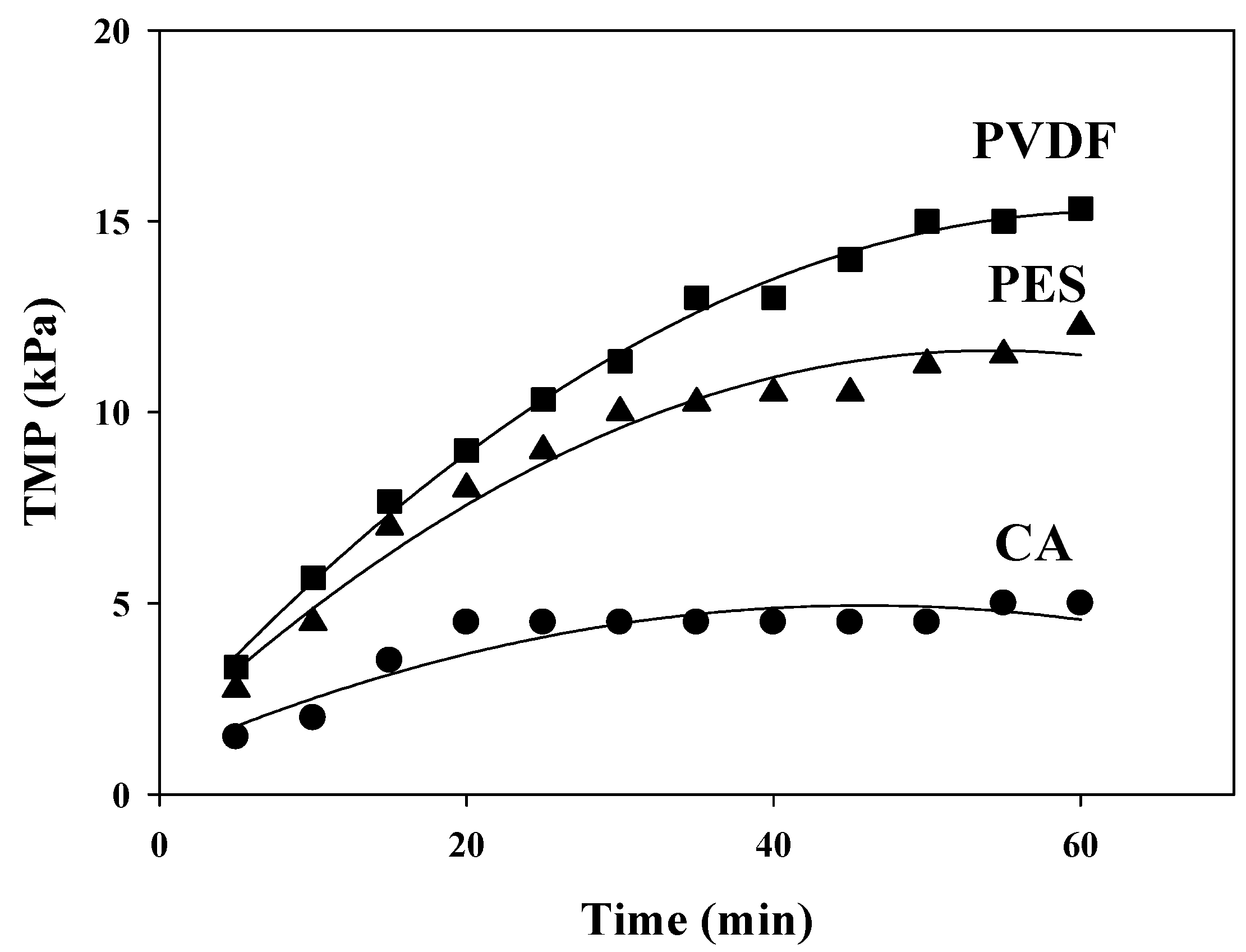
3.2. Fouling Behavior in Batch Filtration Tests
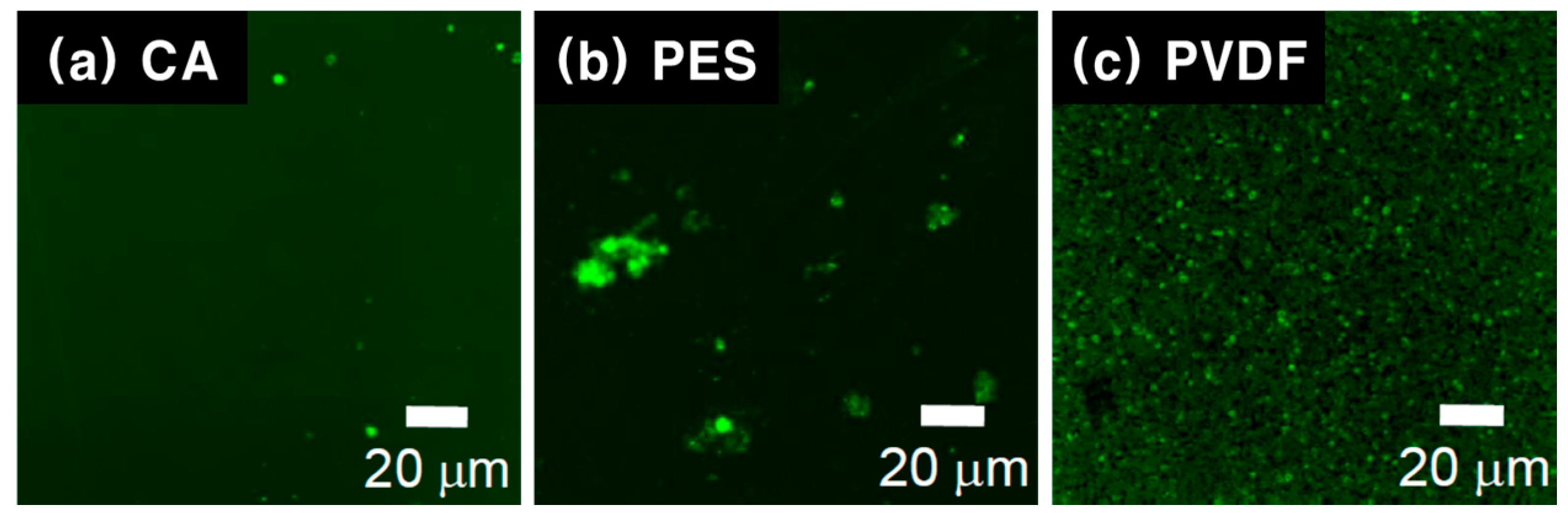
3.3. Visualization of the Biofouling on the Film Surfaces
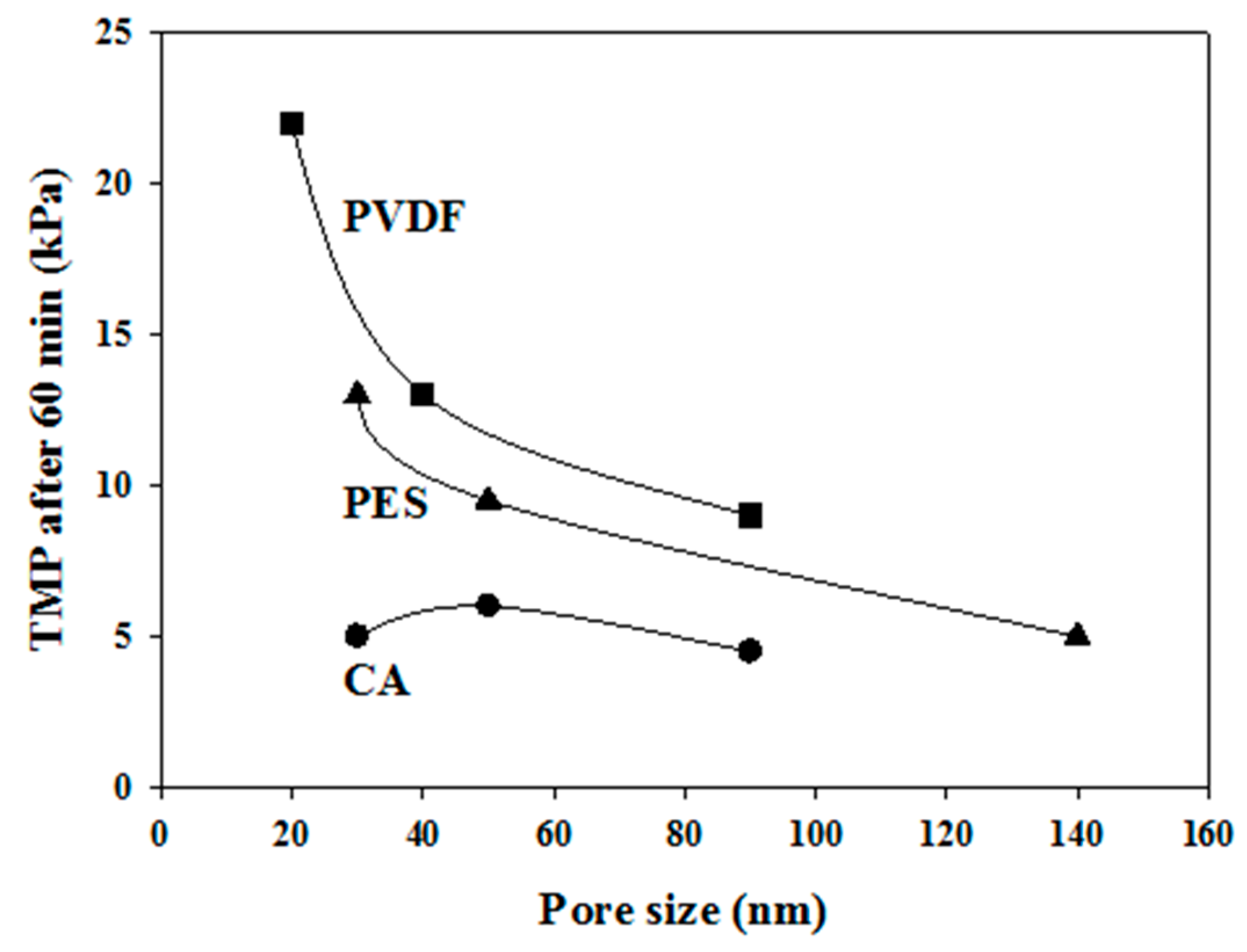
3.4. Effects of Membrane Pore Size on Membrane Fouling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| CA | cellulose acetate |
| CAGR | compound annual growth rate |
| CLSM | confocal laser scanning microscopy |
| DMAc | dimethylacetamide |
| DO | dissolved oxygen |
| EPS | extracellular polymeric substance |
| FE-SEM | field emission scanning electron microscope |
| F/M | food-to-microorganism ratio |
| HRT | hydraulic retention time |
| LiCl | lithium chloride |
| MBR | membrane bioreactor |
| MF | microfiltration |
| MLSS | mixed liquor suspended solids |
| NIPS | non-solvent induced phase separation |
| OsO4 | osmium tetroxide |
| PBS | phosphate buffer solution |
| PES | polyether sulfone |
| PTFE | polytetrafluoroethylene |
| PVDF | polyvinylidene fluoride |
| SMBR | submerged membrane bioreactor |
| SRT | solids retention time |
| TIPS | thermally induced phase separation |
| TMP | transmembrane pressure |
| UF | ultrafiltration |
| WWTP | wastewater treatment plant |
References
- Judd, S. The MBR Book; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Visvanathan, C.; Aim, R.B.; Parameshwaran, K. Membrane separation bioreactors for wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2000, 30, 1–48. [Google Scholar] [CrossRef]
- Cote, P.; Alam, Z.; Penny, J. Hollow fiber membrane life in membrane bioreactors (MBR). Desalination 2012, 288, 145–151. [Google Scholar] [CrossRef]
- Katuri, K.P.; Werner, C.M.; Jimenez-Sandoval, R.J.; Chen, W.; Jeon, S.; Logan, B.E.; Lai, Z.; Amy, G.L.; Saikaly, P.E. A novel anaerobic electrochemical membrane bioreactor (AnEMBR) with conductive hollow-fiber membrane for treatment of low-organic strength solutions. Environ. Sci. Technol. 2014, 48, 12833–12841. [Google Scholar] [CrossRef] [PubMed]
- Hanft, S. Membrane Bioreactors: Global Markets; BCC Research: Wellesley, MA, USA, 2011. [Google Scholar]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, S.-H.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Yamanishi, S.; Miya, A. Modeling of biofouling by extracellular polymers in a membrane separation activated sludge system. Water Sci. Technol. 1998, 38, 497–504. [Google Scholar] [CrossRef]
- Ji, J.; Qiu, J.; Wong, F.-S.; Li, Y. Enhancement of filterability in MBR achieved by improvement of supernatant and floc characteristics via filter aids addition. Water Res. 2008, 42, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.S.; Kim, S.N. Wastewater treatment using membrane filtration-effect of biosolids concentration on cake resistance. Process Biochem. 2005, 40, 1307–1314. [Google Scholar] [CrossRef]
- Ng, H.Y.; Tan, T.W.; Ong, S.L. Membrane fouling of submerged membrane bioreactors: Impact of mean cell residence time and the contributing factors. Environ. Sci. Technol. 2006, 40, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Tan, T.W.; Ong, S.L.; Toh, C.A.; Loo, Z.P. Effects of solid retention time on the performance of submerged anoxic/oxic membrane bioreactor. Water Sci. Technol. 2006, 53, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Malaeb, L.; Katuri, K.P.; Logan, B.E.; Maab, H.; Nunes, S.P.; Kaikaly, P.E. A hybrid microbial fuel cell membrane bioreactor with a conductive ultrafiltration membrane biocathode for wastewater treatment. Environ. Sci. Technol. 2013, 47, 11821–11828. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chun-Te Lin, J.; Huang, C. Application of nanosilver surface modification to RO membrane and spacer for mitigating biofouling in seawater desalination. Water Res. 2009, 43, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, D.; Langner, C.; Ghanem, A.; Rehim, M.A.; Voit, B.; Meier-Haack, J. Hydrogel surface modification of reverse osmosis membranes. J. Membr. Sic. 2015, 476, 264–276. [Google Scholar] [CrossRef]
- Santos, A.; Judd, S. The commercial status of membrane bioreactors for municipal wastewater. Sep. Sci. Technol. 2010, 45, 850–857. [Google Scholar] [CrossRef]
- Kim, J.O.; Jung, J.T.; Yeom, I.T.; Aoh, G.H. Effect of fouling reduction by ozone backwashing in a microfiltration system with advanced new membrane material. Desalination 2007, 202, 361–368. [Google Scholar] [CrossRef]
- Matar, G.; Gonzalez-Gil, G.; Maab, H.; Nunes, S.; Le-Clech, P.; Vrouwenvelder, J.; Saikaly, P.E. Temporal changes in extracellular polymeric substances on hydrophobic and hydrophilic membrane surfaces in a submerged membrane bioreactor. Water Res. 2016, 95, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Park, S.-K.; Ng, H.-Y. Membrane fouling in a submerged membrane bioreactor using track-etched and phase-inversed porous membranes. Sep. Purif. Technol. 2009, 65, 184–192. [Google Scholar] [CrossRef]
- Miyoshi, T.; Yuasa, K.; Ishigami, T.; Rajabzadeh, S.; Kamio, E.; Ohmukai, Y.; Saeki, D.; Ni, J.; Matsuyama, H. Effect of membrane polymeric materials on relationship between surface pore size and membrane fouling in membrane bioreactors. Appl. Surf. Sci. 2015, 330, 351–357. [Google Scholar] [CrossRef]
- Minehara, H.; Dan, K.; Ito, Y.; Takabatake, H.; Henmi, M. Quantitative evaluation of fouling resistance of PVDF/PMMA-g-PEO polymer blend membranes for membrane bioreactor. J. Membr. Sci. 2014, 466, 211–219. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Piassecka, A.; Jishana, P.S.; Naik, P.V.; Vankelecom, I.F.J. Study of PVDF asymmetric membranes in a high-throughput membrane bioreactor (HT-MBR): Influence of phase inversion parameters and filteration performance. Sep. Purif. Technol. 2016, 162, 6–13. [Google Scholar] [CrossRef]
- Nittami, T.; Tokunaga, H.; Satoh, A.; Takeda, M.; Matsumoto, K. Influence of surface hydrophilicity on polytetrafluoroethylene flat sheet membrane fouling in a submerged membrane bioreactor using two activated sludges with different characteristics. J. Membr. Sci. 2014, 463, 183–189. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Sano, R.; Ishigami, T.; Kakihana, Y.; Ohmukai, Y.; Matsuyama, H. Preparation of hydrophilic vinyl chloride copolymer hollow fiber membranes with antifouling properties. Appl. Surf. Sci. 2015, 324, 718–724. [Google Scholar] [CrossRef]
- Zhou, Z.; Rajabzadeh, S.; Shaikh, A.R.; Kakihana, Y.; Ma, W.; Matsuyama, H. Effect of surface properties on antifouling performance of poly(vinyl chloride-co-poly(ethylene glycol)methyl ether methacrylate)/PVC blend membrane. J. Membr. Sci. 2016, 514, 537–546. [Google Scholar] [CrossRef]
- Hashino, M.; Hirami, K.; Ishigami, T.; Ohmukai, Y.; Maruyama, T.; Kubota, N.; Matsuyama, H. Effect of kinds of membrane materials on membrane fouling with BSA. J. Membr. Sci. 2011, 384, 157–165. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)-Part II: Technical aspects. Water Sci. Technol. 2001, 43, 9–16. [Google Scholar] [PubMed]
- Nagaoka, H.; Nemoto, H. Influence of extracellular polymeric substances on nitrogen removal in an intermittently-aerated membrane bioreactor. Water Sci. Technol. 2005, 51, 151–158. [Google Scholar] [PubMed]
- Malamis, S.; Andreadakis, A. Fractionation of proteins and carbohydrates of extracellular polymeric substances in a membrane bioreactor system. Bioresour. Technol. 2009, 100, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, Y.; Cao, C.-Q.; Zhang, J.; Li, Z.-N. Interaction energy evaluation of soluble microbial products (SMP) on different membrane surfaces: Role of the reconstructed membrane topology. Water Res. 2012, 46, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Marel, P.; Zwijnenburg, A.; Kemperman, A.; Wessling, M.; Temmink, H.; Meer, W.B.D. Influence of membrane properties on fouling in submerged membrane bioreactors. J. Membr. Sci. 2010, 348, 66–74. [Google Scholar] [CrossRef]

| Membrane Material | Average Pore Size (μm) | Hydrophilicity | Configuration | Affected by Hydrophilicity | References |
|---|---|---|---|---|---|
| PVDF | 0.047 | Hydrophobic | Flat sheet | Yes | [20] |
| PVDF/PMMA-g-PEG | 0.053–0.089 | Hydrophilic | |||
| PVDF | 0.02–0.3 | Hydrophobic | Flat sheet | No | [21] |
| PE | 0.3 | Hydrophilic | |||
| PC | 0.1–2.5 | Hydrophilic | |||
| PETE | 0.1 | Hydrophobic | Flat sheet | No | [18] |
| PCTE | 0.1 | Hydrophobic | |||
| PTFE | 0.1 | Hydrophobic | |||
| PVDF | 0.08 | Hydrophobic | Flat sheet | Yes | [22] |
| PTFE | 0.3 | Hydrophobic | |||
| PTFE | 0.3 | Hydrophilic | |||
| PVDF | 0.02–0.4 | Hydrophobic | Hollow fiber | No | [19] |
| PVB | 0.02–0.15 | Hydrophobic | |||
| CAB | 0.04–0.2 | Hydrophilic | |||
| PVDF | 0.02–0.09 | Hydrophobic | Hollow fiber | Yes | This work |
| PES | 0.03–0.14 | Hydrophobic | |||
| CA | 0.03–0.09 | Hydrophilic |
| Material | CA | PES | PVDF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pore size | <10–30 nm | <50 nm | <90 nm | <10–30 nm | <50 nm | <140 nm | <10–20 nm | <40 nm | <90 nm |
| Polymer solution | CA-DMAc | PES-DMAc | PES-DMAc 4 wt % water | PVDF-DMAc | PVDF-DMAc 4 wt % LiCl | PVDF-DMAc 4 wt % LiCl-2 wt % water | |||
| Polymer concentration (wt %) | 22 | 20 | 16 | 16 | 14 | 14 | 14 | 14 | 14 |
| Solution temperature (°C) | Room temperature | Room temperature | Room temperature | ||||||
| Polymer solution flow rate (g/min) | 3.8 | 2.08 | 4.0 | 2.78 | 4.2 | 1.92 | 2.78 | 4.2 | 1.92 |
| Inner coagulant (DMAc:Water) | 8:2 | 6:4 | 6:4 | 6:4 | 6:4 | 6:4 | 6:4 | 6:4 | 6:4 |
| Inner coagulant flow rate (g/min) | 2.96 | 3.5 | 2.96 | 3.49 | 3.42 | 3.05 | 3.49 | 3.42 | 3.05 |
| Air gap (mm) | 60 | 50 | 50 | 25 | 25 | 60 | 25 | 25 | 60 |
| Take-up speed (m/s) | 0.034 | 0.025 | 0.046 | 0.024 | 0.03 | 0.027 | 0.024 | 0.03 | 0.027 |
| Membrane | CA | PES | PVDF |
|---|---|---|---|
| Pure water permeability (L/m2·h·bar) | 215 ± 7 | 331 ± 22 | 233 ± 71 |
| Mean pore size (nm) | <10–30 | <10–30 | <10–20 |
| Air bubble contact angle (°) | 121 | 115 | 94 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Rajabzadeh, S.; Okamura, R.; Ishigami, T.; Hasegawa, S.; Kato, N.; Matsuyama, H. The Effect of Membrane Material and Surface Pore Size on the Fouling Properties of Submerged Membranes. Water 2016, 8, 602. https://doi.org/10.3390/w8120602
Jeon S, Rajabzadeh S, Okamura R, Ishigami T, Hasegawa S, Kato N, Matsuyama H. The Effect of Membrane Material and Surface Pore Size on the Fouling Properties of Submerged Membranes. Water. 2016; 8(12):602. https://doi.org/10.3390/w8120602
Chicago/Turabian StyleJeon, Sungil, Saeid Rajabzadeh, Ryo Okamura, Toru Ishigami, Susumu Hasegawa, Noriaki Kato, and Hideto Matsuyama. 2016. "The Effect of Membrane Material and Surface Pore Size on the Fouling Properties of Submerged Membranes" Water 8, no. 12: 602. https://doi.org/10.3390/w8120602







