Effects of Exposed Artificial Substrate on the Competition between Phytoplankton and Benthic Algae: Implications for Shallow Lake Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Analytical Methods
3. Results
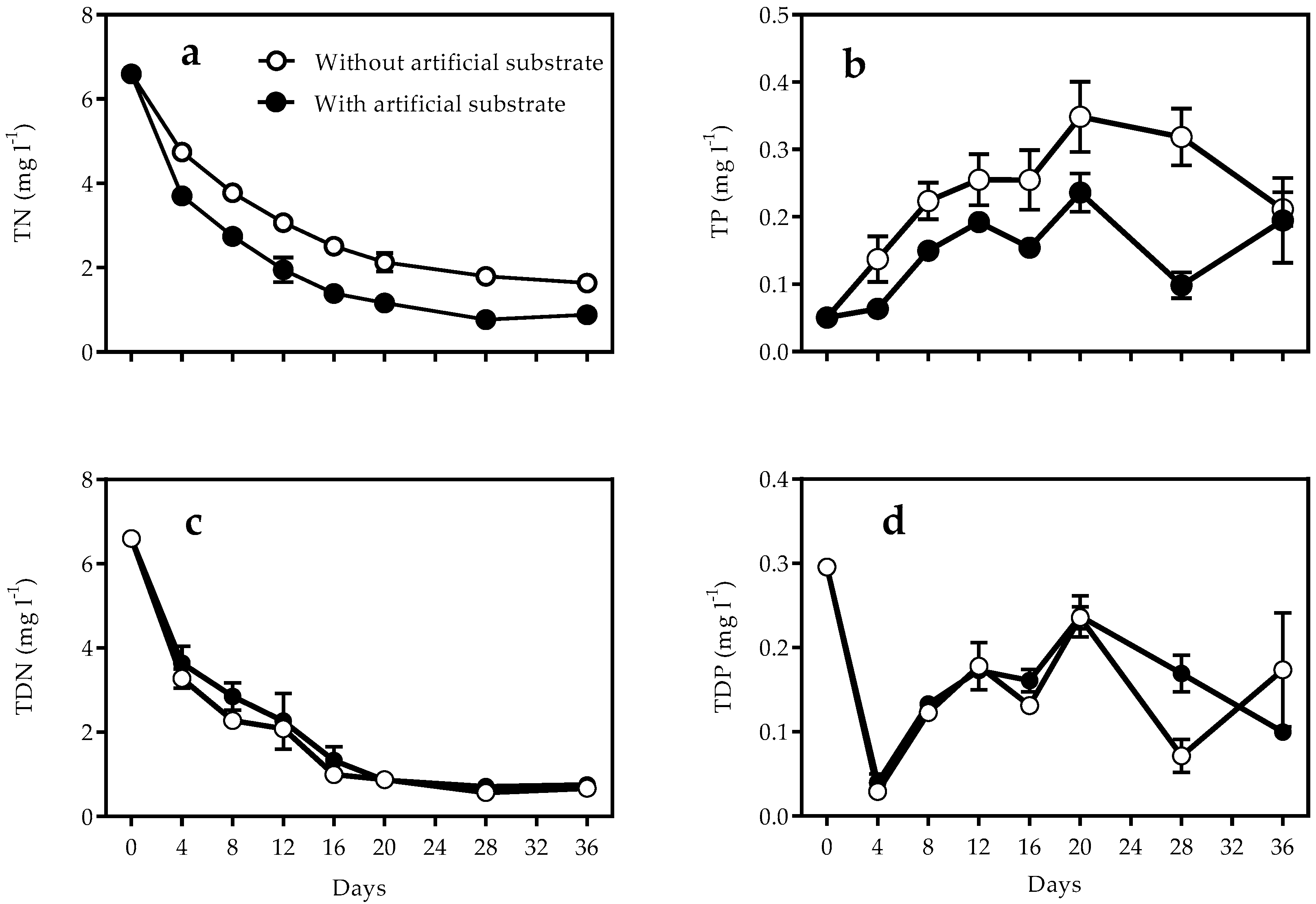
3.1. Nutrients and Turbidity
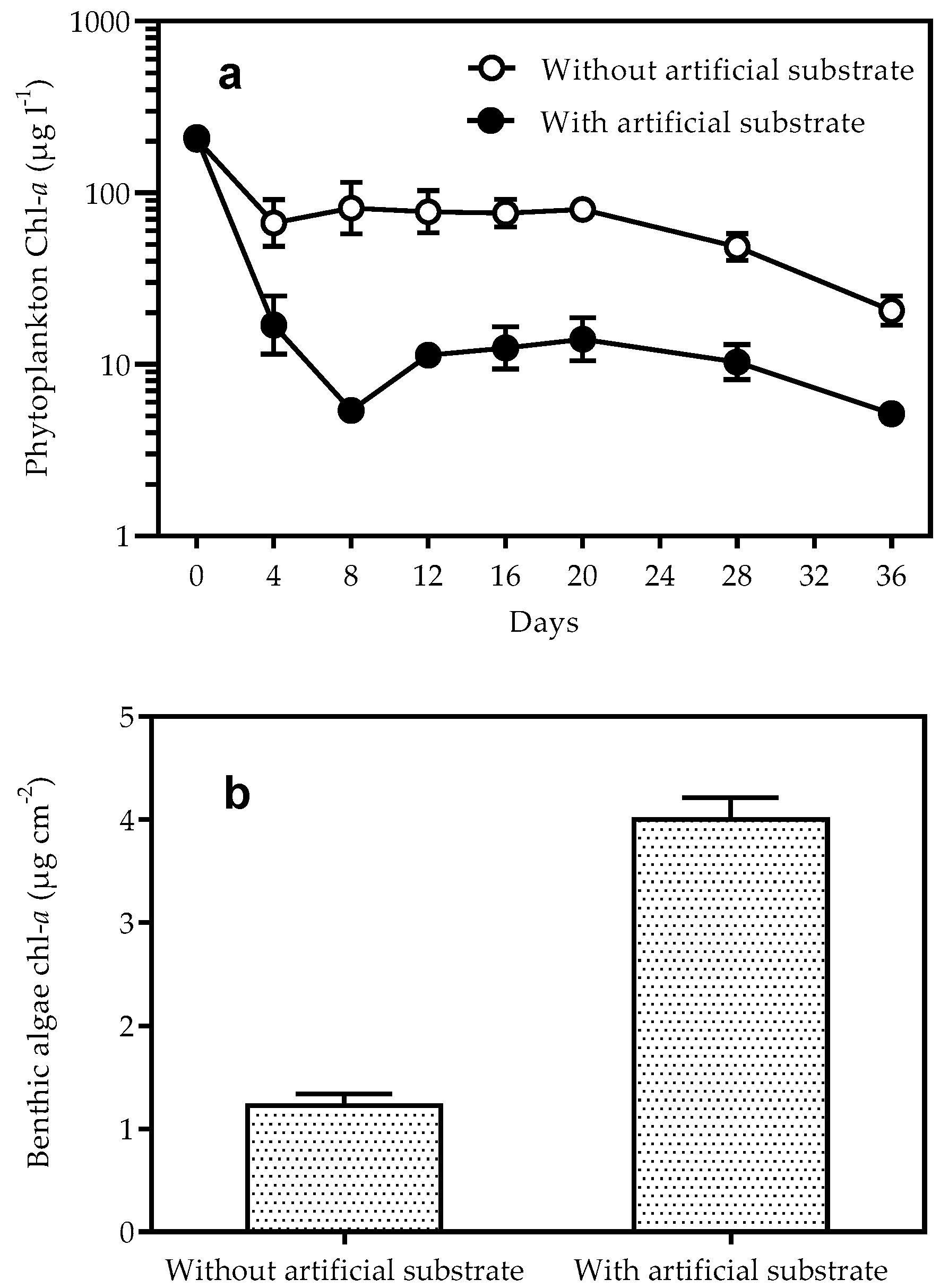
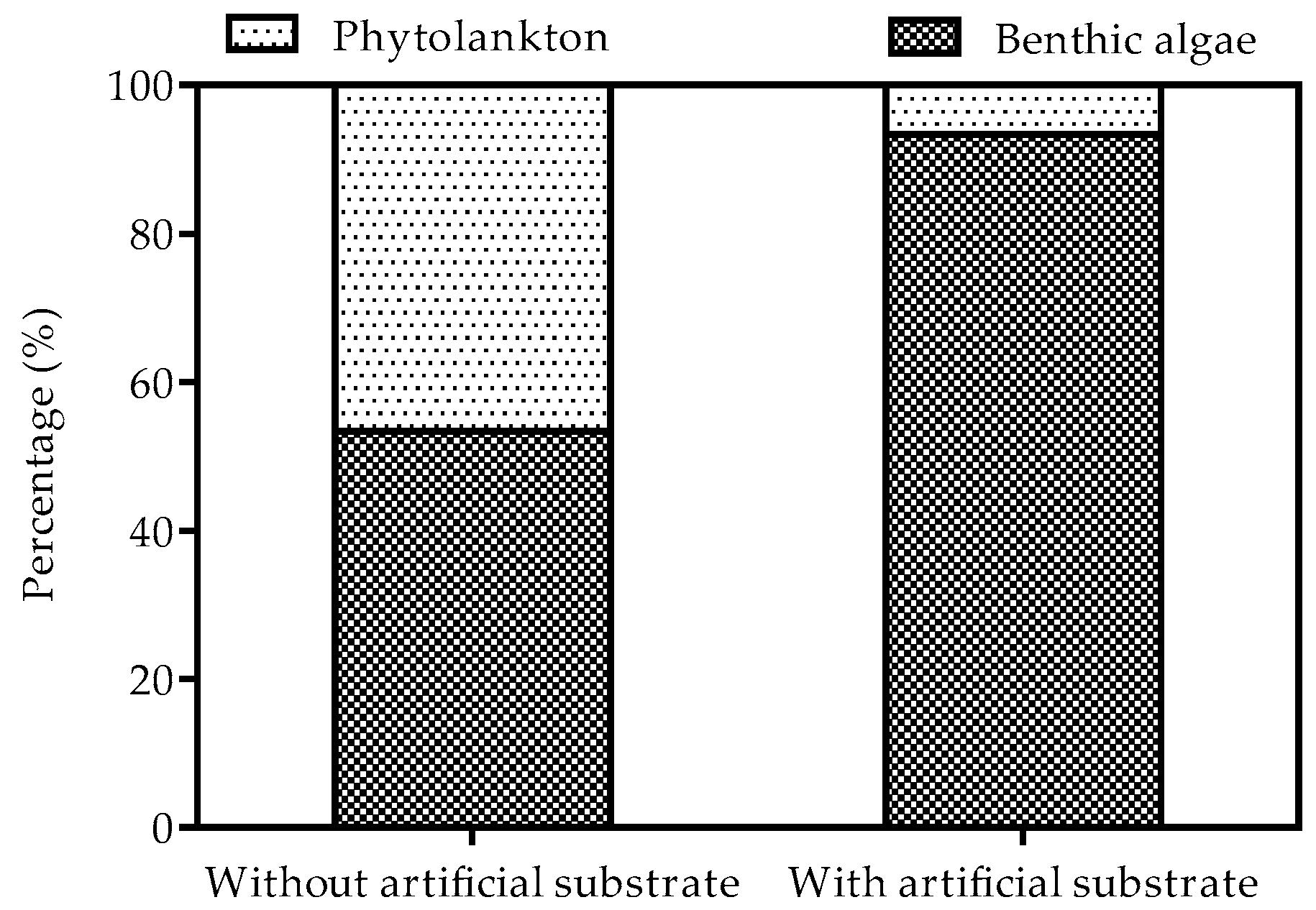
3.2. Biomass of Phytoplankton and Benthic Algae
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Lodge, D.M.; Carpenter, S.R. Whole-lake fertilization effects on distribution of primary production between benthic and pelagic habitats. Ecology 2001, 82, 1065–1077. [Google Scholar] [CrossRef]
- Liboriussen, L.; Jeppesen, E. Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshw. Biol. 2003, 48, 418–431. [Google Scholar] [CrossRef]
- Genkai-Kato, M.; Vadeboncoeur, Y.; Liboriussen, L.; Jeppesen, E. Benthic–planktonic coupling, regime shifts, and whole-lake primary production in shallow lakes. Ecology 2012, 93, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.A. Effects of competitive interactions on the biomass development of planktonic and periphytic algae in lakes. Limnol. Oceanogr. 1988, 33, 121–128. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Borum, J. Interactions among phytoplankton, periphyton, and macrophytes in temperate freshwaters and estuaries. Aquat. Bot. 1991, 41, 137–175. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Steinman, A.D. Periphyton function in lake ecosystems. Sci. World J. 2002, 2, 1449–1468. [Google Scholar] [CrossRef] [PubMed]
- Jäger, C.G.; Diehl, S. Resource competition across habitat boundaries: Asymmetric interactions between benthic and pelagic producers. Ecol. Monogr. 2014, 84, 287–302. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, X.; Gulati, R.D.; Liu, Z. Effects of N and P enrichment on competition between phytoplankton and benthic algae in shallow lakes: A mesocosm study. Environ. Sci. Pollut. Res. 2015, 22, 4418–4424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Gulati, R.D.; Jeppesen, E. The effect of benthic algae on phosphorus exchange between sediment and overlying water in shallow lakes: A microcosm study using 32P as a tracer. Hydrobiologia 2013, 710, 109–116. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Lauridsen, T.L.; Skov, C.; Van Nes, E.H.; Roijackers, R.; Portielje, R.O.B. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2007, 44, 1095–1105. [Google Scholar] [CrossRef]
- Søndergaard, M.; Bjerring, R.; Jeppesen, E. Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 2013, 710, 95–107. [Google Scholar] [CrossRef]
- Chen, F.; Shu, T.; Jeppesen, E.; Liu, Z.; Chen, Y. Restoration of a subtropical eutrophic shallow lake in China: Effects on nutrient concentrations and biological communities. Hydrobiologia 2013, 718, 59–71. [Google Scholar] [CrossRef]
- Vymazal, J. The use of periphyton communities for nutrient removal from polluted streams. Hydrobiologia 1988, 166, 225–237. [Google Scholar] [CrossRef]
- Pei, G.; Wang, Q.; Liu, G. The role of periphyton in phosphorus retention in shallow lakes with different trophic status, China. Aquat. Bot. 2015, 125, 17–22. [Google Scholar] [CrossRef]
- Adey, W.; Luckett, C.; Jensen, K. Phosphorus removal from natural waters using controlled algal production. Restor. Ecol. 1993, 1, 29–39. [Google Scholar] [CrossRef]
- Dodds, W.K. The role of periphyton in phosphorus retention in shallow freshwater aquatic systems. J. Phycol. 2003, 39, 840–849. [Google Scholar] [CrossRef]
- Hansson, L.A. Quantifying the impact of periphytic algae on nutrient availability for phytoplankton. Freshw. Biol. 1990, 24, 265–273. [Google Scholar] [CrossRef]
- Jöbgen, A.; Palm, A.; Melkonian, M. Phosphorus removal from eutrophic lakes using periphyton on submerged artificial substrate. Hydrobiologia 2004, 528, 123–142. [Google Scholar] [CrossRef]
- Sansone, U.; Belli, M.; Riccardi, M.; Alonzi, A.; Jeran, Z.; Radojko, J.; Cavolo, F. Adhesion of water-borne particulates on freshwater biota. Sci. Total Environ. 1998, 219, 21–28. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Hansen, C.M. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 2003, 426, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Salant, N.L. ‘Sticky business’: The influence of streambed periphyton on particle deposition and infiltration. Geomorphology 2011, 126, 350–363. [Google Scholar] [CrossRef]
- Jones, J.I.; Duerdoth, C.P.; Collins, A.L.; Naden, P.S.; Sear, D.A. Interactions between diatoms and fine sediment. Hydrol. Process. 2014, 28, 1226–1237. [Google Scholar] [CrossRef]
- Qin, B.Q.; Xu, P.Z.; Wu, Q.L.; Luo, L.C.; Zhang, Y.L. Environmental issues of lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Ning, X.Y.; Gu, J.; Tan, B.C.; Zhu, X.L.; Li, K.Y. Effects of artificial substrate materials on eutrophic water quality improvement. Shanghai Environ. Sci. 2015, 34, 231–234. (In Chinese) [Google Scholar]
- Jin, X.C.; Tu, Q.Y. The Standard Methods for Observation and Analysis in Lake Eutrophication, 2nd ed.; Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- State Environmental Protection Administration (SEPA). Monitoring and Analytical Methods for Water and Wastewater, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Hansen, K.S.; Møller, P.H.; Rasmussen, H.U.; Larsen, S.E. Does resuspension prevent a shift to a clear state in shallow lakes during reoligotrophication? Limnol. Oceanogr. 2003, 48, 1913–1919. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.W.; Qin, B.Q.; Li, Y.P.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Gulati, R.D.; Pires, L.M.D.; Van Donk, E. Lake restoration studies: Failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 2008, 38, 233–247. [Google Scholar] [CrossRef]
- Schou, M.O.; Risholt, C.; Lauridsen, T.L.; Søndergaard, M.; Grønkjær, P.; Jacobsen, L.; Berg, S.; Skov, C.; Brucet, S.; Jeppesen, E. Restoring lakes by using artificial plant beds: Habitat selection of zooplankton in a clear and a turbid shallow lake. Freshw. Biol. 2009, 54, 1520–1521. [Google Scholar] [CrossRef]
- Boll, T.; Balayla, D.; Andersen, F.Ø.; Jeppesen, E. Can artificial plant beds be used to enhance macroinvertebrate food resources for perch (Perca fluviatilis L.) during the initial phase of lake restoration by cyprinid removal? Hydrobiologia 2012, 679, 175–186. [Google Scholar] [CrossRef]
- Balayla, D.; Boll, T.; Trochine, C.; Jeppesen, E. Does artificial plant beds facilitate microcrustacean development during biomanipulation of eutrophic shallow lakes? Hydrobiologia 2017. in revision. [Google Scholar]

| Artificial Substrate | Time | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | DF | p | F | DF | p | F | DF | p | |
| TN | 121.86 | 1 | <0.001 | 299.75 | 7 | <0.001 | 13.44 | 7 | <0.001 |
| TP | 41.57 | 1 | 0.003 | 118.95 | 7 | <0.001 | 13.71 | 7 | <0.001 |
| TDN | 11.43 | 1 | 0.015 | 282.03 | 7 | <0.001 | 1.15 | 7 | 0.352 |
| TDP | 11.47 | 1 | 0.028 | 96.71 | 7 | <0.001 | 9.47 | 7 | 0.006 |
| Turb | 13.26 | 1 | 0.011 | 29.38 | 7 | <0.001 | 4.12 | 7 | 0.044 |
| TSS | 30.12 | 1 | 0.002 | 22.77 | 7 | <0.001 | 6.89 | 7 | 0.012 |
| OSS | 61.19 | 1 | <0.001 | 20.18 | 7 | <0.001 | 8.59 | 7 | <0.001 |
| ISS | 7.71 | 1 | 0.038 | 24.13 | 7 | <0.001 | 2.77 | 7 | 0.095 |
| Chl-a | 39.18 | 1 | 0.001 | 50.74 | 7 | <0.001 | 8.32 | 7 | 0.002 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Luo, X.; Jin, H.; Gu, J.; Jeppesen, E.; Liu, Z.; Li, K. Effects of Exposed Artificial Substrate on the Competition between Phytoplankton and Benthic Algae: Implications for Shallow Lake Restoration. Water 2017, 9, 24. https://doi.org/10.3390/w9010024
He H, Luo X, Jin H, Gu J, Jeppesen E, Liu Z, Li K. Effects of Exposed Artificial Substrate on the Competition between Phytoplankton and Benthic Algae: Implications for Shallow Lake Restoration. Water. 2017; 9(1):24. https://doi.org/10.3390/w9010024
Chicago/Turabian StyleHe, Hu, Xuguang Luo, Hui Jin, Jiao Gu, Erik Jeppesen, Zhengwen Liu, and Kuanyi Li. 2017. "Effects of Exposed Artificial Substrate on the Competition between Phytoplankton and Benthic Algae: Implications for Shallow Lake Restoration" Water 9, no. 1: 24. https://doi.org/10.3390/w9010024






