Removal of Uranium from Contaminated Water by Clay Ceramics in Flow-Through Columns
Abstract
:1. Introduction
2. Materials and Methods
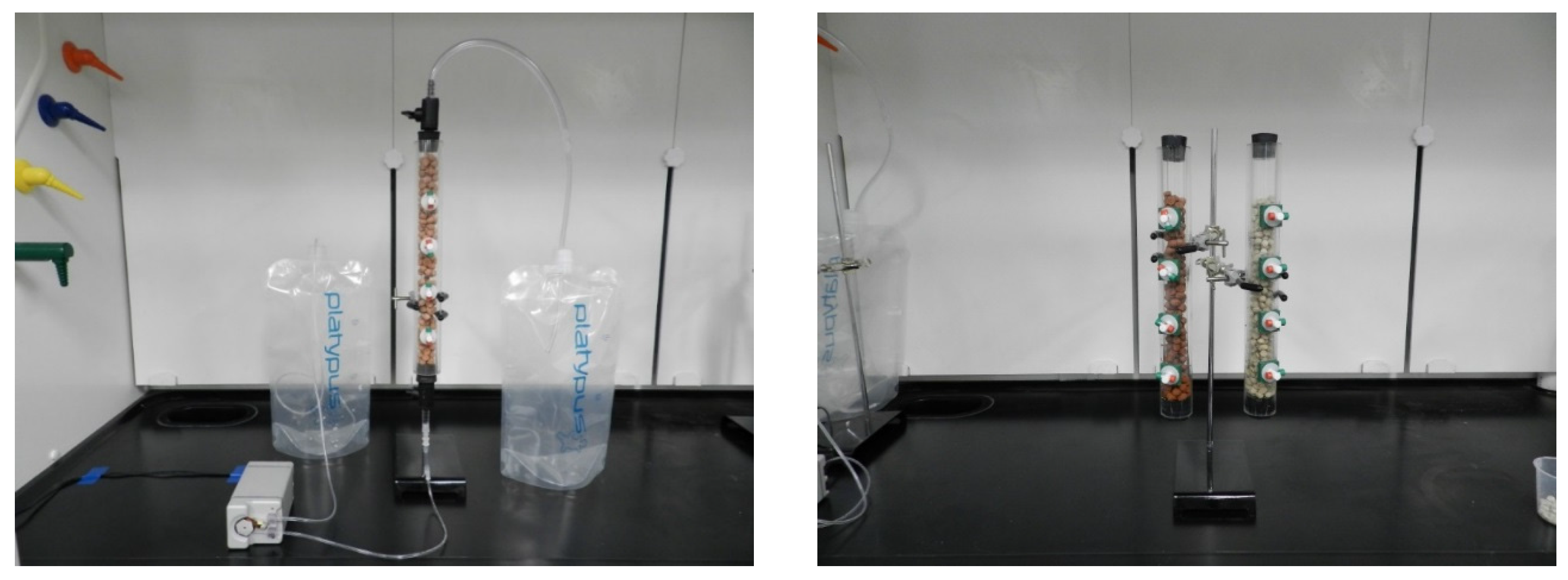
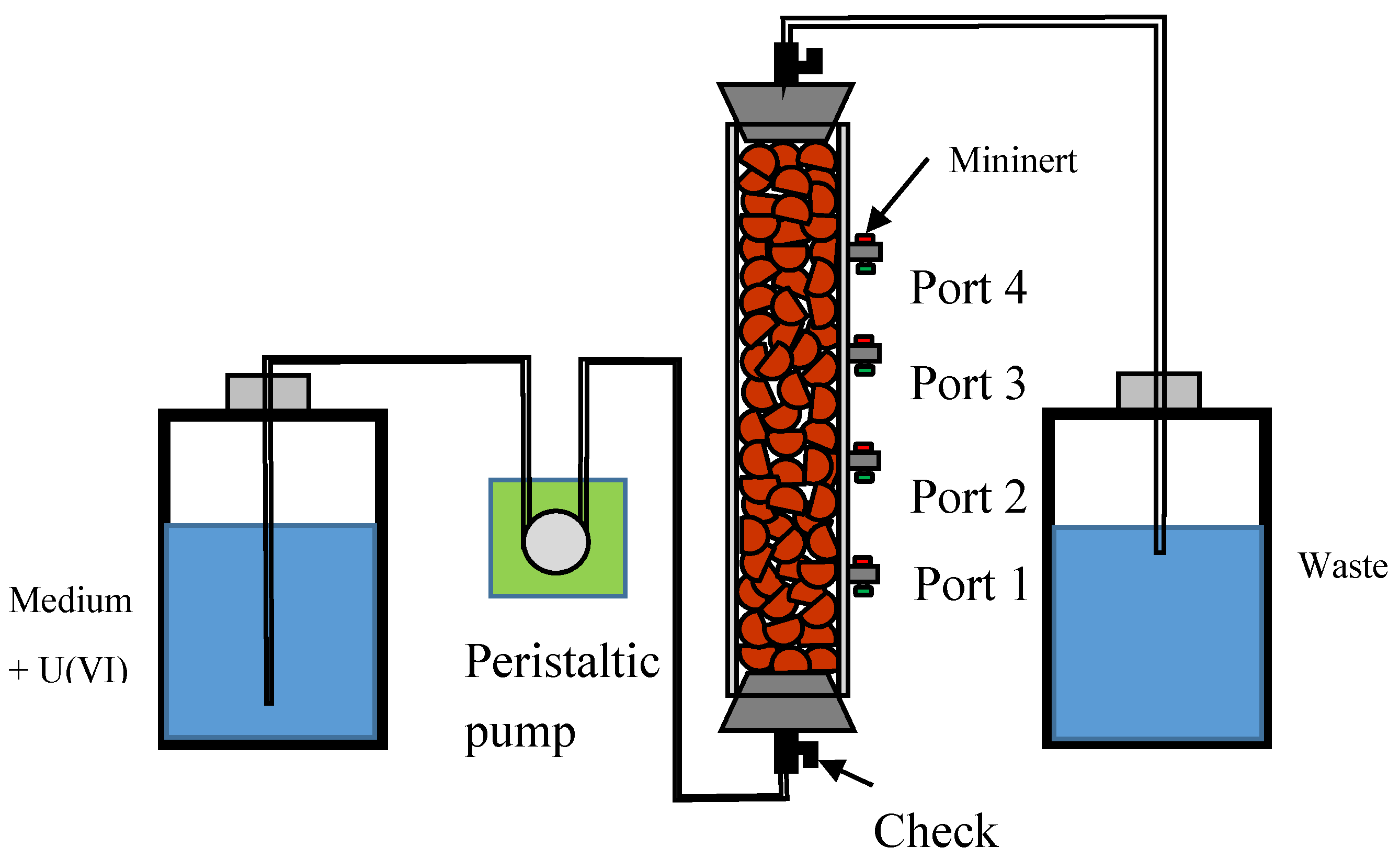
2.1. Fabrication of Clay Pellet and Flow-Through Column
2.2. Experimental Procedure and Sampling
3. Results and Discussion
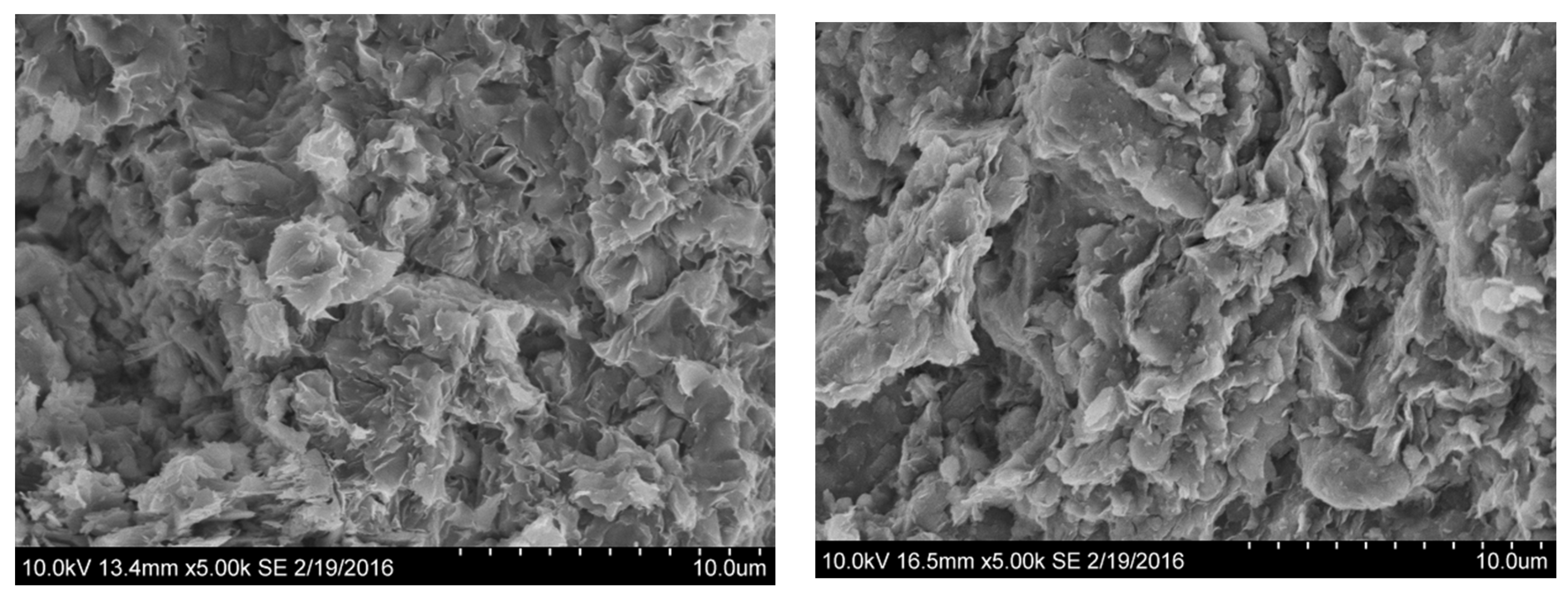
3.1. Clay Characterization
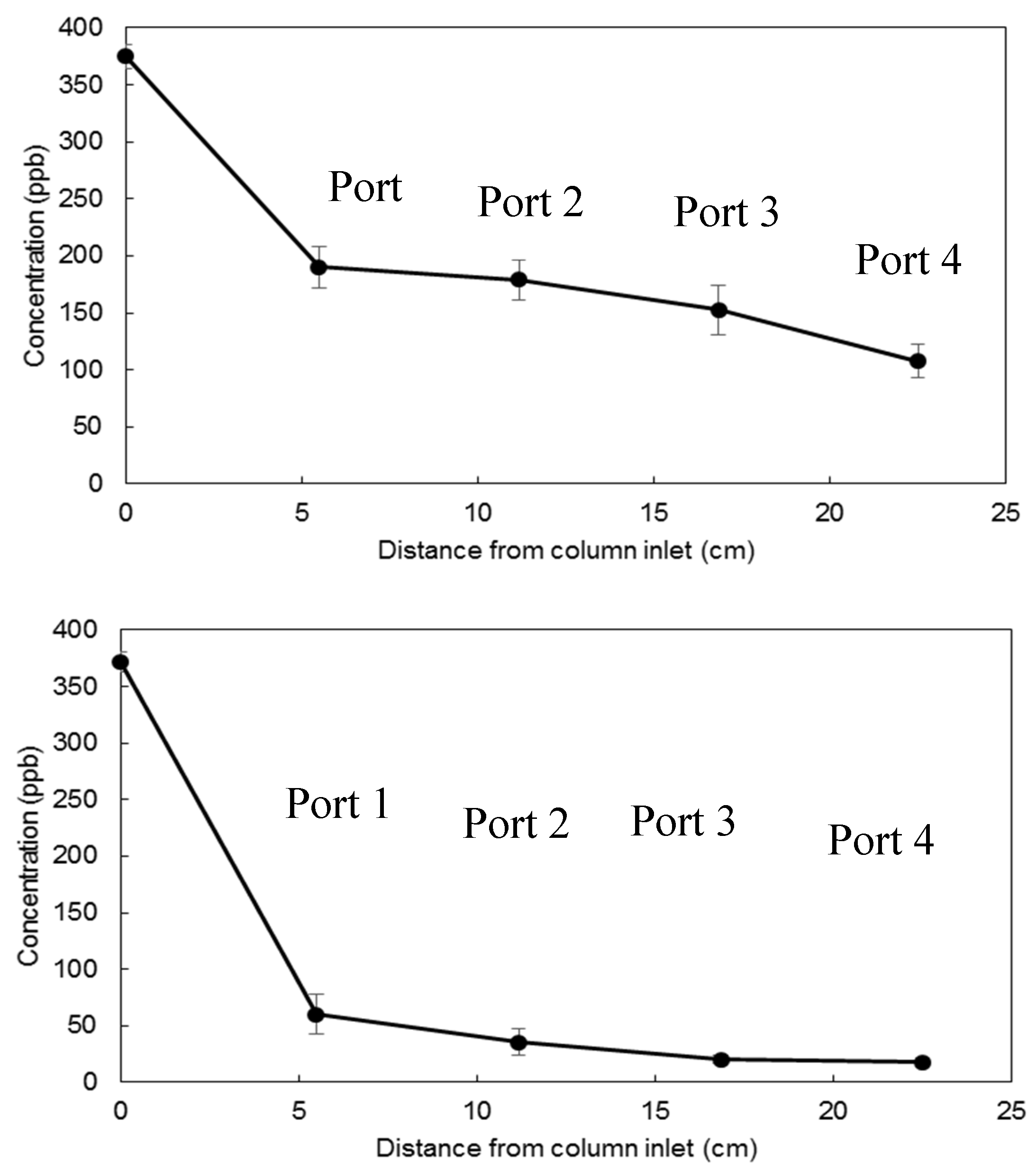
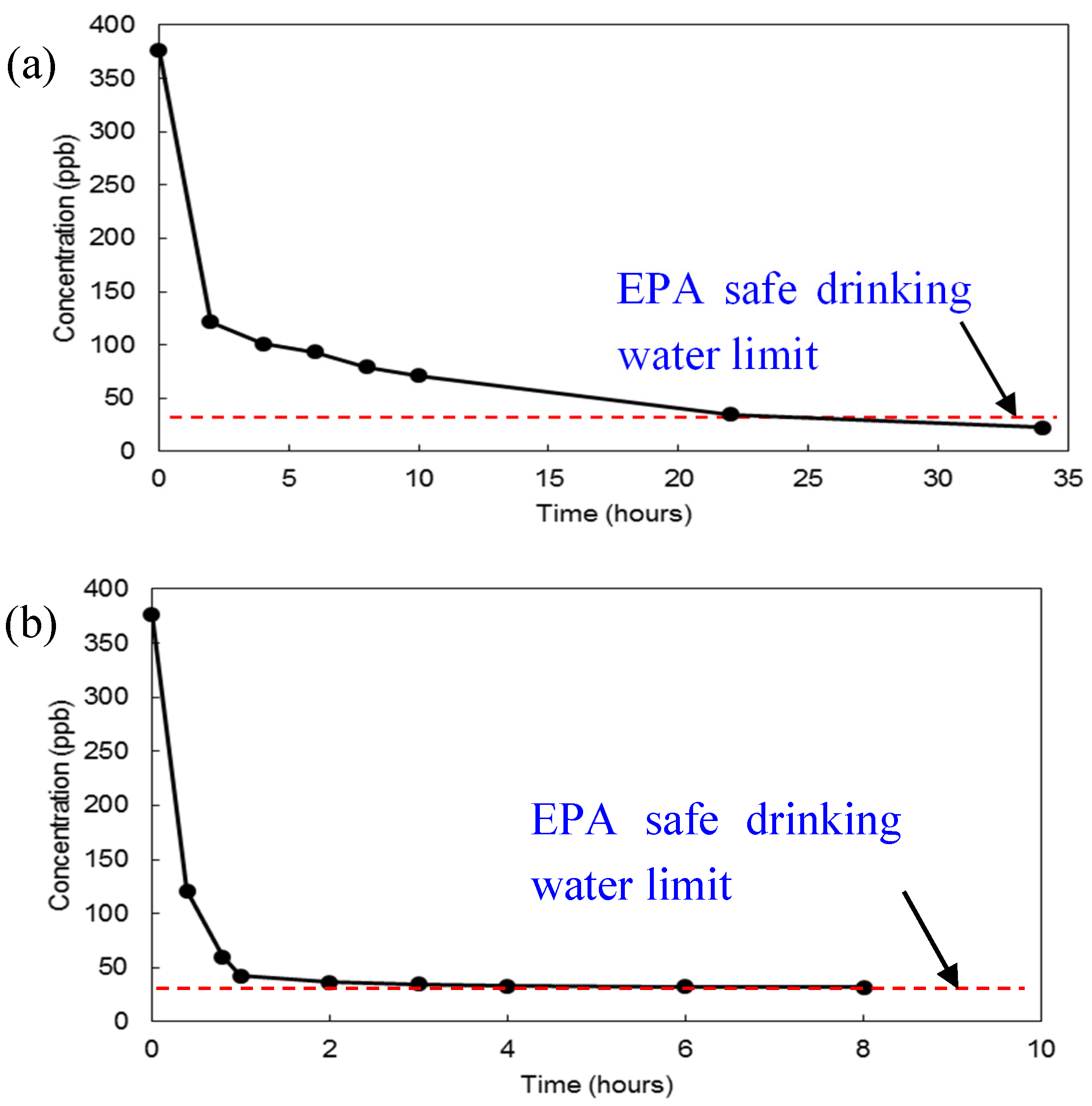
3.2. Flow-Through Experiments for Uranium Removal from Groundwater
3.3. Water Purification Experiments for Uranium Removal from Drinking Water
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeLemos, J.L.; Burgge, D.; Cajero, M.; Downs, M.; Durant, J.L.; George, C.M.; Henio-Adeky, S.; Nez, T.; Manning, T.; Rock, T.; et al. Development of risk maps to minimize uranium exposures in the Native Churchrock Mining District. Environ. Health 2009, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Arzuaga, X.; Rieth, S.H.; Bathija, A. Cooper, G.S. Renal effects of exposure to natural and depleted uranium: A review of the epidemiologic and experimental data. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R. Uranium and Other Radioactive Elements in Jefferson County Ground Water; U.S. Geological Survey: Helena, MT, USA, 2008.
- Kurttio, P.; Auvinen, A.; Salonen, L.; Saha, H.; Pekkanen, J.; Makelainen, I.; Vaisanen, S.B.; Penttila, I.M.; Komulainen, H. Renal Effects of Uranium in Drinking Water. Environ. Health Perspect. 2002, 110, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Orloff, K.G.; Mistry, K.; Charp, P.; Metcalf, S.; Marino, R.; Shelly, T.; Melaro, E.; Donohoe, A.M.; Jones, R.L. Human exposure to uranium in groundwater. Environ. Res. 2004, 94, 319–326. [Google Scholar] [CrossRef]
- Moore-Nall, A. The legacy of uranium development on or near Indian reservations and health implications rekindling public awareness. Geosciences 2015, 5, 15–29. [Google Scholar] [CrossRef]
- Farrell, J.; Bostick, W.D.; Jarabek, R.J.; Fiedor, J.N. Uranium Removal from Ground Water Using Zero Valent Iron Media. Groundwater 1999, 37, 618–624. [Google Scholar] [CrossRef]
- Darnault, C.J.G. Overexploitation and Contamination of Shared Groundwater Resources; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Freethey, G.F.; Naftz, D.L.; Rowland, R.C.; Davis, J.A. Deep Aquifer Remediation Tools: Theory, Design, and Performance Modeling. In Handbook of Groundwater Remediation Using Permeable Reactive Barriers; Naftz, D.L., Morrison, S.J., Davis, J.A., Fuller, C.C., Eds.; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Naidu, R.; Birke, V. Permeable Reactive Barrier: Sustainable Groundwater Remediation; Chemical Rubber Company CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Gu, B.; Liang, L.; Dickey, M.J.; Yin, X.; Dai, S. Reductive Precipitation of Uranium (VI) by Zero-Valent Iron. Environ. Sci. Technol. 1998, 32, 3366–3373. [Google Scholar] [CrossRef]
- Morrison, S.J.; Metzler, D.R.; Carpenter, C.E. Uranium Precipitation in a Permeable Reactive Barrier by Progressive Irreversible Dissolution of Zero-Valent Iron. Environ. Sci. Technol. 2001, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.N.; Bostick, W.D.; Jarabek, R.J.; Farrell, J. Understanding the Mechanism of Uranium Removal from Groundwater by Zero-Valent Iron Using X Ray Photoelectron Spectroscopy. Environ. Sci. Technol. 1998, 32, 1466–1473. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Merkel, B.J. Investigating the Mechanism of Uranium Removal by Zero-Valent Iron. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas, L. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Jiuhui, Q.U. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Carrott, M.M.L.; Suhas, L. Low-cost adsorbents: Growing approach to wastewater treatment—A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Kushwaha, S.; Soni, H.; Ageethe, V.; Padmaja, P. An insight into the production, characterization and mechanisms of action of low-cost adsorbents for removal of organics from aqueous solution. Crit. Rev. Environ. Sci. Technol. 2013, 43, 443–549. [Google Scholar] [CrossRef]
- Svinka, R.; Svinka, V.; Petersone, E. The sorption properties of Latvian clays and use for the water purification. Latvian J. Chem. 1994, 3, 280–285. [Google Scholar]
- Luukkonen, T.; Veznikova, K.; Tolonen, E.T.; Runtti, H.; Yliniemi, J.; Hu, T.; Kemppainen, K.; Lassi, U. Removal of ammonium from municipal wastewater with powdered and gradulated metakaolin geopolymer. Environ. Technol. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lakevics, V.; Ruplis, A. Sorption properties of Latvian clays and research of clays innovative application. Sci. J. Riga Tech. Univ. Mater. Sci. Appl. Chem. 2011, 24, 20–25. [Google Scholar]
- Svinka, R.; Svinka, V.; Pudze, I.; Damberga, M. Clay ceramic pellets for water treatment. Mater. Sci. Appl. Chem. 2015, 32, 39–44. [Google Scholar]
- Sposito, G. The Surface Chemistry of Soils; Oxford University Press: New York, NY, USA, 1984. [Google Scholar]
- Davis, J.A.; Kent, D.B. Surface Complexation Modeling in Aqueous Geochemistry; Hochella, M.F., White, A.F., Eds.; Mineral-Water Interface Geochemistry, Mineralogical Society of America: Chantilly, VA, USA, 1990; Volume 3, pp. 177–260. [Google Scholar]
- Kam, S.; Zerbo, L.; Bathiebo, J.; Soro, J.; Naba, S.; Wenmenga, U.; Traore, K.; Gomina, M.; Blanchart, P. Permeability to water of sintered clay ceramics. Appl. Clay Sci. 2009, 6, 351–357. [Google Scholar] [CrossRef]
- Caylor, E.; Rasmussen, C.; Dhakal, P. Lithologic Control on Secondary Clay Mineral Formation in the Valles Caldera, New Mexico. In Proceedings of the Abstract EP31B-1000 Presented at 2015 Fall Meeting, American Geophysical Union (AGU), San Francisco, CA, USA, 14–18 December 2015; Available online: http://adsabs.harvard.edu//abs/2015AGUFMEP31B1000C (accessed on 1 October 2017).
- Auebach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Bukka, K.; Miller, J.D. FTIR study of deuterated montmorillonites: Structural features relevant to pillared clay stability. Clays Clay Miner. 1992, 40, 92–103. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Minitab 17 Documentation. 2006. Available online: https://www.minitab.com/uploadedFiles/Documents/getting-started/Minitab17_GettingStarted-en.pdf (accessed on 30 September 2017).

| Clay Type | Cheto Clay | Gallup Clay | ||
|---|---|---|---|---|
| Port location | Port 1 | Port 4 | Port 1 | Port 4 |
| Mean value | 190.1 | 107.5 | 60.1 | 17.54 |
| Standard deviation | 18.2 | 14.7 | 17.8 | 4.61 |
| Standard error mean | 8.2 | 6.6 | 8.9 | 2.3 |
| p-value | 0.000 | 0.019 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florez, C.; Park, Y.H.; Valles-Rosales, D.; Lara, A.; Rivera, E. Removal of Uranium from Contaminated Water by Clay Ceramics in Flow-Through Columns. Water 2017, 9, 761. https://doi.org/10.3390/w9100761
Florez C, Park YH, Valles-Rosales D, Lara A, Rivera E. Removal of Uranium from Contaminated Water by Clay Ceramics in Flow-Through Columns. Water. 2017; 9(10):761. https://doi.org/10.3390/w9100761
Chicago/Turabian StyleFlorez, Charles, Young Ho Park, Delia Valles-Rosales, Antonio Lara, and Emilio Rivera. 2017. "Removal of Uranium from Contaminated Water by Clay Ceramics in Flow-Through Columns" Water 9, no. 10: 761. https://doi.org/10.3390/w9100761




