Effects of Climate, Limnological Features and Watershed Clearcut Logging on Long-Term Variation in Zooplankton Communities of Boreal Shield Lakes
Abstract
:1. Introduction
2. Materials and Methods
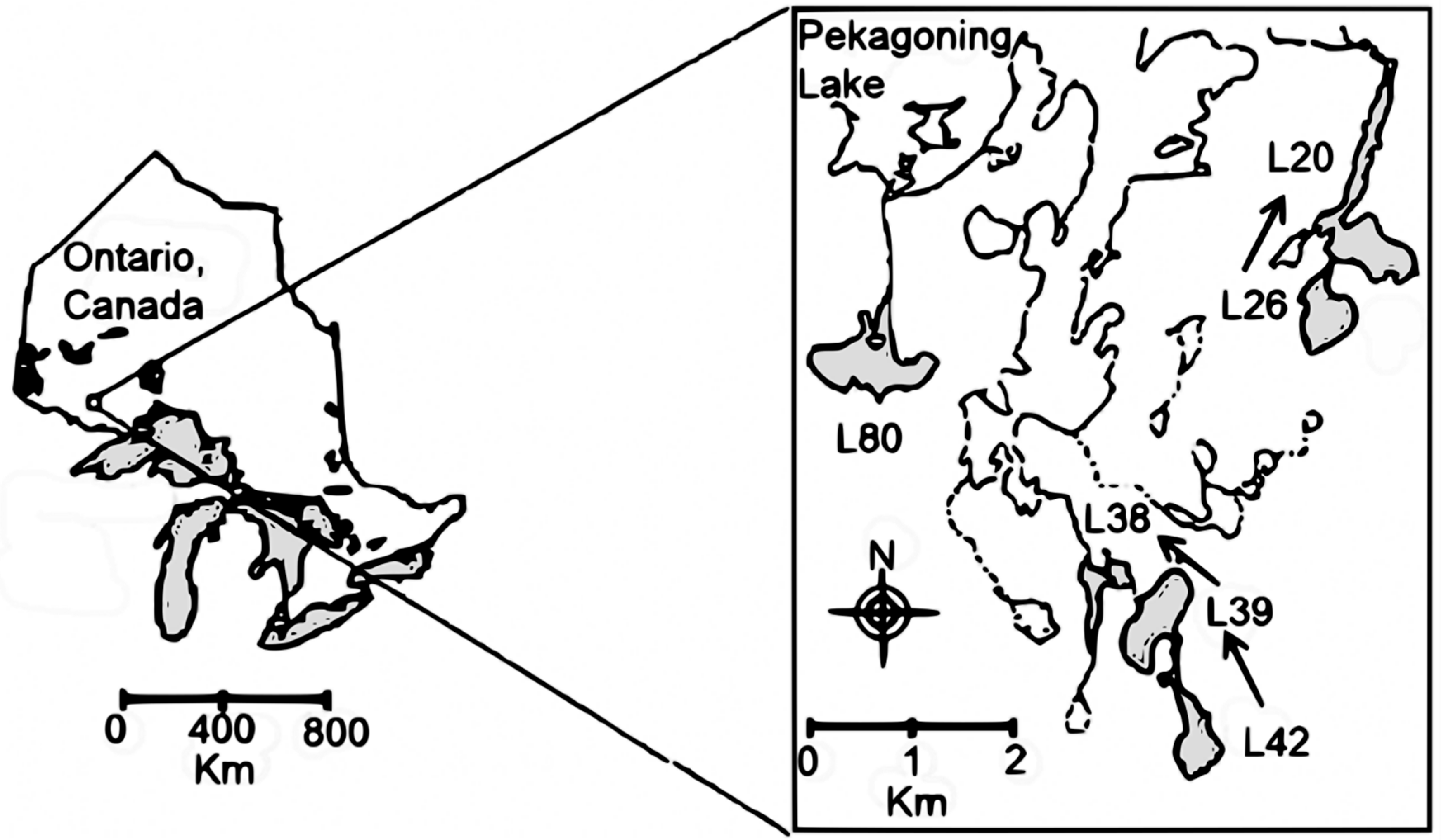
2.1. Study Area, Multiple Before/After-Control-Impact (MBACI) Experimental Logging Design and Limnological Features
2.2. Zooplankton Sampling and Analysis
2.3. Statistical Analysis
2.3.1. Natural Environmental Heterogeneity and Logging Disturbance Impact
All Lakes Grouped
Two Groups: Undisturbed and Harvested Lakes
2.3.2. Influence of Natural Environmental Variation and Clearcut Logging on Zooplankton Community
2.3.3. Analysis of Zooplankton Stability through Time in Individual Lakes
3. Results
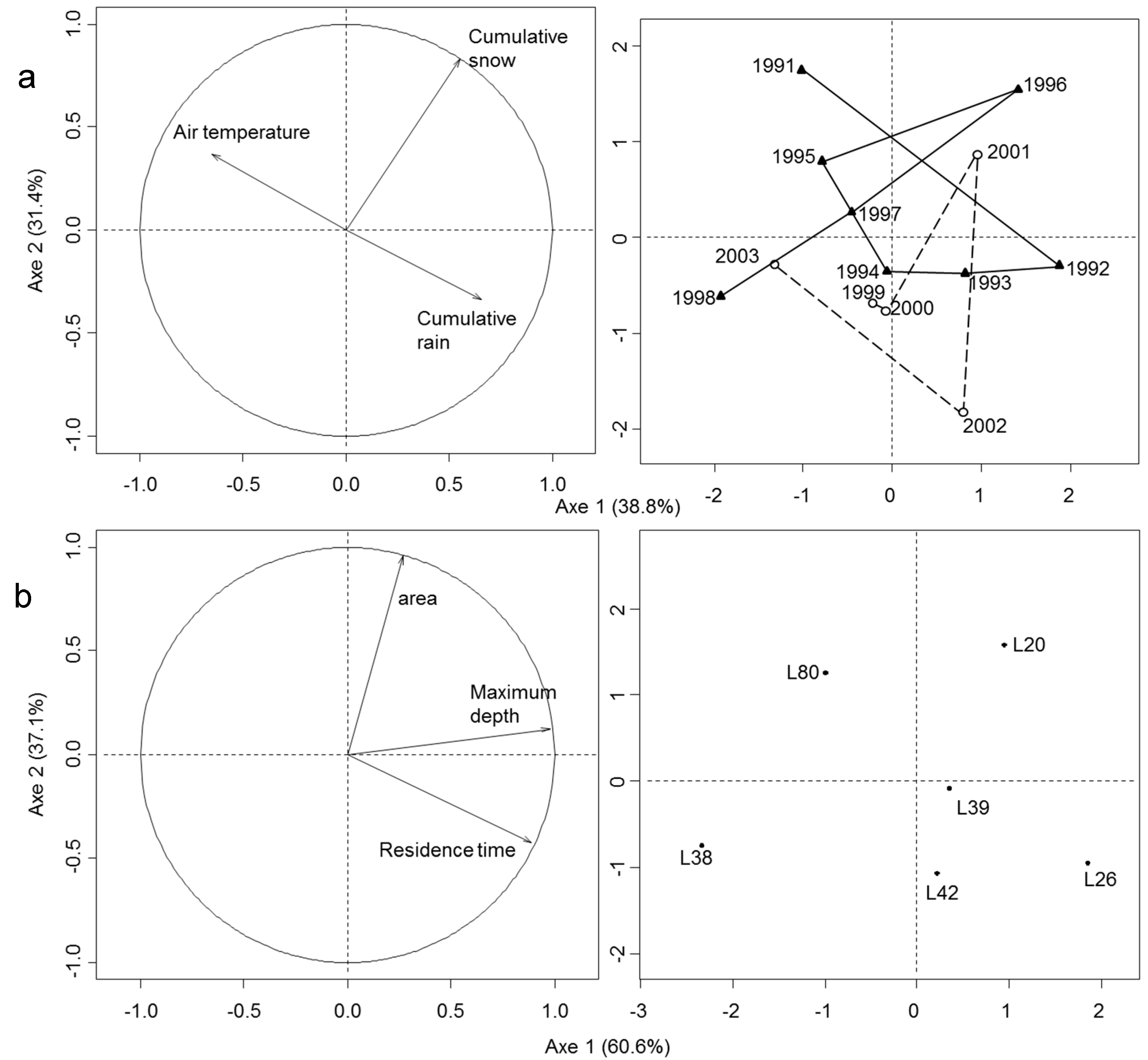
3.1. Space/Time Variation in Climate and Limnological Features
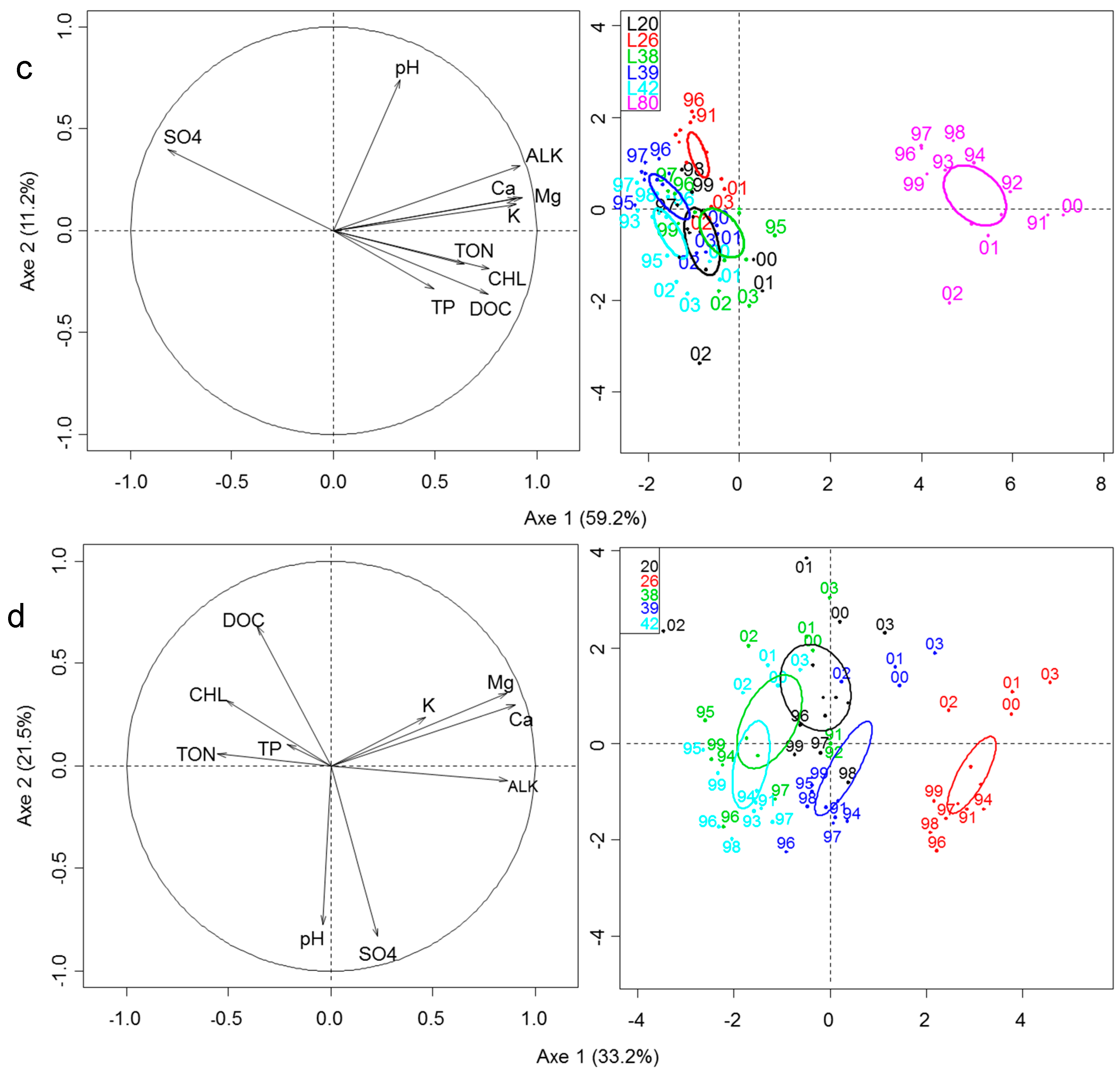
3.2. Space-Time Variation in Zooplankton Community
3.3. Space-Time Interaction in Harvested and Undisturbed Lakes
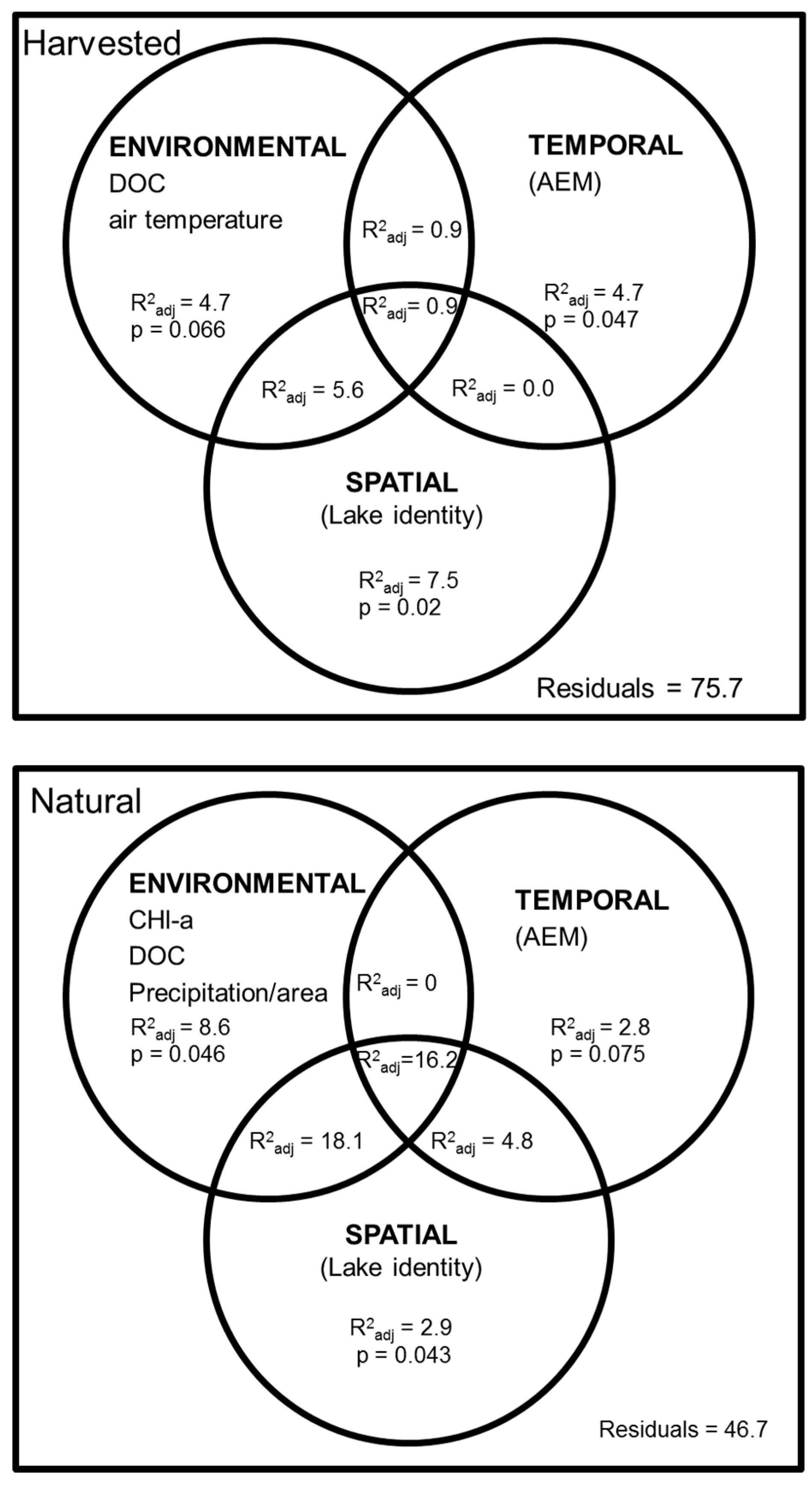
3.4. Influence of Environment and Logging Disturbance on Zooplankton Space-Time Variation
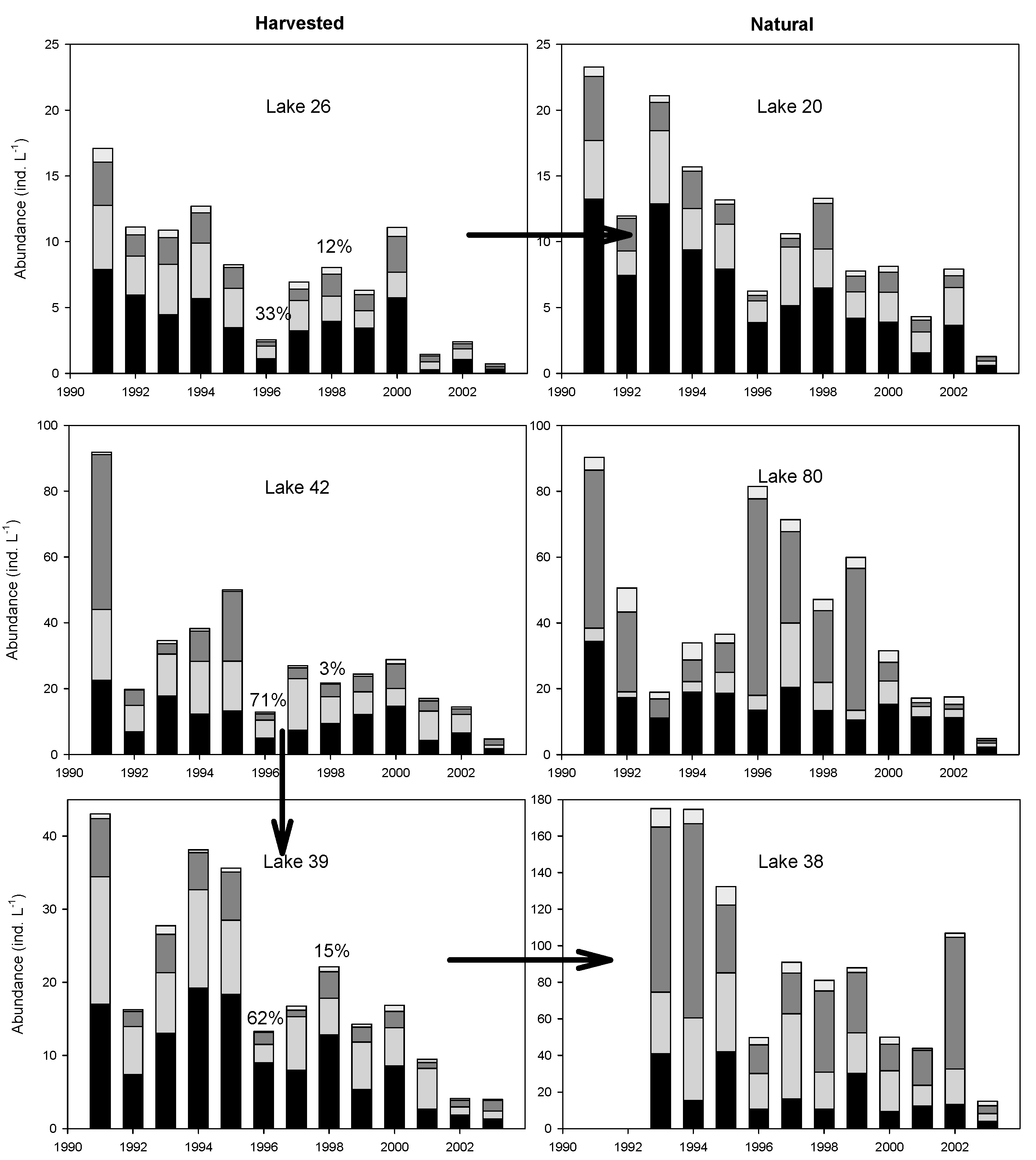
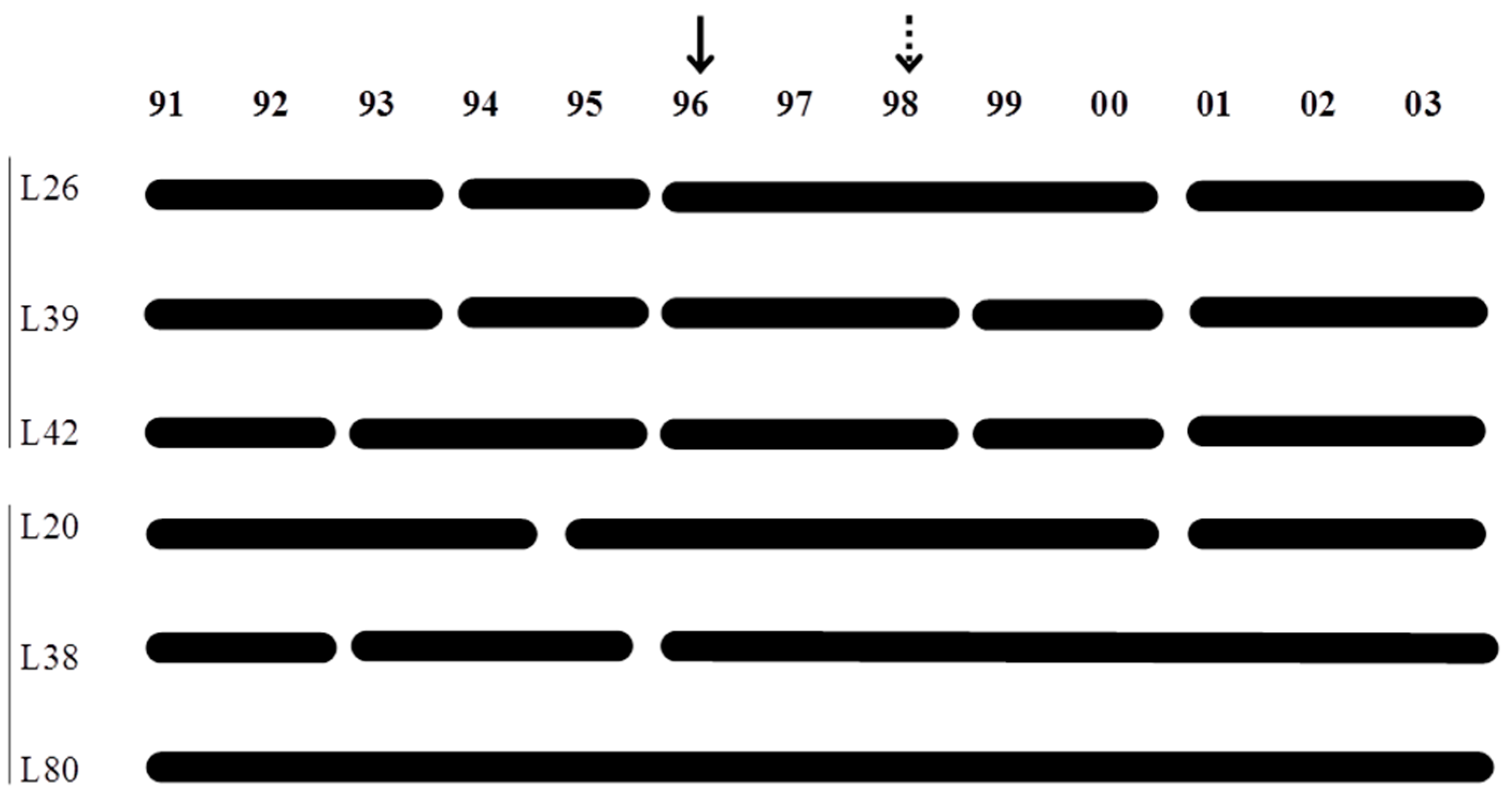
3.5. Temporal Patterns of Variation in Zooplankton Abundance in Each Lake
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carignan, R.; Steedman, R.J. Impacts of major watershed perturbations on aquatic ecosystems. Can. J. Fish. Aquat. Sci. 2000, 57, 1–4. [Google Scholar] [CrossRef]
- Schindler, D.W. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millenium. Can. J. Fish. Aquat. Sci. 2001, 58, 18–29. [Google Scholar] [CrossRef]
- Kernan, M.; Battarbee, R.W.; Moss, B. Climate Change Impacts on Freshwater Ecosystems; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Adrian, R.; O’Reilly, C.M.; Zagarese, H.; Baines, S.B.; Hessen, D.O.; Keller, W.; Livingstone, D.M.; Sommaruga, R.; Straile, D.; Van Donk, E.; et al. Lakes as sentinels of climate change. Limnol. Oceanogr. 2009, 54, 2283–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, C.E.; Saros, J.E.; Vincent, W.F.; Smol, J.P. Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol. Oceanogr. 2009, 54, 2273–2282. [Google Scholar] [CrossRef]
- Moss, B. Cogs in the endless machine: Lakes, climate change and nutrient cycles: A review. Sci. Total Environ. 2012, 434, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Stemberger, R.S.; Larsen, D.P.; Kincaid, T.M. Sensitivity of zooplankton for regional lake monitoring. Can. J. Fish. Aquat. Sci. 2001, 58, 2222–2232. [Google Scholar] [CrossRef]
- Kreutzweiser, D.; Beall, F.; Webster, K.; Thompson, D.; Creed, I. Impacts and prognosis of natural resource development on aquatic biodiversity in Canada’s boreal zone. Environ. Rev. 2013, 21, 227–259. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Lazorchak, J.M. Zooplankton assemblage responses to disturbance gradients. Can. J. Fish. Aquat. Sci. 1994, 51, 2435–2447. [Google Scholar] [CrossRef]
- Christensen, M.R.; Graham, M.D.; Vinebrooke, R.D.; Findlay, D.L.; Paterson, M.J.; Turner, M.A. Multiple anthropogenic stressors cause ecological surprises in lakes. Glob. Chang. Biol. 2006, 12, 2316–2322. [Google Scholar] [CrossRef]
- Prepas, E.E.; Pinel-Alloul, B.; Planas, D.; Méthot, G.; Paquet, S.; Reedyk, S. Forest harvest impacts on water quality and aquatic biota on the Boreal Plain: Introduction to the TROLS Lake Program. Can. J. Fish. Aquat. Sci. 2001, 58, 421–436. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Prepas, E.; Planas, D.; Steedman, R.; Charette, T. Watershed impacts of logging and wildfire: Case studies in Canada. Lake Reserv. Manag. 2002, 18, 307–318. [Google Scholar] [CrossRef]
- Schindler, D.W. Replication versus realism: The need for ecosystem-scale experiments. Ecosystems 1998, 1, 323–334. [Google Scholar] [CrossRef]
- Carpenter, S.; Cole, J.J.; Essington, T.E.; Hodgson, J.R.; Houser, J.N.; Kitchell, J.F.; Pace, M.L. Evaluating alternative explanations in ecosystem experiments. Ecosystems 1998, 1, 335–344. [Google Scholar] [CrossRef]
- Özkan, K.; Jeppesen, E.; Davidson, T.A.; Bjerring, R.; Johansson, L.S.; Søndergaard, M.; Lauridsen, T.L.; Svenning, J.-C. Long-term trends and temporal synchrony in plankton richness, diversity and biomass driven by re-oligotrophication and climate across 17 Danish lakes. Water 2016, 8, 427. [Google Scholar] [CrossRef]
- Steedman, R.J. Effects of experimental clearcut logging on water quality in three small boreal forest lake trout (Salvelinus namaycush) lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 92–96. [Google Scholar] [CrossRef]
- Winkler, G.; Leclerc, V.; Sirois, P.; Archambault, P.; Berube, P. Short-term impact of forest harvesting on water quality and zooplankton communities in oligotrophic headwater lakes of the eastern Canadian Boreal Shield. Boreal Environ. Res. 2009, 14, 323–337. [Google Scholar]
- Rask, M.; Nyberg, K.; Markkanen, S.-L.; Ojala, A. Forestry in catchments: Effects on water quality, plankton, zooplankton and fish in small lakes. Boreal Environ. Res. 1998, 3, 75–86. [Google Scholar]
- Carignan, R.; D’Arcy, P.; Lamontagne, S. Comparative impacts of fire and forest harvesting on water quality in Boreal Shield lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 105–117. [Google Scholar] [CrossRef]
- Planas, D.; Desrosiers, M.; Groulx, S.-R.; Paquet, S.; Carignan, R. Pelagic and benthic algal responses in eastern Canadian Boreal Shield lakes following harvesting and wildfires. Can. J. Fish. Aquat. Sci. 2000, 57, 136–145. [Google Scholar] [CrossRef]
- Patoine, A.; Pinel-Alloul, B.; Prepas, E.E.; Carignan, R. Do logging and forest fires influence zooplankton biomass in Canadian Boreal Shield lakes? Can. J. Fish. Aquat. Sci. 2000, 57, 155–164. [Google Scholar] [CrossRef]
- Patoine, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of catchment perturbations by logging and wildfires on zooplankton species richness and composition in Boreal Shield lakes. Freshw. Biol. 2002, 47, 1996–2014. [Google Scholar] [CrossRef]
- Patoine, A.; Pinel-Alloul, B.; Prepas, E.E. Influence of catchment deforestation by logging and natural forest fires on crustacean community size structure in lakes of the Eastern Boreal Canadian forest. J. Plankton. Res. 2002, 24, 601–616. [Google Scholar] [CrossRef]
- Steedman, R.J.; Kushneriuk, R. Effects of experimental claercut logging on thermal stratification, dissolved oxygen, and lake trout (Salvelinus namaycush) habitat volume in three small boreal forest lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 82–91. [Google Scholar] [CrossRef]
- Steedman, R.J. Littoral fish response to experimental logging around small Boreal Shield lakes. N. Am. J. Fish. Manag. 2003, 23, 392–403. [Google Scholar] [CrossRef]
- Nicholls, K.H.; Steedman, R.J.; Carney, E.C. Changes in phytoplankton communities following logging in the drainage basins of three boreal forest lakes in northwestern Ontario (Canada), 1991–2000. Can. J. Fish. Aquat. Sci. 2003, 60, 43–54. [Google Scholar] [CrossRef]
- Tonn, W.M.; Paszkowski, C.A.; Scrimgeour, G.J.; Aku, P.K.M.; Lange, M.; Prepas, E.E.; Wescott, K. Effects of forest harvesting and fire on fish assemblages in Boreal Plain lakes: A reference condition approach. Trans. Am. Fish. Soc. 2003, 132, 514–523. [Google Scholar] [CrossRef]
- Leclerc, V.; Sirois, P.; Bérubé, P. Impact of forest harvesting on larval and juvenile growth of yellow perch (Perca flavescens) in boreal lakes. Boreal Environ. Res. 2011, 16, 417–429. [Google Scholar]
- Rask, M.; Arvola, L.; Salonen, K. Effects of catchment deforestation and burning on the limnology of a small forest in southern Finland. Verh. Int. Ver. Limnol. 1993, 25, 525–528. [Google Scholar]
- Glaz, P.; Sirois, P.; Archambault, P.; Nozais, C. Impact of Forest Harvesting on Trophic Structure of Eastern Canadian Boreal Shield Lakes: Insights from Stable Isotope Analyses. PLoS ONE 2014, 9, e96143. [Google Scholar] [CrossRef] [PubMed]
- Pinel-Alloul, B.; Niyosenga, T.; Legendre, P. Spatial and environmental components of freshwater zooplankton structure. Ecoscience 1995, 2, 1–19. [Google Scholar] [CrossRef]
- Bennett, L.T.; Adams, M.A. Assessment of ecological effects due to forest harvesting: Approach and statistical issues. J. Appl. Ecol. 2004, 41, 585–598. [Google Scholar] [CrossRef]
- Håkanson, L. Lumbering operations, lake humidification and consequences for the structure of the lake foodweb: A case study using the LakeWeb-model for Lake Stora Kröntjärn, Sweden. Aquat. Sci. 2002, 64, 185–197. [Google Scholar] [CrossRef]
- Rusak, J.A.; Yan, N.D.; Somers, K.M.; Cottingham, K.L.; Micheli, F.; Carpenter, S.; Frost, T.M.; Paterson, M.J.; McQueen, D.J. Temporal, spatial, and taxonomic patterns of crustacean zooplankton variability in unmanipulated north-temperate lakes. Limnol. Oceanogr. 2002, 47, 613–625. [Google Scholar] [CrossRef]
- Schindler, D.W. Experimental perturbations of whole lakes as tests of hypotheses concerning ecosystem structure and function. Oikos 1990, 57, 25–41. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L. Beyond BACI: Optimization of environmental sampling designs: Through monitoring and simulation. Ecol. Appl. 2001, 11, 783–799. [Google Scholar] [CrossRef]
- Underwood, A.J. On beyond BACI: Sampling designs that might reliably detect environmental disturbances. Ecol. Appl. 1994, 4, 3–15. [Google Scholar] [CrossRef]
- Stewart-Oaten, A.; Bence, J.R. Temporal and spatial variation in environmental impact assessment. Ecol. Monogr. 2001, 71, 305–339. [Google Scholar] [CrossRef]
- Ward, J. A description of new zooplankton counter. Q. J. Microsc. Sci. 1955, 96, 371–373. [Google Scholar]
- Edmondson, W.T. Rotifera. In Freshwater Biology; Ward, H.B., Whipple, G.C., Eds.; John Wiley and Sons: New York, NY, USA, 1959; pp. 420–494. [Google Scholar]
- Stemberger, R.S. A Guide to Rotifers of the Laurentian Great Lakes; EPA-600/4-79-021; Environmental Monitoring and Support Laboratory, Office of Research and Development, Environmental Protection Agency: Washington, DC, USA, 1979; 185p. [Google Scholar]
- Chengalath, R.; Koste, W. Rotifera from Northwestern Canada. Hydrobiologia 1987, 147, 49–56. [Google Scholar] [CrossRef]
- Nogrady, T.; Pourriot, R.; Segers, H. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; SPB Academic Publishing: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Brooks, J.L. The systematics of North American Daphnia. Mem. Conn. Acad. Art Sci. 1957, 13, 1–180. [Google Scholar]
- Brooks, J.L. Cladocera. In Freshwater Biology; Ward, H.B., Whipple, G.C., Eds.; John Wiley and Sons: New York, NY, USA, 1959; pp. 587–656. [Google Scholar]
- Brandlova, J.; Brandl, Z.; Fernando, C.H. The Cladocera of Ontario with remarks on some species and distribution. Can. J. Zool. 1972, 50, 1373–1403. [Google Scholar] [CrossRef]
- Amoros, C. Introduction Pratique à la Systématique des Eaux Continentales Françaises. 5. Crustacés Cladocères; Association Française de Limnologie: Paris, France, 1984. [Google Scholar]
- De Melo, R.; Hebert, P.D.N. A taxonomic reevaluation of North American Bosminidae. Can. J. Zool. 1994, 72, 1808–1825. [Google Scholar] [CrossRef]
- Hebert, P.D.N. The Daphnia of North America: An Illustrated Fauna. CD-ROM; University of Guelph: Guelph, ON, Canada, 1995. [Google Scholar]
- Smith, K.; Fernando, C.H. A Guide to the Freshwater Calanoid and Cyclopoid Copepod Crustacea of Ontario; Biology Series; University of Waterloo: Waterloo, ON, Canada, 1978; 74p. [Google Scholar]
- Dussart, B. Les Copépodes des eaux Continentales d’Europe Occidentale. Tome I. Calanoïdes et Harpacticoïdes; Editions N Boublée & Cie: Paris, France, 1967. [Google Scholar]
- Dussart, B. Les Copépodes des eaux Continentales d’Europe occidentale. Tome II. Cyclopoïdes et Biologie Quantitative; Editions N Boublée & Cie: Paris, France, 1969. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Legendre, P.; Gauthier, O. Statistical methods for temporal and space-time analysis of community composition data. Proc. R. Soc. B-Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth International Group: Belmont, CA, USA, 1984. [Google Scholar]
- Legendre, P.; De Caceres, M.; Borcard, D. Community surveys through space and time: Testing the space-time interaction in the absence of replication. Ecology 2010, 91, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Modelling directional spatial processes in ecological data. Ecol. Model. 2008, 215, 325–336. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; ISBN 3-900051-07-0. [Google Scholar]
- Husson, F.; Josse, J.; Le, S.; Mazet, J. Multivariate Exploratory Data Analysis and Data Mining with R. 2010. Available online: http://cran.r-project.org/web/packages/FactoMineR/index.html (accessed on 15 August 2016).
- Oksanen, J.; Blanchet, G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-10. 2013. Available online: http://cran.r-project.org/package=vegan (accessed on 15 August 2016).
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer Science: New York, NY, USA, 2011. [Google Scholar]
- Schindler, D.W.; Bayley, S.E.; Parker, B.R.; Beaty, K.G.; Cruikshank, D.R.; Fee, E.J.; Schindler, E.U.; Stainton, M.P. The effects of climate warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Ontario. Limnol. Oceanogr. 1996, 41, 1004–1017. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Méthot, G.; Verreault, G.; Vigneault, Y. Zooplankton species associations in Quebec lakes: Variation with abiotic factors, including natural and anthropogenic acidification. Can. J. Fish. Aquat. Sci. 1990, 47, 110–121. [Google Scholar] [CrossRef]
- Pinto-Coelho, R.; Pinel-Alloul, B.; Méthot, G.; Havens, K.E. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: Variation with trophic status. Can. J. Fish. Aquat. Sci. 2005, 62, 348–361. [Google Scholar] [CrossRef]
- Prepas, E.E.; Pinel-Alloul, B.; Steedman, R.J.; Planas, D.; Charette, T. Impacts of forest disturbance on boreal surface waters in Canada. Chapter 10; In Towards Sustainable Management of the Boreal Forest; Burton, P.J., Messier, C., Smith, D.W., Adamowics, W.L., Eds.; NRC Research Press: Ottawa, ON, Canada, 2003. [Google Scholar]
- Price, D.T.; Alfaro, R.I.; Brown, K.J.; Falnnigan, M.D.; Fleming, R.A.; Hogg, E.H.; Girardin, M.P.; Lakista, T.; Johnston, M.; McKenney, D.W.; et al. Anticipating the consequences of climate for Canada’s boreal forest ecosystems. Environ. Rev. 2013, 21, 332–365. [Google Scholar] [CrossRef]
| Limnological Features | Undisturbed Lakes | Harvested Lakes | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Morphometry/Hydrology | ||||
| Max depth (m) | 16 ± 15 | 3–32 | 26 ± 10 | 18–37 |
| Mean depth (m) | 9 ± 6 | 2–13 | 9 ± 3 | 6–12 |
| Lake perimeter (km) | 5.1 ± 2.2 | 3.1–7.5 | 3.0 ± 0.5 | 2.5–3.4 |
| Lake volume (106 m3) | 4.2 ± 5.0 | 0.4–9.8 | 3.6 ± 1.3 | 2.2–4.6 |
| Lake elevation (m) | 421 ± 12 | 411–435 | 433 ± 11 | 420–440 |
| Lake surface (ha) | 46 ± 24 | 18–64 | 31 ± 7 | 26–39 |
| Drainage basin (ha) | 386 ± 127 | 300–532 | 123 ± 64 | 70–194 |
| Water renewal time (years−1) | 3.0 ± 3.0 | 0.5–6.3 | 10.6 ± 2.5 | 8.2–13.1 |
| Water quality | ||||
| Alkalinity (mg L−1) | 9.1 ± 4.3 | 3.9–17 | 6.5 ± 1.5 | 4.6–9.4 |
| Potassium K (mg L−1) | 0.37 ± 0.17 | 0.12–0.68 | 0.29 ± 0.06 | 0.10–0.40 |
| Magnesium Mg (mg L−1) | 0.57 ± 0.22 | 0.28–1.03 | 0.43 ± 0.08 | 0.30–0.60 |
| Calcium Ca (mg L−1) | 2.3 ± 1.1 | 0.9–4.6 | 1.8 ± 0.5 | 1.0–2.8 |
| Chlorophyll-a (Chl-a) (µg L−1) | 3.3 ± 2.0 | 1.2–9.0 | 2.2 ± 0.7 | 1.2–4.5 |
| Dissolved organic carbon DOC (mg C L−1) a | 3.9 ± 0.6 | 2.6–5.2 | 2.4 ± 0.4 | 1.8–3.2 |
| pH | 6.7 ± 0.3 | 6.0–7.2 | 6.5 ± 0.3 | 5.9–7.1 |
| Water transparency Secchi (m−1) * | 4.9 ± 1.2 | 2.3–7.0 | 6.0 ± 1.9 | 2.8–8.1 |
| Total organic nitrogen TON (µg N L−1) a | 320 ± 130 | 102–682 | 215 ± 54 | 119–355 |
| Total phosphorus TP (µg P L−1) | 12 ± 7 | 1–39 | 9 ± 4 | 3–22 |
| Sulfates SO4 (mg L−1) a | 1.8 ± 0.5 | 1.0–2.7 | 2.5 ± 0.2 | 2.1–3.1 |
| Groups | Lake | Logging 1996 | Total | Rotifera | Cladocera | Calanoida | Cyclopoida |
|---|---|---|---|---|---|---|---|
| Harvested lakes | L26 | before | 17.0 ± 5.0 | 2.8 ± 1.3 | 0.4 ± 0.2 | 3.7 ± 1.4 | 10.2 ± 2.7 |
| after | 7.4 ± 3.7 | 1.2 ± 1.0 | 0.3 ± 0.1 | 2.3 ± 1.2 | 3.7 ± 1.9 | ||
| L39 | before | 32.2 ± 10.5 | 5.4 ± 2.2 | 0.6 ± 0.4 | 11.2 ± 4.3 | 15.0 ± 4.9 | |
| after | 12.6 ± 6.4 | 1.7 ± 0.9 | 0.4 ± 0.2 | 4.3 ± 2.4 | 6.2 ± 4.1 | ||
| L42 | before | 46.9 ± 27.3 | 17.0 ± 18.2 | 0.6 ± 0.3 | 14.7 ± 4.9 | 14.5 ± 5.9 | |
| after | 18.9 ± 8.1 | 3.5 ± 1.9 | 0.6 ± 0.3 | 7.2 ± 4.2 | 7.6 ± 4.3 | ||
| Undisturbed Lakes | L20 | before | 12.0 ± 3.3 | 2.2 ± 0.7 | 0.6 ± 0.3 | 3.8 ± 0.8 | 5.5 ± 1.7 |
| after | 4.9 ± 3.7 | 1.0 ± 0.9 | 0.3 ± 0.2 | 1.3 ± 0.8 | 2.4 ± 2.0 | ||
| L38 | before | 160.7 ± 24.5 | 77.9 ± 36.2 | 9.4 ± 1.3 | 40.7 ± 6.1 | 32.8 ± 15.1 | |
| after | 65.7 ± 30.8 | 28.2 ± 21.5 | 3.5 ± 1.7 | 20.8 ± 12.2 | 13.3 ± 7.6 | ||
| L80 | before | 46.1 ± 27.2 | 18.7 ± 18.0 | 4.2 ± 2.1 | 3.2 ± 2.3 | 20.0 ± 8.7 | |
| after | 41.4 ± 28.0 | 20.2 ± 22.2 | 2.7 ± 1.3 | 6.2 ± 5.9 | 12.2 ± 5.1 |
| Effects | Harvested Lakes | Undisturbed Lakes | ||
|---|---|---|---|---|
| R2 | p Value | R2 | p Value | |
| Space (Lake) | 0.23 | 0.001 * | 0.55 | 0.001 * |
| Time (Year) | 0.42 | 0.003 * | 0.17 | 0.002 * |
| Space × time | 0.04 | 0.527 | 0.03 | 0.205 |
| Groups | Lake | Logging Intensity (% of Watershed) | Linear Model (Regression) | Non-Linear Model (AEM) | ||
|---|---|---|---|---|---|---|
| P | R2 adj (%) | P | R2 adj (%) | |||
| Harvested Lakes | L26 | 45 | 0.010 | 23.5 | 0.088 | |
| L39 | 77 | 0.405 | 0.260 | |||
| L42 | 74 | 0.489 | 0.040 | 46.8 | ||
| Undisturbed Lakes | L20 | 0 | 0.257 | 0.093 | ||
| L38 | 0 | 0.931 | 0.900 | |||
| L80 | 0 | 0.065 | 0.115 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lévesque, D.; Pinel-Alloul, B.; Méthot, G.; Steedman, R. Effects of Climate, Limnological Features and Watershed Clearcut Logging on Long-Term Variation in Zooplankton Communities of Boreal Shield Lakes. Water 2017, 9, 733. https://doi.org/10.3390/w9100733
Lévesque D, Pinel-Alloul B, Méthot G, Steedman R. Effects of Climate, Limnological Features and Watershed Clearcut Logging on Long-Term Variation in Zooplankton Communities of Boreal Shield Lakes. Water. 2017; 9(10):733. https://doi.org/10.3390/w9100733
Chicago/Turabian StyleLévesque, David, Bernadette Pinel-Alloul, Ginette Méthot, and Robert Steedman. 2017. "Effects of Climate, Limnological Features and Watershed Clearcut Logging on Long-Term Variation in Zooplankton Communities of Boreal Shield Lakes" Water 9, no. 10: 733. https://doi.org/10.3390/w9100733





