Hydrogeochemistry and Genesis Analysis of Thermal and Mineral Springs in Arxan, Northeastern China
Abstract
:1. Introduction
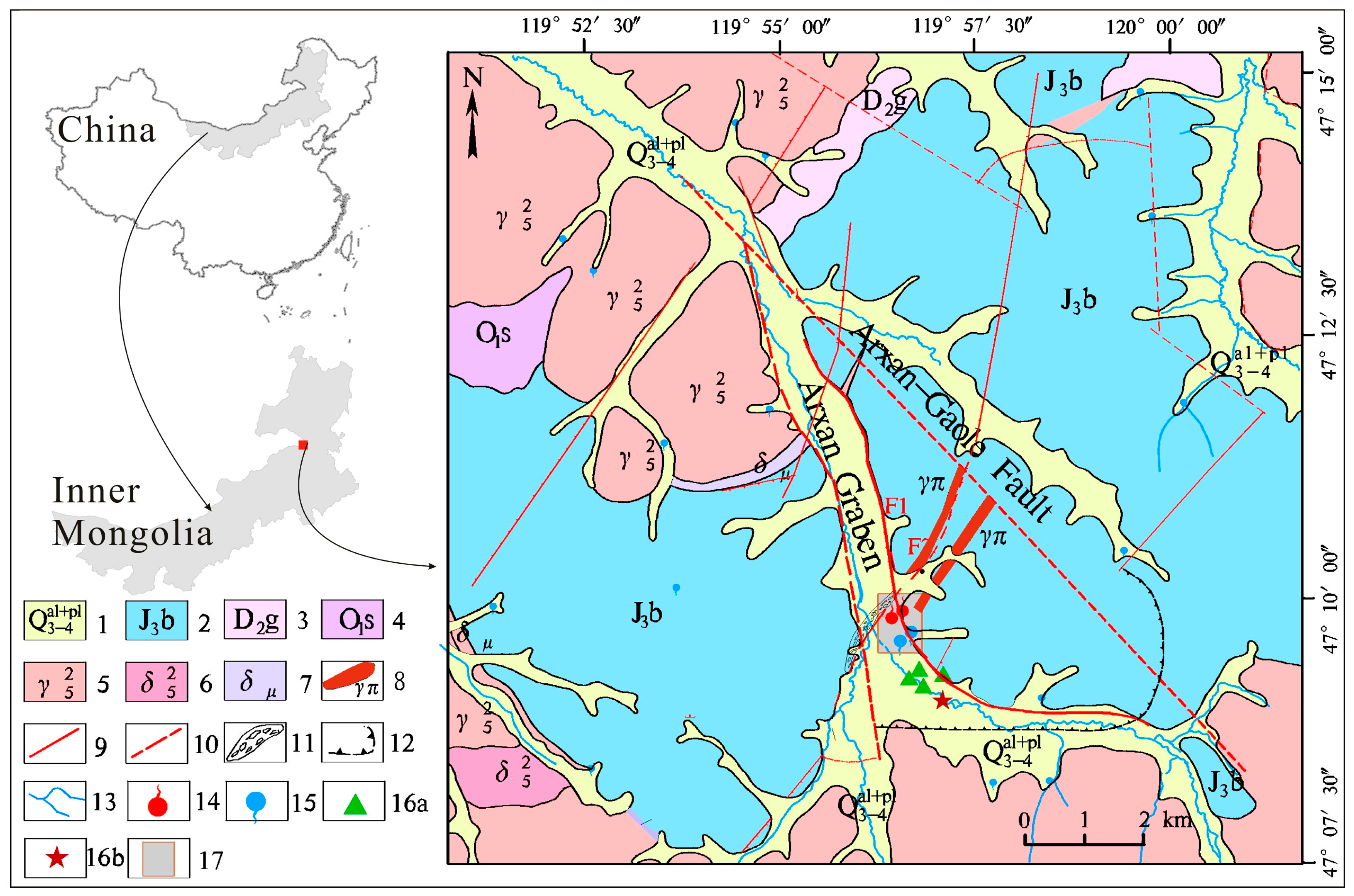
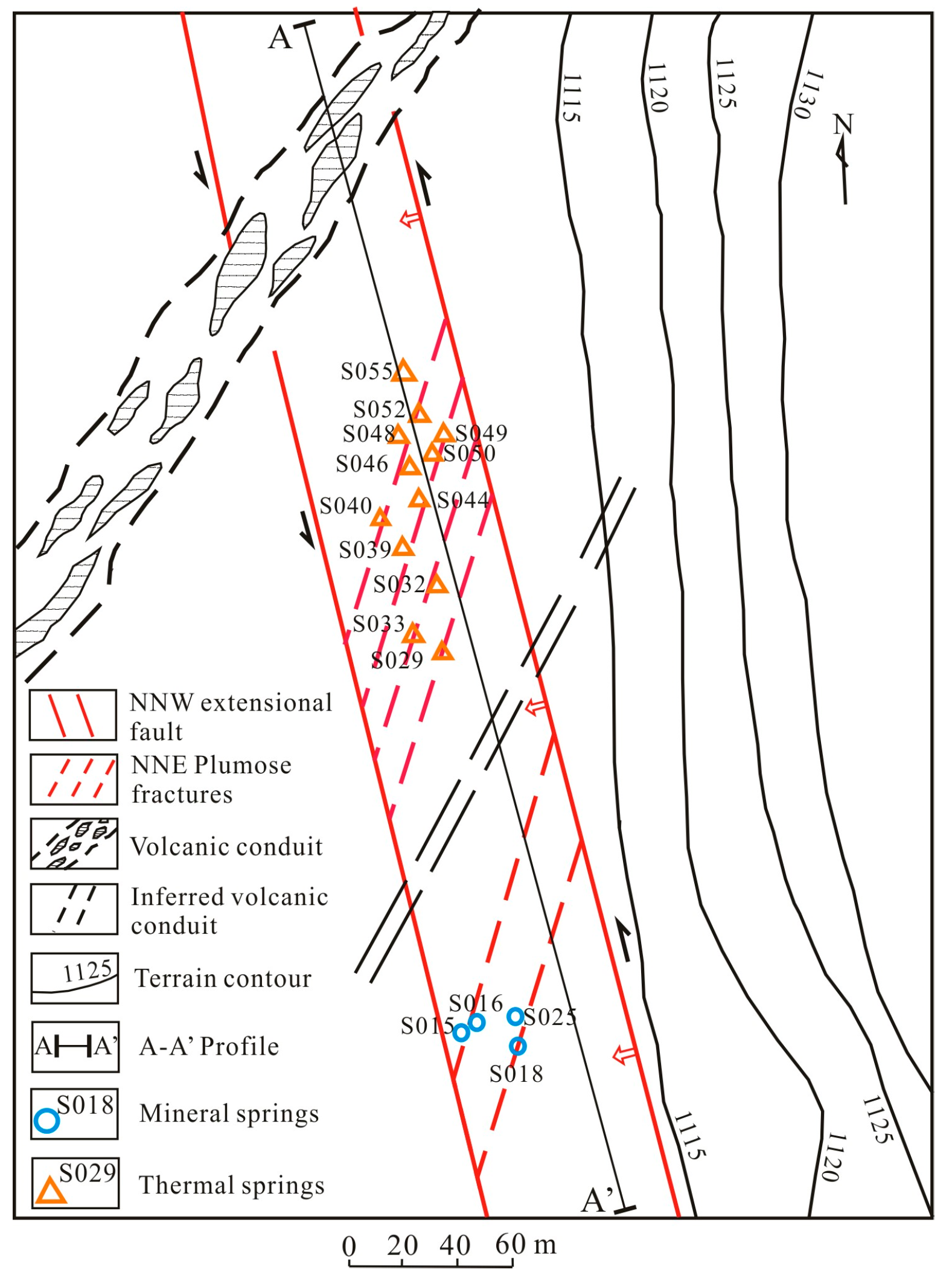
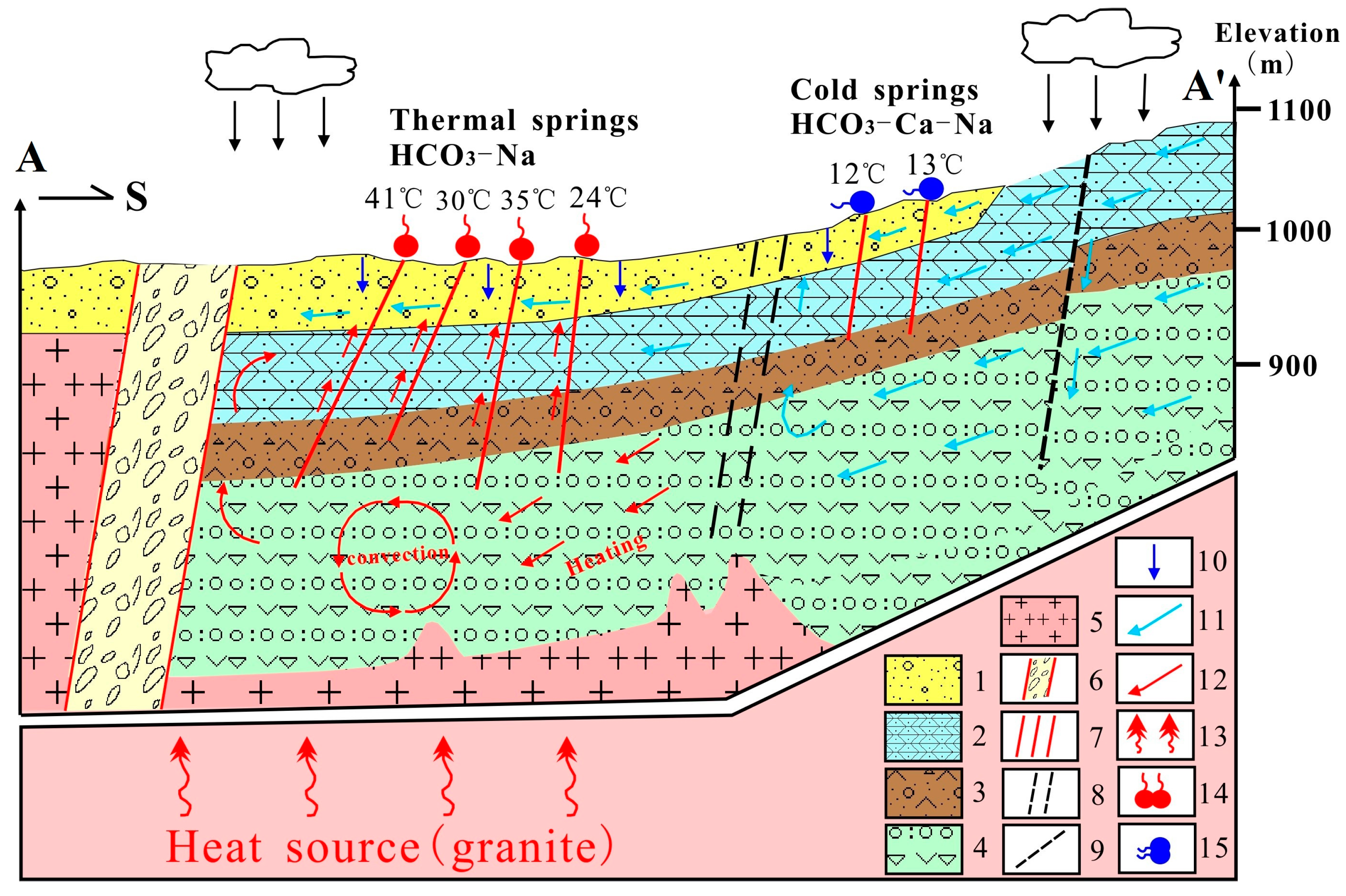
2. Geological and Hydrogeological Setting
3. Materials and Methods
4. Results and Discussion
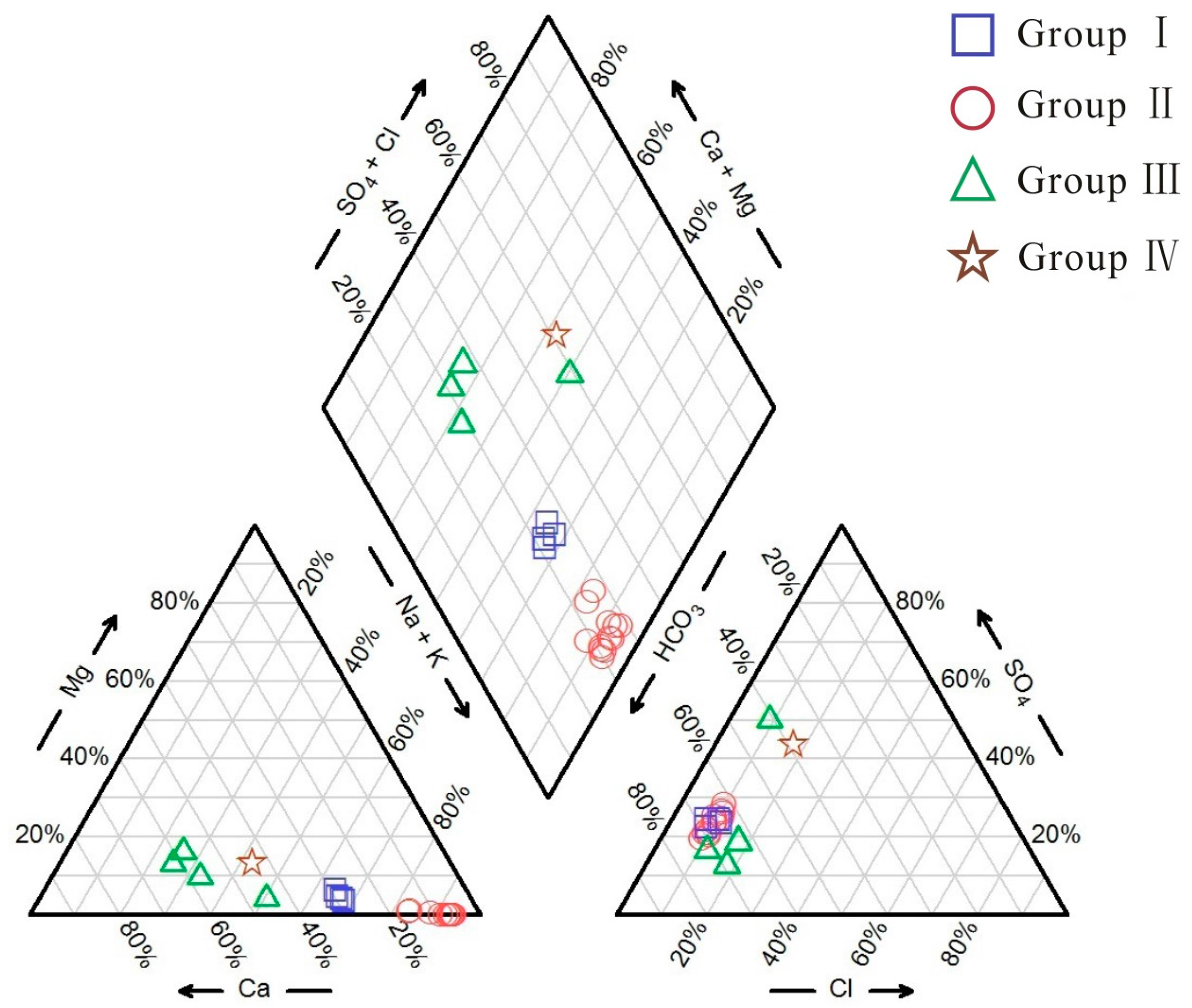
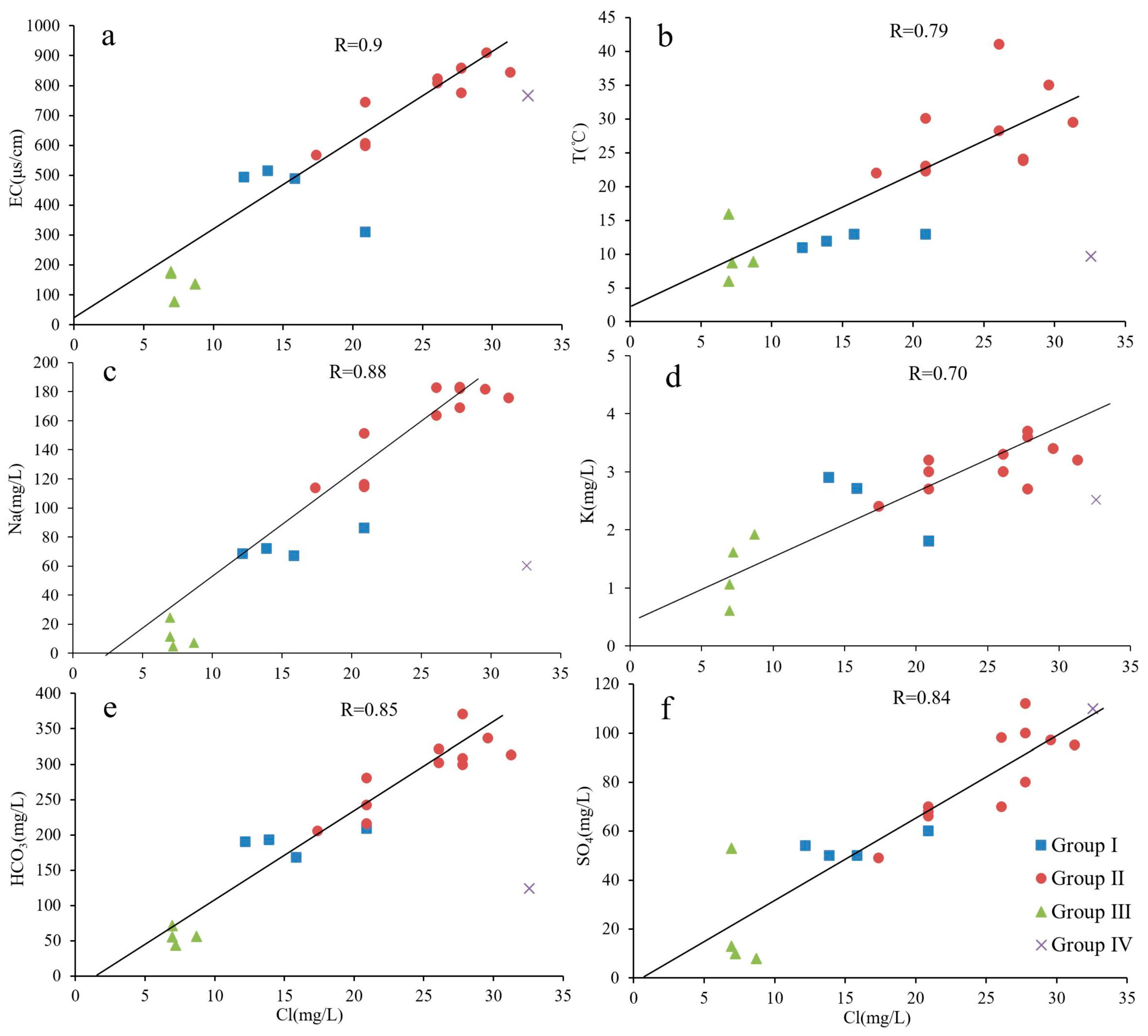
4.1. Hydrochemical Characteristics
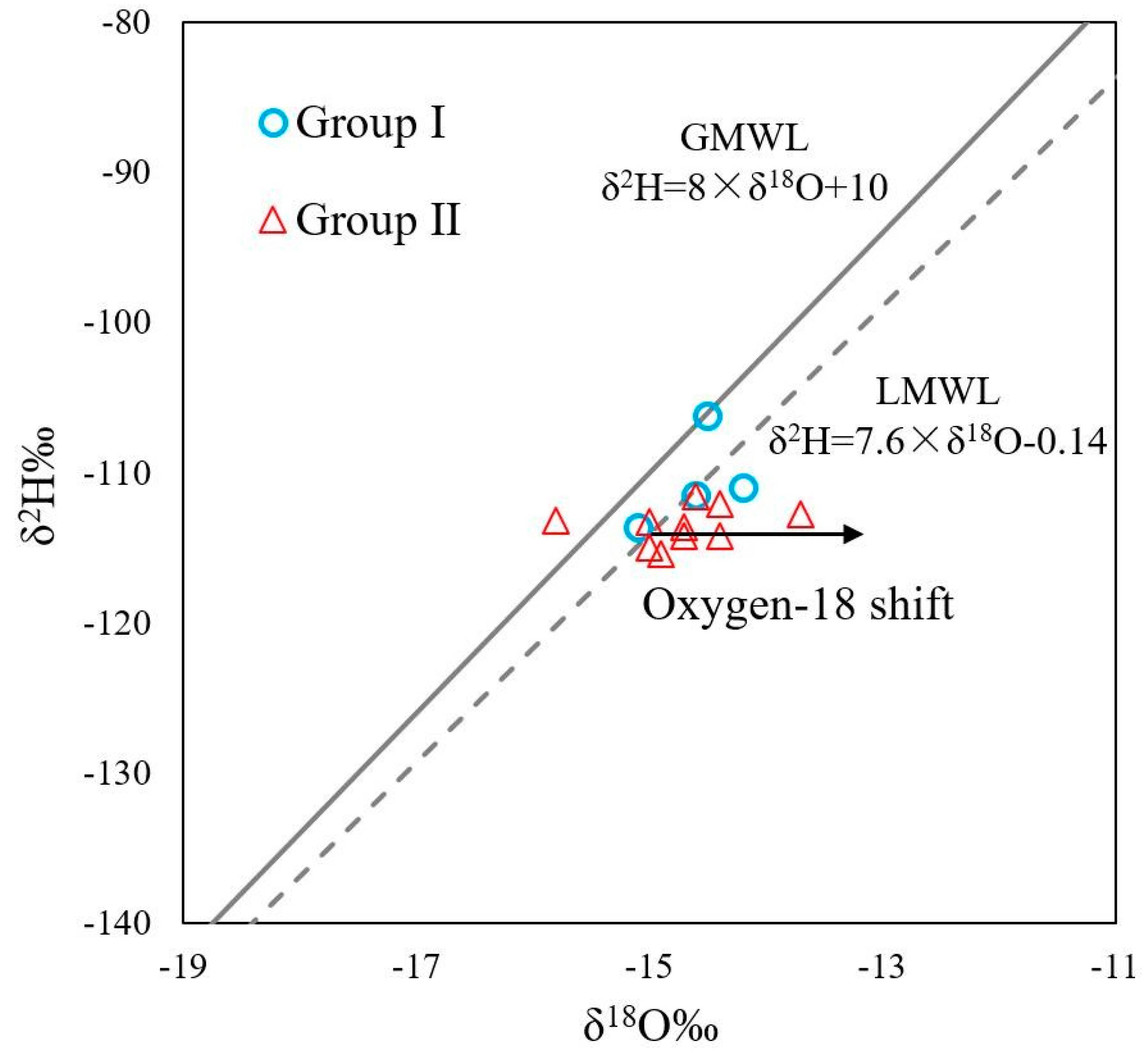
4.2. Source of Recharge
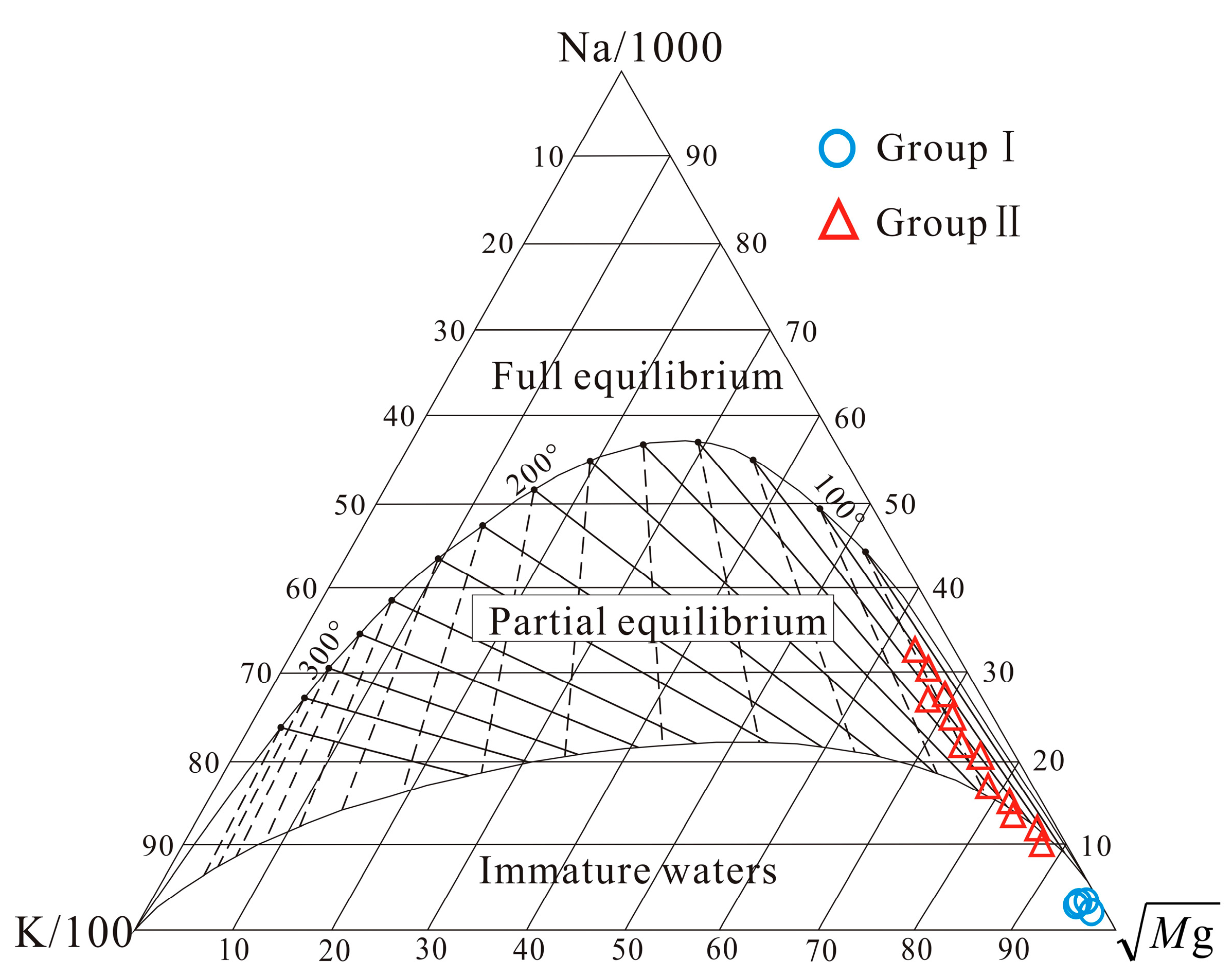
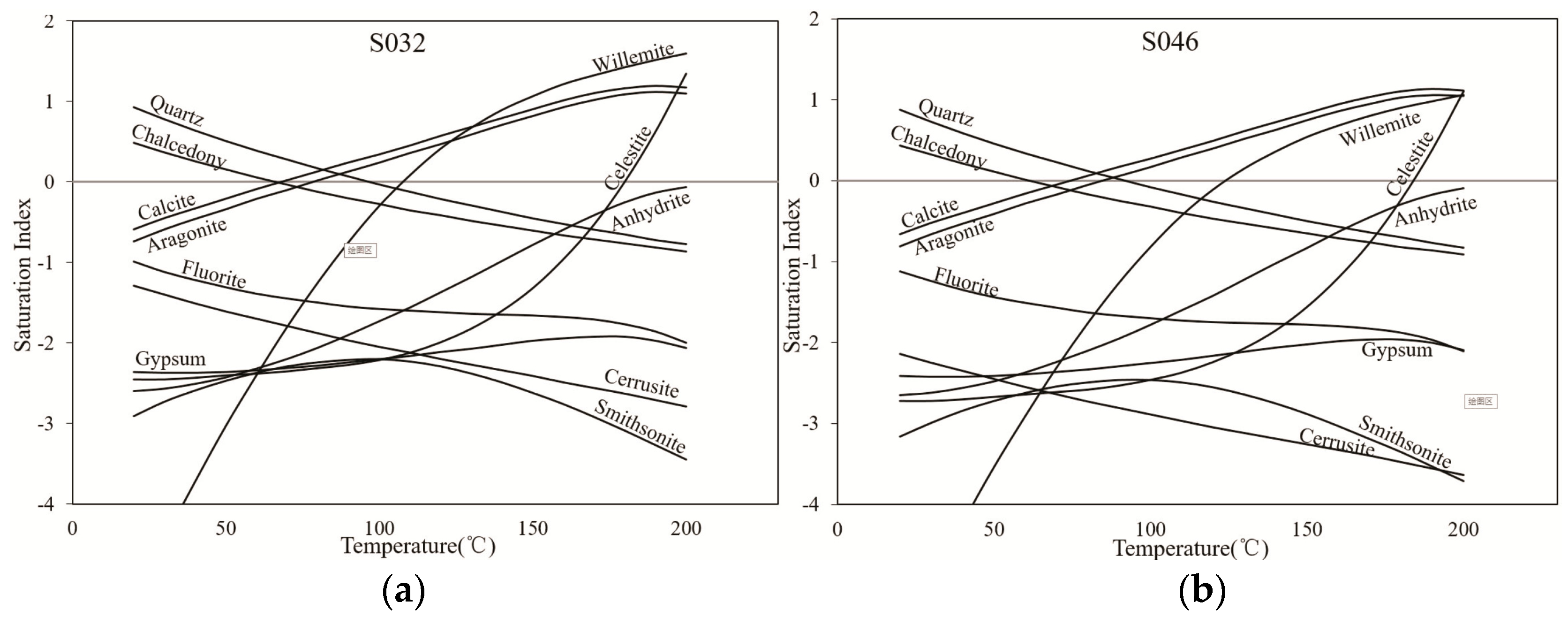
4.3. Hydrogeochemical Thermometry
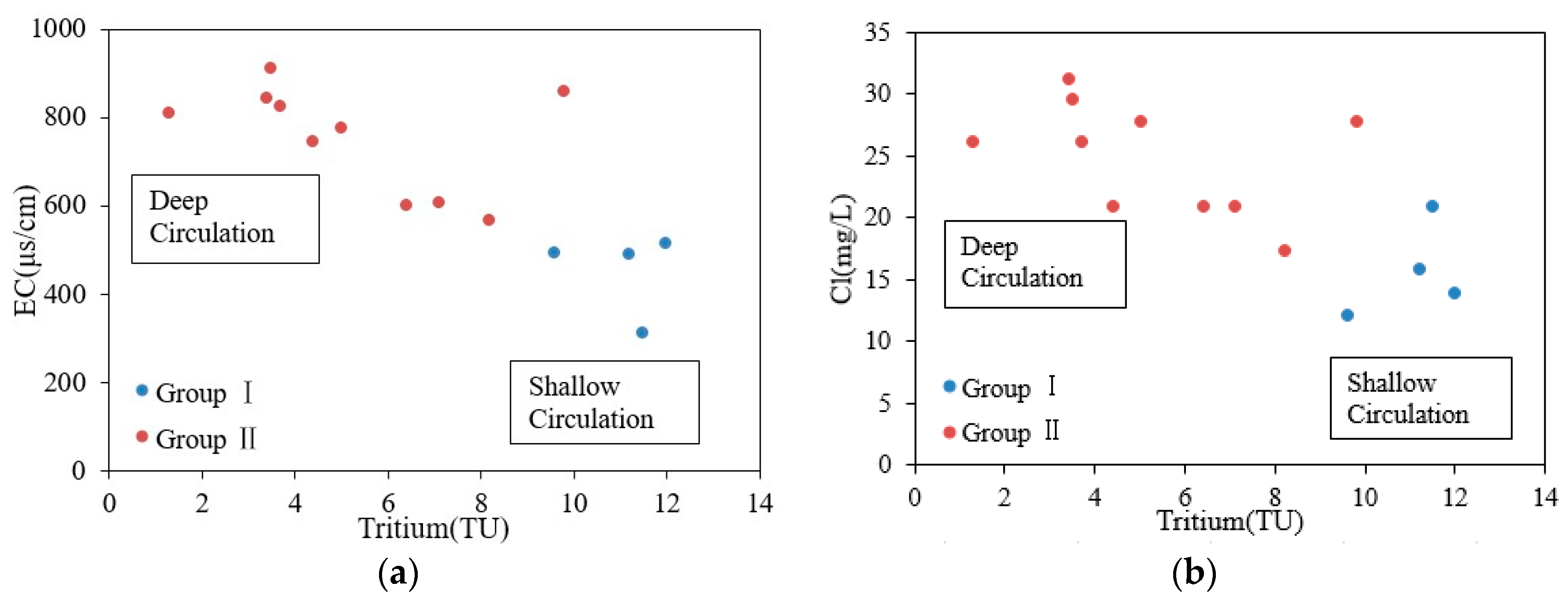
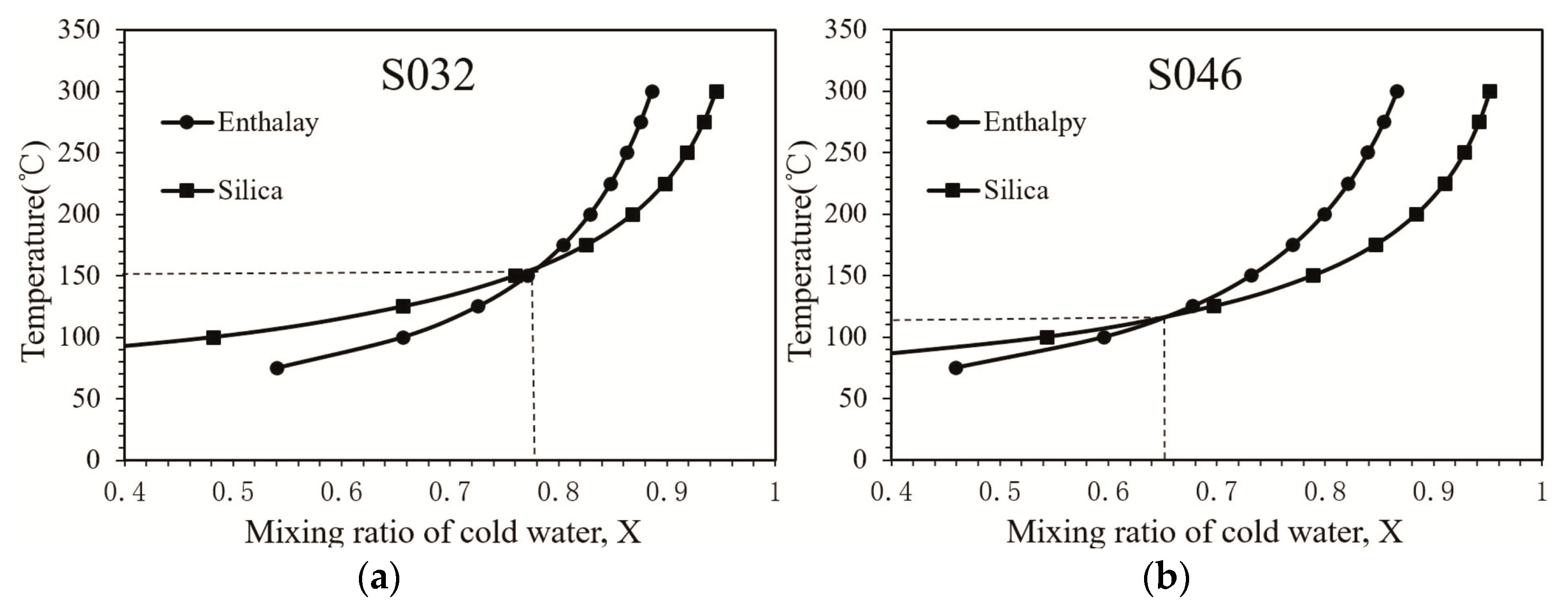
4.4. Mixing of Cold and Thermal Water
4.5. Conceptual Model of the Geothermal System
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, X. Groundwater Science Monographs; Geology Publishing House: Beijing, China, 2010; pp. 104–105. (In Chinese) [Google Scholar]
- Minissale, A.; Mattash, M.A.; Vaselli, O.; Tassi, F.; Al-Ganad, I.N.; Selmo, E.; Shawki, N.M.; Tedesco, D.; Poreda, R.; Ad-Dukhain, A.M.; et al. Thermal springs, fumaroles and gas vents of continental Yemen: Their relation with active tectonics, regional hydrology and the country’s geothermal potential. Appl. Geochem. 2007, 22, 799–820. [Google Scholar] [CrossRef]
- De Filippis, L.; Billi, A. Morphotectonics of fissure ridge travertines from geothermal areas of Mammoth Hot Springs (Wyoming) and Bridgeport (California). Tectonophysics 2012, 548–549, 34–48. [Google Scholar] [CrossRef]
- Zaher, M.A.; Saibi, H.; Nishijima, J.; Fujimitsu, Y.; Mesbah, H.; Ehara, S. Exploration and assessment of the geothermal resources in the Hammam Faraun hot spring, Sinai Peninsula, Egypt. J. Asian Earth Sci. 2012, 45, 256–267. [Google Scholar] [CrossRef]
- Mao, X.; Wang, Y.; Zhan, H.; Feng, L. Geochemical and isotopic characteristics of geothermal springs hosted by deep-seated faults in Dongguan Basin, Southern China. J. Geochem. Explor. 2015, 158, 112–121. [Google Scholar] [CrossRef]
- Pasvanoğlu, S. Hydrogeochemical study of the thermal and mineralized waters of the Banaz (Hamamboğazi) area, western Anatolia, Turkey. Environ. Earth Sci. 2012, 65, 741–752. [Google Scholar] [CrossRef]
- Katsanou, K.; Siavalas, G.; Lambrakis, N. The thermal and mineral springs of Aitoloakarnania Prefecture: Function mechanism and origin of groundwater. Environ. Earth Sci. 2012, 65, 2351–2364. [Google Scholar] [CrossRef]
- Cai, J.; Chu, Y.; Guo, H. The distribution and charactistics of Arxan geothermal sturcture. Heilongjiang Sci. Technol. Water Conserv. 2008, 36, 61–62. (In Chinese) [Google Scholar]
- Qi, F.; Zhang, F.; Lu, S.; Sun, Y. The geothermal geological features of Arxan mountains. Jilin Geol. 2012, 31, 109–112. (In Chinese) [Google Scholar]
- Giggenbach, W.F. Geothermal solute equilibria-derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Guo, F.; Fan, W.; Li, C.; Zhao, L.; Li, H.; Yang, J. Multi-stage crust–mantle interaction in SE China: Temporal, thermal and compositional constraints from the Mesozoic felsic volcanic rocks in eastern Guangdong–Fujian provinces. Lithos 2012, 150, 62–84. [Google Scholar] [CrossRef]
- Mottl, M.J.; Seewald, J.S.; Wheat, C.G.; Tivey, M.K.; Michael, P.J.; Proskurowski, G.; McCollom, T.M.; Reeves, E.; Sharkey, J.; You, C.F.; et al. Chemistry of hot springs along the Eastern Lau Spreading Center. Geochim. Cosmochim. Acta 2011, 75, 1013–1038. [Google Scholar] [CrossRef]
- Li, J.; Guo, H. The Geological Characteristics and Formation of Arxan Geothermal system. Heilongjiang Land Resour. 2012, 8, 47. (In Chinese) [Google Scholar]
- Tang, S. Characteristics of the Late Jurassic Volcanic System in Arxan and the Formation Mechanism of Arxan Hot Springs. Jilin Geol. 1984, 3, 54–64. (In Chinese) [Google Scholar]
- Han, X.; Jin, X.; Sun, C. Geothermal Structure of Aershan Hot Springs, Inner Mongolia. AC TA Geosci. Tia Sin. 2001, 22, 259–264. (In Chinese) [Google Scholar]
- GB/T8538-2008. Drinking Natural Mineral Water; China Standards Press: Beijing, China, 2008.
- GB12375-1990. Analytical Method of Tritium in Water; China Standards Press: Beijing, China, 1990.
- Michard, G. Behaviour of major elements and some trace elements (Li, Rb, Cs, Sr, Fe, Mn, W, F) in deep hot waters from granitic areas. Chem. Geol. 1990, 89, 117–134. [Google Scholar] [CrossRef]
- Han, D.M.; Liang, X.; Jin, M.G.; Currell, M.J.; Song, X.F.; Liu, C.M. Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou Basin. J. Volcanol. Geotherm. Res. 2010, 189, 92–104. [Google Scholar] [CrossRef]
- Shakeri, A.; Moore, F.; Kompani-Zare, M. Geochemistry of the thermal springs of Mount Taftan, southeastern Iran. J. Volcanol. Geotherm. Res. 2008, 178, 829–836. [Google Scholar] [CrossRef]
- Müller, G. Diagenesis in Argillaceous Sediments. Dev. Sedimentol. 1967, 8, 127–177. [Google Scholar]
- Schäffer, R.; Sass, I. The thermal springs of Jordan. Environ. Earth Sci. 2013, 72, 171–187. [Google Scholar] [CrossRef]
- Wang, H. An Introduction to Isotopic Hydrogeology; Geological Publishing House: Beijing, China, 1991; p. 191. [Google Scholar]
- Craig, H. Isotopic Variation in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Sun, X.X.; Gu, W.Z.; Tan, H.B.; Rao, W.B.; Dong, H.Z.; Liu, X.Y.; Su, Z.G. Isotopic and hydrochemical data to restrict the origin of the groundwater in the Badain Jaran Desert, Northern China. Geochem. Int. 2012, 50, 455–465. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Su, Y.; Xie, J.; Jia, X.; Hu, Y. δ18O shifts of geothermal waters in the central of Weihe Basin, NW China. Environ. Earth Sci. 2009, 59, 995–1008. [Google Scholar] [CrossRef]
- Capasso, G.; Dongarrà, G.; Favara, R.; Hauser, S.; Valenza, M. Isotope composition of rain water, well water and fumarole steam on the island of Vulcano, and their implications for volcanic surveillance. J. Volcanol. Geotherm. Res. 1992, 49, 147–155. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Thomas, J.; Rose, T. Environmental Isotopes in Hydrogeology; Boca Raton Fla Lewis Publishers: Boca Raton, FL, USA, 1997; Volume 80, p. 532. [Google Scholar]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Ravikumar, P.; Somashekar, R.K. Environmental Tritium (3 H) and hydrochemical investigations to evaluate groundwater in Varahi and Markandeya river basins, Karnataka, India. J. Environ. Radioact. 2011, 102, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Günay, G. Hydrology and hydrogeology of Sakaryabaşı Karstic springs, Çifteler, Turkey. Environ. Geol. 2006, 51, 229–240. [Google Scholar] [CrossRef]
- Çelmen, O.; Çelik, M. Hydrochemistry and environmental isotope study of the geothermal water around Beypazarı granitoids, Ankara, Turkey. Environ. Geol. 2009, 58, 1689–1701. [Google Scholar] [CrossRef]
- Gultekin, F.; Hatipoglu, E.; Ersoy, A.F. Hydrogeochemistry, environmental isotopes and the origin of the Hamamayagi-Ladik thermal spring (Samsun, Turkey). Environ. Earth Sci. 2011, 62, 1351–1360. [Google Scholar] [CrossRef]
- Stanley, W.D. Geothermal systems, principles and case histories. Earth-Sci. Rev. 1982, 18, 91–92. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Li, J.; Liu, M.; Wang, Y.; Yang, Y. Estimation of reservoir temperature using silica and cationic solutes geothermometers: A case study in the Tengchong geothermal area. Chin. J. Geochem. 2015, 34, 233–240. [Google Scholar] [CrossRef]
- Spycher, N.; Peiffer, L.; Sonnenthal, E.L.; Saldi, G.; Reed, M.H.; Kennedy, B.M. Integrated multicomponent solute geothermometry. Geothermics 2014, 51, 113–123. [Google Scholar] [CrossRef]
- Verma, M. Silica solubility geothermometers for hydrothermal systems. In Proceedings of the 10th International Symposium on Water–Rock Interaction, WRI-10, Villasimius, Italy, 10–15 July 2001.
- Dulanya, Z.; Morales-Simfors, N.; Sivertun, Å. Comparative study of the silica and cation geothermometry of the Malawi hot springs: Potential alternative energy source. J. Afr. Earth Sci. 2010, 57, 321–327. [Google Scholar] [CrossRef]
- Pope, L.A.; Hajash, A.; Popp, R.K. An experimental investigation of the quartz, Na-K, Na-K-Ca geothermometers and the effects of fluid composition. J. Volcanol. Geotherm. Res. 1987, 31, 151–161. [Google Scholar] [CrossRef]
- Baioumy, H.; Nawawi, M.; Wagner, K.; Arifin, M.H. Geochemistry and geothermometry of non-volcanic hot springs in West Malaysia. J. Volcanol. Geotherm. Res. 2015, 290, 12–22. [Google Scholar] [CrossRef]
- PHREEQC; version 2.12.5; (Computer File) Water Chemistry, Computer Programs Geochemistry; U.S. Geological Survey: Denver, CO, USA, 1999.
- Surveys, G. User’s Guide to PHREEQC—A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1995.
- Tole, M.P.; Ármannsson, H.; Zhong-He, P.; Arnórsson, S. Fluid/mineral equilibrium calculations for geothermal fluids and chemical geothermometry. Geothermics 1993, 22, 17–37. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Deng, Z.; Fang, B.; Tsutomu, Y.; Zhao, J.; Wang, X. Hydrochemical characteristics and genesis analysis of the Jifei hot spring in Yunnan, southwestern China. Geothermics 2015, 53, 38–45. [Google Scholar] [CrossRef]
- Arnórsson, S. The use of mixing models and chemical geothermometers for estimating underground temperatures in geothermal systems. J. Volcanol. Geotherm. Res. 1985, 23, 299–335. [Google Scholar] [CrossRef]
- Truesdell, A.; Fournier, R. Procedure for estimating temperature of a hot-water component in a mixed water by using a plot of dissolved silica versus enthalpy. J. Res. U.S. Geol. Surv. 1977, 5, 49–52. [Google Scholar]
- Keenan, J.H.; Keyes, F.G.; Hill, P.H.; Moore, J.G. Steam tables. J. Appl. Mech. 1978, 37, 567. [Google Scholar] [CrossRef]
- Gupta, H.K.; Roy, S. Geothermal Energy: An Alternative Resource for the 21st Century; Elsevier Science & Technology: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Fournier, R.O.; Truesdell, A.H. An empirical Na-K-Ca geothermometer for natural waters. Geochim. Cosmochim. Acta 1973, 37, 1543–1550. [Google Scholar] [CrossRef]
| No. | T (°C) | pH | TDS (mg/L) | EC (μS/cm) | DO (mg/L) | Na (mg/L) | K (mg/L) | Ca (mg/L) | Mg (mg/L) | Cl (mg/L) | SO4 (mg/L) | HCO3 (mg/L) | SiO2 (mg/L) | δ18O (‰SMOW) | δ2H (‰SMOW) | Tritium (TU) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I | ||||||||||||||||
| S015 | 10.9 | 7.2 | 295 | 493 | 73 | 68 | 5.8 | 28 | 2.79 | 12 | 54 | 190 | 29 | −14.2 | −111 | 9.6 ± 0.5 |
| S016 | 11.9 | 7.0 | 294 | 515 | 94 | 72 | 2.9 | 27 | 2.43 | 14 | 50 | 193 | 29 | −15.1 | −113.6 | 12.0 ± 0.5 |
| S018 | 12.9 | 7.1 | 382 | 310 | 144 | 86 | 1.8 | 34 | 4.72 | 21 | 60 | 209 | 32 | −14.6 | −111.5 | 11.5 ± 0.5 |
| S025 | 12.9 | 7.0 | 279 | 488 | 76 | 67 | 2.7 | 25 | 1.97 | 16 | 50 | 168 | 32 | −14.5 | −106.2 | 11.2 ± 0.5 |
| Group II | ||||||||||||||||
| S033 | 22.0 | 7.3 | 345 | 567 | 56 | 114 | 2.4 | 8.2 | 0.1 | 17 | 49 | 206 | 35 | −15.0 | −115.0 | 8.2 ± 0.5 |
| S039 | 23.0 | 7.2 | 380 | 606 | 58 | 114 | 3.2 | 18 | 0.9 | 21 | 70 | 216 | 32 | −14.4 | −112.1 | 7.1 ± 0.5 |
| S040 | 22.3 | 7.2 | 403 | 598 | 65 | 116 | 3.0 | 19 | 0.8 | 21 | 68 | 242 | 32 | −15.4 | −113.2 | 6.4 ± 0.4 |
| S050 | 23.8 | 7.5 | 572 | 857 | 83 | 183 | 3.6 | 19 | 0.87 | 28 | 80 | 371 | 39 | −14.6 | −111.6 | 9.8 ± 0.5 |
| S055 | 24.0 | 7.9 | 505 | 858 | 87 | 169 | 3.7 | 11 | 0.14 | 28 | 100 | 299 | 44 | n.a. | n.a. | n.a. |
| S029 | 24.0 | 7.4 | 536 | 775 | 31 | 182 | 2.7 | 10 | 0.01 | 28 | 112 | 308 | 38 | −15.0 | −113.3 | 5.0 ± 0.4 |
| S052 | 28.2 | 7.4 | 505 | 823 | 45 | 164 | 3.3 | 11 | 0.09 | 26 | 70 | 302 | 47 | −14.7 | −113.6 | 3.7 ± 0.4 |
| S049 | 29.5 | 7.5 | 548 | 843 | 38 | 176 | 3.2 | 15 | 0.00 | 31 | 95 | 313 | 38 | −14.4 | −114.2 | 3.4 ± 0.4 |
| S044 | 30.1 | 7.4 | 446 | 744 | 40 | 151 | 2.7 | 8.7 | 0.03 | 21 | 66 | 280 | 41 | −13.7 | −112.8 | 4.4 ± 0.4 |
| S048 | 34.0 | 7.5 | 463 | 760 | 25 | 155 | 3.2 | 9.2 | 0.03 | 24 | 75 | 302 | 45 | n.a. | n.a. | n.a. |
| S032 | 35.0 | 7.4 | 556 | 909 | 5.9 | 182 | 3.4 | 11 | 0.23 | 30 | 97 | 336 | 45 | −14.9 | −115.4 | 3.5 ± 0.4 |
| S046 | 41.0 | 7.4 | 530 | 807 | 5.8 | 183 | 3.0 | 10 | 0.01 | 26 | 98 | 321 | 40 | −14.7 | −114.2 | 1.3 ± 0.4 |
| Group III | ||||||||||||||||
| Z001 | 6.0 | 6.8 | 175 | 177 | 92 | 25 | 0.6 | 20 | 1.09 | 6.9 | 53 | 55 | 21 | n.a. | n.a. | n.a. |
| Z002 | 15.9 | 6.6 | 173 | 173 | 65 | 12 | 1.1 | 19 | 1.93 | 6.9 | 13 | 72 | 24 | n.a. | n.a. | n.a. |
| Z003 | 8.9 | 8.2 | 136 | 136 | 242 | 7 | 1.9 | 16 | 2.70 | 8.7 | 8 | 56 | 18 | n.a. | n.a. | n.a. |
| Z004 | 8.7 | 7.3 | 85 | 77 | 219 | 4.5 | 1.6 | 11 | 1.48 | 0.1 | 10 | 44 | 17 | n.a. | n.a. | n.a. |
| Group IV | ||||||||||||||||
| Z005 | 9.7 | 7.8 | 768 | 766 | 192 | 60 | 2.5 | 55 | 10.3 | 33 | 110 | 125 | 30 | n.a. | n.a. | n.a. |
| Samples | Na/Cl (Mean) | K/Cl (Mean) | Ca/Mg (Mean) | SO4/Cl (Mean) | HCO3/Cl (Mean) |
|---|---|---|---|---|---|
| Mineral springs (Group I) | 4.8 | 0.2 | 10.3 | 3.5 | 12.5 |
| Thermal springs (Group II) | 6.3 | 0.1 | 95.1 | 3.3 | 11.6 |
| Shallow groundwater (Group III) | 1.7 | 0.2 | 10.3 | 3.0 | 7.7 |
| Deep groundwater (Group IV) | 1.8 | 0.1 | 5.3 | 3.4 | 3.8 |
| Sample | Temperature (°C) | Na–K | K–Mg | Chalcedony | Quartz No-Steam Loss | Quartz-Max, Steam Loss |
|---|---|---|---|---|---|---|
| S033 | 22.0 | 107.4 | 87.6 | 54.9 | 85.9 | 88.7 |
| S039 | 23.0 | 102.1 | 68.8 | 50.9 | 82.1 | 85.4 |
| S040 | 22.3 | 116.7 | 68.9 | 50.9 | 82.1 | 85.4 |
| S050 | 23.8 | 103.4 | 71.7 | 59.9 | 90.6 | 92.8 |
| S055 | 24.0 | 108.1 | 95.2 | 65.6 | 95.9 | 97.5 |
| S029 | 24.0 | 91.9 | 100.1 | 58.7 | 89.4 | 91.8 |
| S032 | 35.0 | 101.0 | 86.5 | 66.7 | 97.0 | 98.4 |
| S044 | 30.1 | 100.0 | 108.4 | 62.2 | 92.8 | 94.7 |
| S046 | 41.0 | 95.5 | 127.0 | 61.1 | 91.7 | 93.8 |
| S048 | 34.0 | 106.2 | 113.2 | 66.7 | 97.0 | 98.4 |
| S049 | 29.5 | 100.9 | 130.0 | 58.7 | 89.4 | 91.8 |
| S052 | 28.2 | 104.6 | 98.2 | 68.8 | 98.9 | 100.1 |
| No. | Anhydrite | Aragonite | Calcite | Chalcedony | Dolomite | Gypsum | Halite | Quartz | SiO2(a) |
|---|---|---|---|---|---|---|---|---|---|
| Group I | |||||||||
| S015 | −2.41 | −0.88 | −0.73 | 0.41 | −2.29 | −2.15 | −7.61 | 0.88 | −0.48 |
| S016 | −2.45 | −1.04 | −0.89 | 0.39 | −2.67 | −2.19 | −7.53 | 0.87 | −0.49 |
| S018 | −2.31 | −0.89 | −0.74 | 0.42 | −2.16 | −2.05 | −7.29 | 0.89 | −0.46 |
| S025 | −2.47 | −1.13 | −0.98 | 0.42 | −2.89 | −2.22 | −7.50 | 0.89 | −0.46 |
| Group II | |||||||||
| S033 | −2.96 | −1.15 | −1.01 | 0.35 | −3.62 | −2.73 | −7.26 | 0.79 | −0.50 |
| S039 | −2.49 | −0.87 | −0.72 | 0.30 | −2.43 | −2.26 | −7.19 | 0.74 | −0.55 |
| S040 | −2.49 | −0.82 | −0.67 | 0.31 | −2.41 | −2.26 | −7.18 | 0.75 | −0.54 |
| S050 | −2.47 | −0.32 | −0.17 | 0.38 | −1.36 | −2.24 | −6.88 | 0.81 | −0.47 |
| S055 | −2.60 | −0.30 | −0.15 | 0.42 | −1.87 | −2.37 | −6.91 | 0.86 | −0.42 |
| S029 | −2.59 | −0.73 | −0.59 | 0.36 | −3.85 | −2.37 | −6.88 | 0.80 | −0.48 |
| S032 | −2.54 | −0.53 | −0.39 | 0.31 | −0.88 | −2.37 | −7.21 | 0.71 | −0.49 |
| S044 | −2.83 | −0.77 | −0.63 | 0.33 | −3.32 | −2.62 | −7.07 | 0.74 | −0.5 |
| S046 | −2.55 | −0.52 | −0.39 | 0.2 | −0.84 | −2.41 | −6.91 | 0.58 | −0.59 |
| S048 | −2.64 | −0.57 | −0.43 | 0.32 | −2.92 | −2.56 | −7.02 | 0.73 | −0.49 |
| S049 | −2.48 | −0.42 | −0.28 | 0.3 | −4.35 | −2.28 | −6.84 | 0.72 | −0.52 |
| S052 | −2.74 | −0.68 | −0.54 | 0.41 | −2.78 | −2.53 | −6.94 | 0.83 | −0.42 |
| Group III | |||||||||
| Z001 | −2.48 | −1.93 | −1.77 | 0.33 | −4.75 | −2.22 | −8.27 | 0.82 | −0.58 |
| Z002 | −3.08 | −1.93 | −1.78 | 0.26 | −4.31 | −2.84 | −8.61 | 0.72 | −0.61 |
| Z003 | −3.34 | −0.61 | −0.46 | 0.22 | −1.58 | −3.09 | −8.71 | 0.70 | −0.68 |
| Z004 | −3.35 | −1.75 | −1.59 | 0.20 | −3.97 | −3.10 | −10.84 | 0.69 | −0.70 |
| Group IV | |||||||||
| Z005 | −1.88 | −0.24 | −0.08 | 0.44 | −0.78 | −1.63 | −6.97 | 0.91 | −0.46 |
| No. | Outlet Temperature (°C) | Reservoir Temperature (°C) | Mixing Ratio of Cold Water |
|---|---|---|---|
| S046 | 41.0 | 82.0 | 0.54 |
| S033 | 22.0 | 114.0 | 0.83 |
| S040 | 22.3 | 93.0 | 0.79 |
| S039 | 23.0 | 87.0 | 0.76 |
| S050 | 23.8 | 136.0 | 0.86 |
| S029 | 24.0 | 122.0 | 0.83 |
| S055 | 24.0 | 165.0 | 0.87 |
| S052 | 28.2 | 150.0 | 0.84 |
| S049 | 29.5 | 103.0 | 0.74 |
| S044 | 30.1 | 118.0 | 0.78 |
| S048 | 34.0 | 122.0 | 0.76 |
| S032 | 35.0 | 116 | 0.72 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Zhang, Q.; Cui, Y.; Shao, J.; Xiao, Y.; Zhang, P.; Liu, J. Hydrogeochemistry and Genesis Analysis of Thermal and Mineral Springs in Arxan, Northeastern China. Water 2017, 9, 61. https://doi.org/10.3390/w9010061
Gu X, Zhang Q, Cui Y, Shao J, Xiao Y, Zhang P, Liu J. Hydrogeochemistry and Genesis Analysis of Thermal and Mineral Springs in Arxan, Northeastern China. Water. 2017; 9(1):61. https://doi.org/10.3390/w9010061
Chicago/Turabian StyleGu, Xiaomin, Qiulan Zhang, Yali Cui, Jingli Shao, Yong Xiao, Peng Zhang, and Jinxing Liu. 2017. "Hydrogeochemistry and Genesis Analysis of Thermal and Mineral Springs in Arxan, Northeastern China" Water 9, no. 1: 61. https://doi.org/10.3390/w9010061






