Characterization of a Microbial Community in an Anammox Process Using Stored Anammox Sludge
Abstract
:1. Introduction
2. Materials and Methods
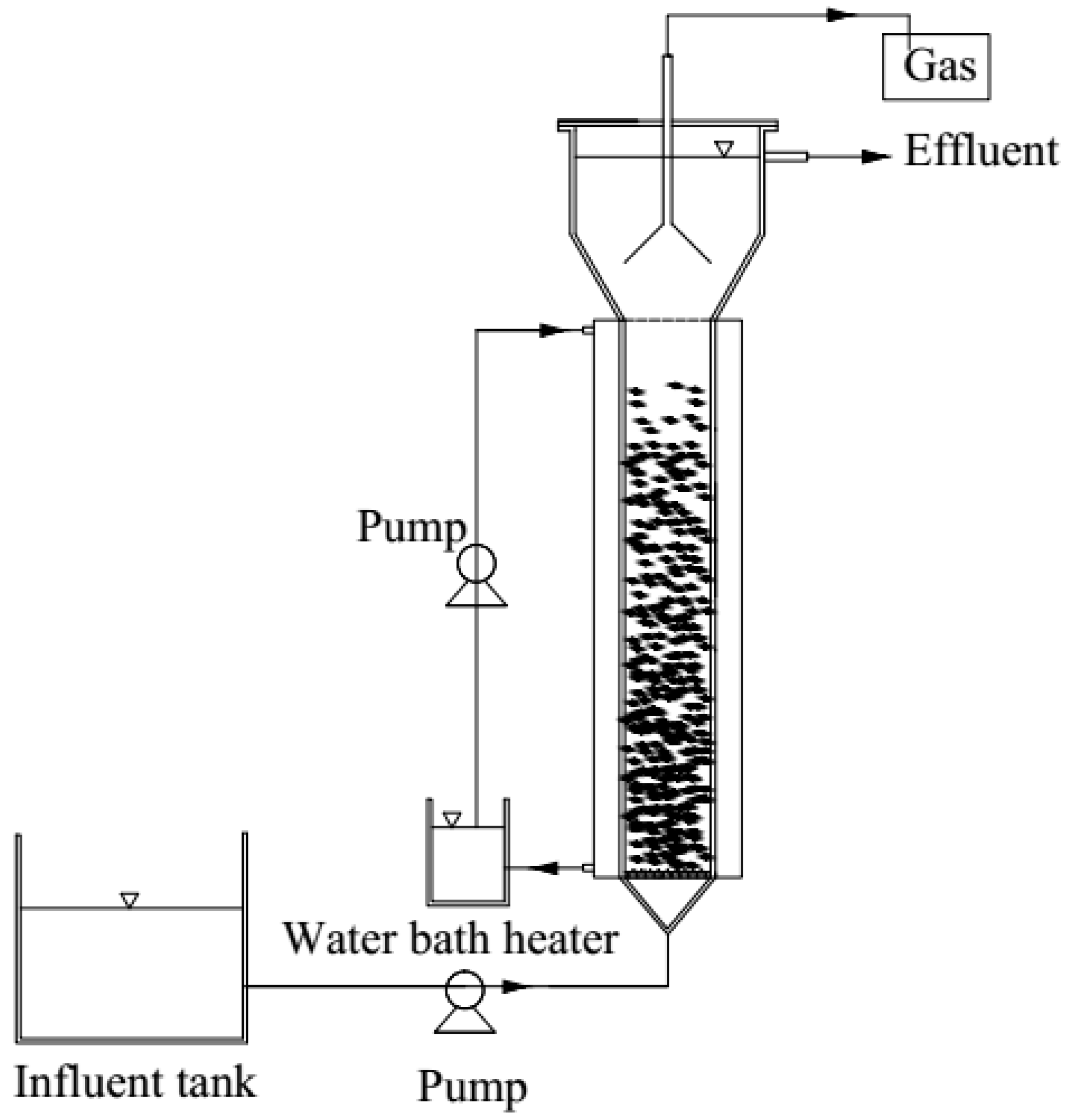
2.1. UASB Configuration
2.2. Inoculation
2.3. Synthetic Wastewater
2.4. Anammox Operation Strategy
2.5. Analytical Methods
2.6. High-Throughput Sequencing
3. Results and Discussion
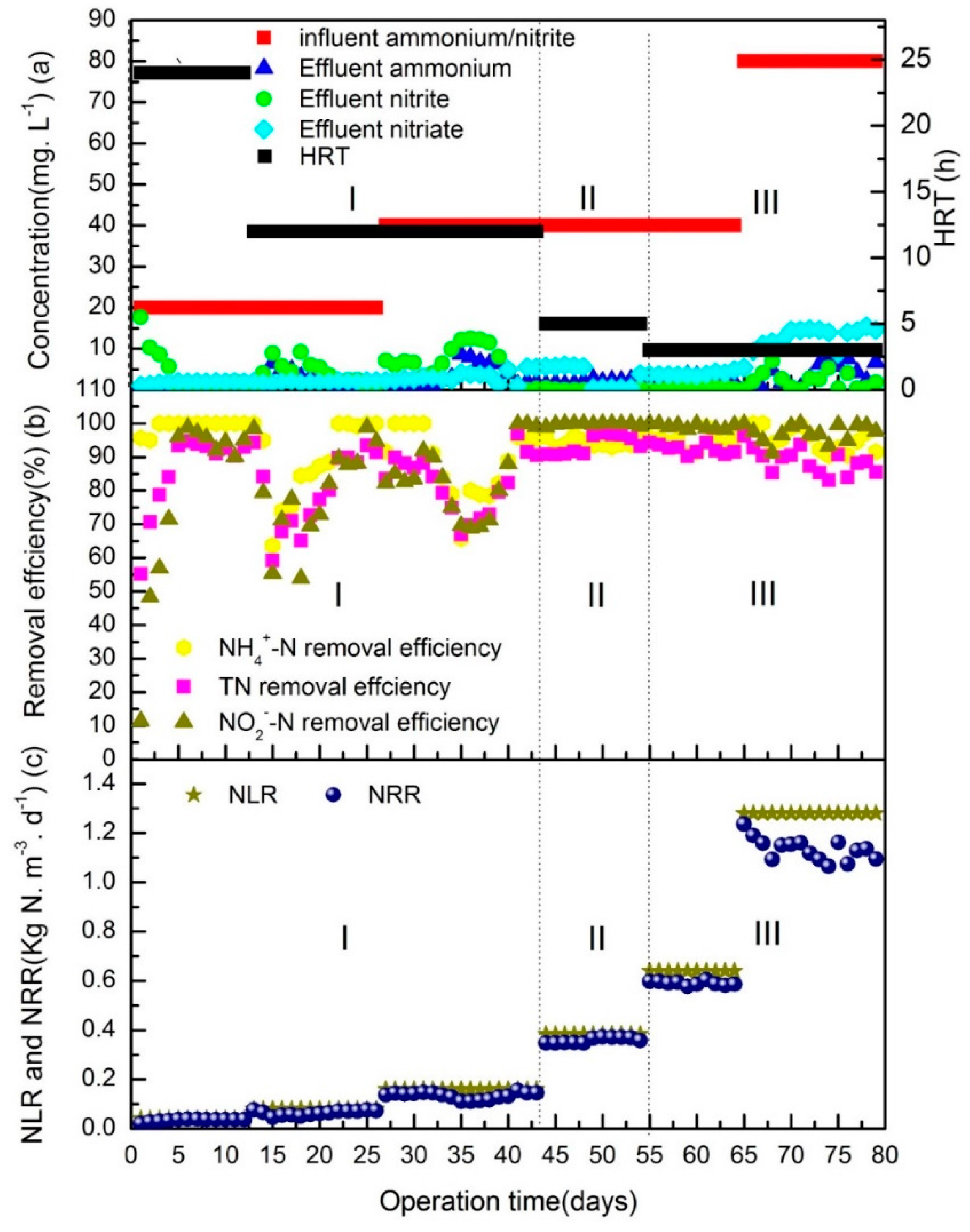
3.1. Rapid Start-Up of the Anammox Process (Phases I and II)
3.2. Performance Improvement (Phase III)
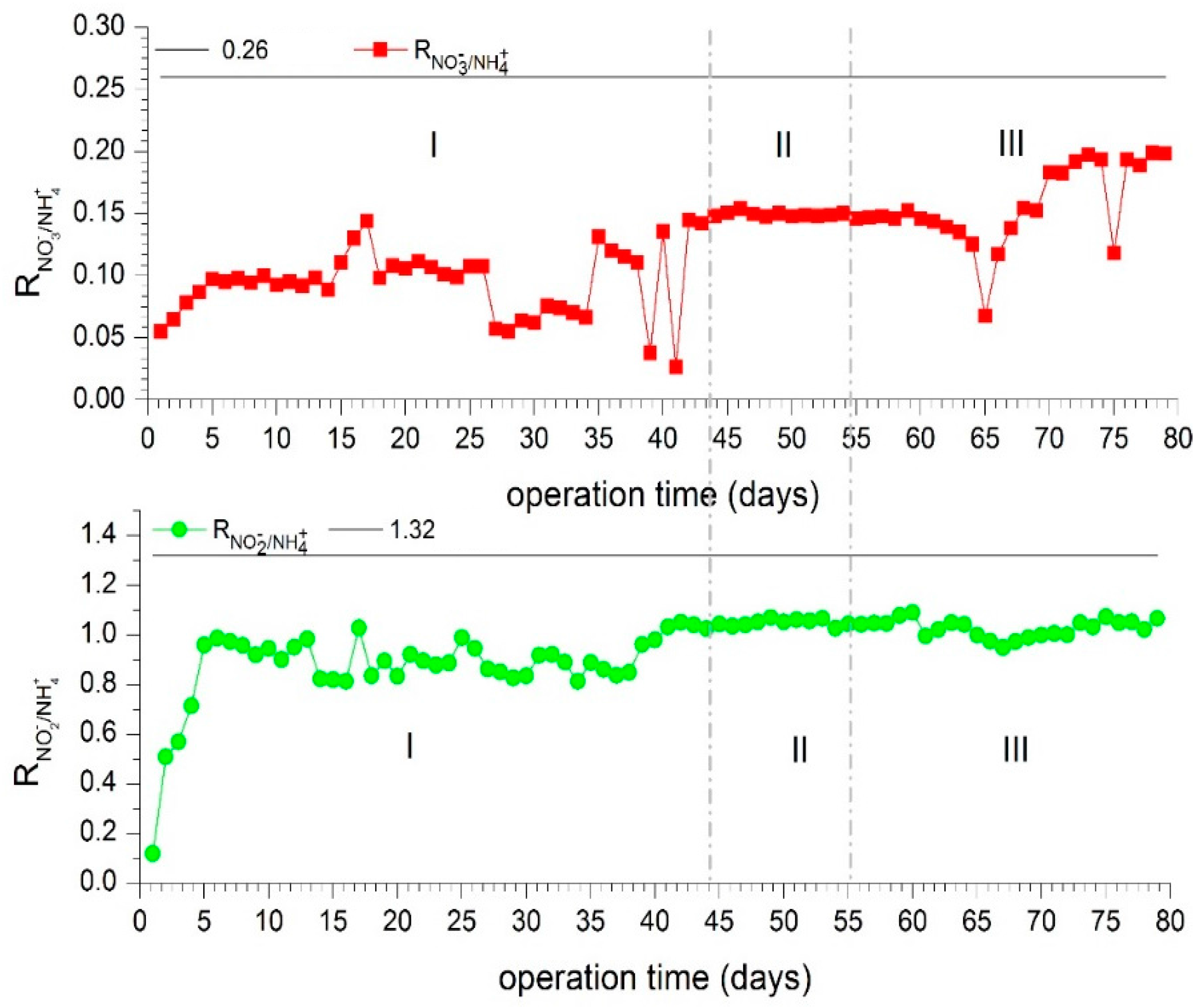
3.3. Stoichiometry
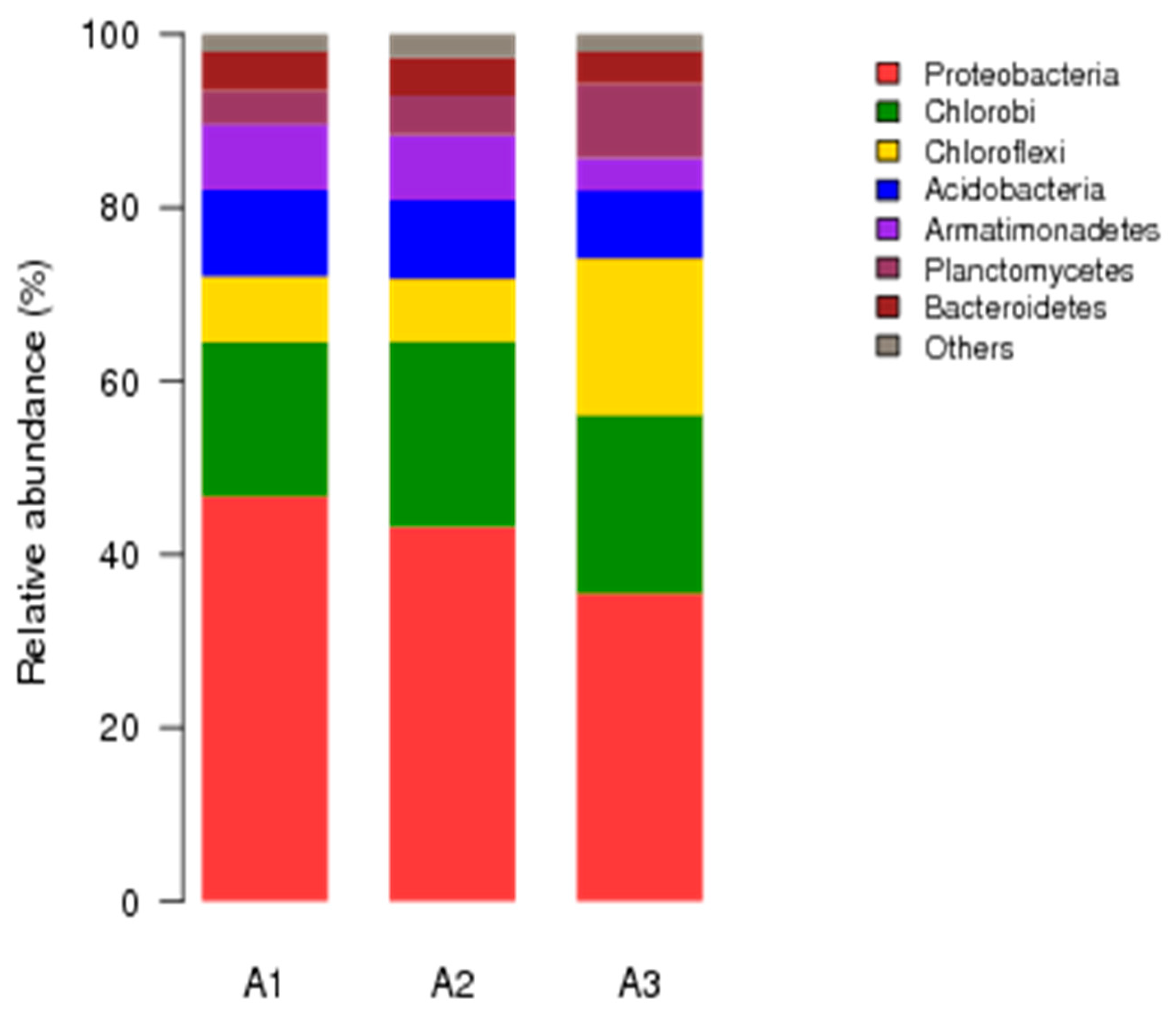
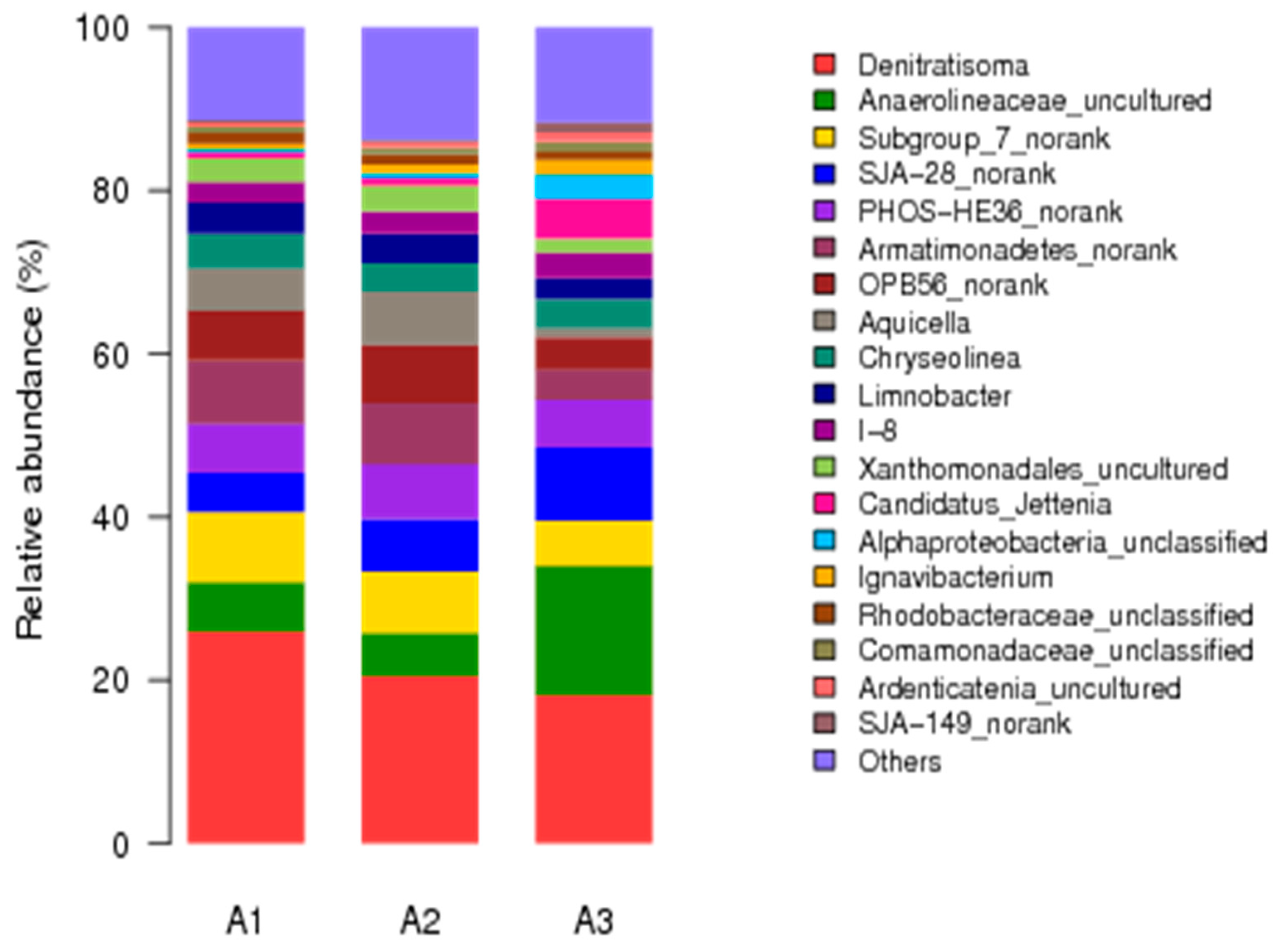
3.4. Microbial Community Structures of Anammox Sludge
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Mulder, A.; Vandegraaf, A.A.; Robertson, L.A.; Kuenen, J.G. Anaerobic Ammonium Oxidation Discovered in a Denitrifying Fluidized-Bed Reactor. FEMS Microbiol. Ecol. 1995, 16, 177–183. [Google Scholar] [CrossRef]
- Lotti, T.; Kleerebezem, R.; Hu, Z.; Kartal, B.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Simultaneous partial nitritation and anammox at low temperature with granular sludge. Water Res. 2014, 66, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.A.; Su, G.Y.; Hu, Y.F.; Lu, H.; Huang, L.N.; Chen, G.H. Improving nitrogen removal in an ANAMMOX reactor using a permeable reactive biobarrier. Water Res. 2014, 58, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C.M. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Salzgeber, D.; Eugster, J.; Konig, R.; Rottermann, K.; Burger, S.; Fabijan, P.; Leumann, S.; Mohn, J.; Siegrist, H. Full-scale nitrogen removal from digester liquid with partial nitritation and anammox in one SBR. Environ. Sci. Technol. 2009, 43, 5301–5306. [Google Scholar] [CrossRef] [PubMed]
- Van der Star, W.R.L.; Abma, W.R.; Blommers, D.; Mulder, J.W.; Tokutomi, T.; Strous, M.; Picioreanu, C.; van Loosdrecht, M.C.M. Startup of reactors for anoxic ammonium oxidation: Experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007, 41, 4149–4163. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Biswas, R.; Nandy, T. Start-up and stabilization of an Anammox process from a non-acclimatized sludge in CSTR. J. Ind. Microbiol. Biotechnol. 2010, 37, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Zekker, I.; Rikmann, E.; Tenno, T.; Lemmiksoo, V.; Menert, A.; Loorits, L.; Vabamae, P.; Tomingas, M.; Tenno, T. Anammox enrichment from reject water on blank biofilm carriers and carriers containing nitrifying biomass: Operation of two moving bed biofilm reactors (MBBR). Biodegradation 2012, 23, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Abma, W.R.; Schultz, C.E.; Mulder, J.W.; van der Star, W.R.L.; Strous, M.; Tokutomi, T.; van Loosdrecht, M.C.M. Full-scale granular sludge Anammox process. Water Sci. Technol. 2007, 55, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, B.; Garcia, M.C.; Karakashev, D.; Angelidaki, I. Anammox for ammonia removal from pig manure effluents: Effect of organic matter content on process performance. Bioresour. Technol. 2009, 100, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Van Hulle, S.W.H.; Vandeweyer, H.J.P.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Third, K.A.; Sliekers, A.O.; Kuenen, J.G.; Jetten, M.S.M. The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: Interaction and competition between three groups of bacteria. Syst. Appl. Microbiol. 2001, 24, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Gu, J.D. More refined diversity of anammox bacteria recovered and distribution in different ecosystems. Appl. Microbiol. Biotechnol. 2013, 97, 3653–3663. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.Q.; Gao, B.Y.; Wang, C.C.; Lin, J.G.; Sung, S.W. Fast start-up, performance and microbial community in a pilot-scale anammox reactor seeded with exotic mature granules. Bioresour. Technol. 2011, 102, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, D.; Zeng, H.P.; Chang, X.Y.; Zhang, J. Microbial characteristics of a CANON reactor during the start-up period seeding conventional activated sludge. Water Sci. Technol. 2013, 67, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qu, Y.Y.; Shen, W.L.; Zhang, Z.J.; Wang, J.W.; Liu, Z.Y.; Li, D.X.; Li, H.J.; Zhou, J.T. Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing. Bioresour. Technol. 2015, 179, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Shen, K.; Li, A.M.; Shi, P.; Li, Y.; Shi, Q.; Wang, Z. High-nitrate wastewater treatment in an expanded granular sludge bed reactor and microbial diversity using 454 pyrosequencing analysis. Bioresour. Technol. 2013, 134, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.R.; Wang, K.; Li, X.K.; Zhu, M.T.; Yang, L.; Zhang, J. Microbial characterization of aggregates within a one-stage nitritation-anammox system using high-throughput amplicon sequencing. Chem. Eng. J. 2015, 262, 41–48. [Google Scholar] [CrossRef]
- Tang, C.J.; Zheng, P.; Wang, C.H.; Mahmood, Q.; Zhang, J.Q.; Chen, X.G.; Zhang, L.; Chen, J.W. Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res. 2011, 45, 135–144. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; Volume 47, pp. 859–869. [Google Scholar]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Chamchoi, N.; Nitisoravut, S. Anammox enrichment from different conventional sludges. Chemosphere 2007, 66, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, H.M.; Yang, F.L.; Liu, S.T.; Fu, Z.M.; Chen, H.H. Start-up of the Anammox process from the conventional activated sludge in a membrane bioreactor. Bioresour. Technol. 2009, 100, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, H.M.; Gao, D.W.; Yang, F.L.; Yang, S.A.; Jiang, T.; Zhang, G.Y. Enrichment of Anammox bacteria in seed sludges from different wastewater treating processes and start-up of Anammox process. Desalination 2011, 271, 193–198. [Google Scholar] [CrossRef]
- Tang, C.J.; Zheng, P.; Mahmood, Q.; Chen, J.W. Start-up and inhibition analysis of the Anammox process seeded with anaerobic granular sludge. J. Ind. Microbiol. Biotechnol. 2009, 36, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Bettazzi, E.; Caffaz, S.; Vannini, C.; Lubello, C. Nitrite inhibition and intermediates effects on Anammox bacteria: A batch-scale experimental study. Process Biochem. 2010, 45, 573–580. [Google Scholar] [CrossRef]
- Daverey, A.; Chen, Y.C.; Dutta, K.; Huang, Y.T.; Lin, J.G. Start-up of simultaneous partial nitrification, anammox and denitrification (SNAD) process in sequencing batch biofilm reactor using novel biomass carriers. Bioresour. Technol. 2015, 190, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, H.M.; Yang, F.L. Performance of Anammox process and low-oxygen adaptability of Anammox biofilms in a FBR with small ring non-woven carriers. Ecol. Eng. 2016, 86, 126–134. [Google Scholar] [CrossRef]
- Ahn, Y.H. Sustainable nitrogen elimination biotechnologies: A review. Process Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- Ma, B.; Peng, Y.; Zhang, S.; Wang, J.; Gan, Y.; Chang, J.; Wang, S.; Wang, S.; Zhu, G. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour. Technol. 2013, 129, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; Wang, Z.W.; Yang, Y.; Mei, X.J.; Wu, Z.C. Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res. 2013, 47, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Loman, N.J.; Misra, R.V.; Dallman, T.J.; Constantinidou, C.; Gharbia, S.E.; Wain, J.; Pallen, M.J. Performance comparison of benchtop high-throughput sequencing platforms. Nat. Biotechnol. 2012, 30, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Du, R.; Li, B.; Ren, N.; Peng, Y. High-throughput profiling of microbial community structures in an ANAMMOX-UASB reactor treating high-strength wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6457–6467. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Sliekers, A.O.; Lavik, G.; Schmid, M.; Jorgensen, B.B.; Kuenen, J.G.; Damste, J.S.S.; Strous, M.; Jetten, M.S.M. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 2003, 422, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Kindaichi, T.; Yuri, S.; Ozaki, N.; Ohashi, A. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci. Technol. 2012, 66, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Du, B.; Fu, H.X.; Wang, R.F.; Shi, J.H.; Wang, Y.; Jetten, M.S.; Quan, Z.X. The bacterial diversity in an anaerobic ammonium-oxidizing (anammox) reactor community. Syst. Appl. Microbiol. 2009, 32, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, B.; Richter, M.; Schuler, M.; Pinhassi, J.; Acinas, S.G.; Gonzalez, J.M.; Pedros-Alio, C. Ecology of marine Bacteroidetes: A comparative genomics approach. ISME J. 2013, 7, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Sonthiphand, P.; Hall, M.W.; Neufeld, J.D. Biogeography of anaerobic ammonia-oxidizing (anammox) bacteria. Front. Microbiol. 2014, 5, 399. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, D.; Li, X.; Yang, L.; Ding, A.; Zhang, J. High-rate nitrogen removal and microbial community of an up-flow anammox reactor with ceramics as biomass carrier. Chemosphere 2014, 113, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.L.; Zheng, P.; Tang, C.J.; Chen, J.W.; van der Biezen, E.; Zhang, L.; Ni, B.J.; Jetten, M.S.M.; Yan, J.; Yu, H.Q.; Kartal, B. Identification and quantification of anammox bacteria in eight nitrogen removal reactors. Water Res. 2010, 44, 5014–5020. [Google Scholar] [CrossRef] [PubMed]
| Seed Sludge | SS (g L−1) | VSS (g L−1) | VSS/SS |
|---|---|---|---|
| Anammox activated sludge | 23.24 | 17.24 | 0.74 |
| Phases | Days | Inlet NH4+-N and NO2−-N (mg N L−1) | HRT (h) | NLR (kg N m−3 day−1) |
|---|---|---|---|---|
| I | 1–12 | 20 | 24 | 0.04 |
| 13–26 | 20 | 12 | 0.08 | |
| 27–43 | 40 | 12 | 0.16 | |
| II | 44–54 | 40 | 5 | 0.38 |
| III | 55–64 | 40 | 3 | 0.64 |
| 65–79 | 80 | 3 | 1.28 |
| Samples | Reads | 0.97 | |||||
|---|---|---|---|---|---|---|---|
| OTU | Ace | Chao | Coverage | Shannon | Simpson | ||
| A1 | 24189 | 240 | 261 | 264 | 0.9985 | 3.16 | 0.0983 |
| A2 | 22456 | 247 | 259 | 262 | 0.9988 | 3.36 | 0.0757 |
| A3 | 26310 | 237 | 248 | 250 | 0.9991 | 3.52 | 0.0624 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Dai, X.; Cao, D.; Hu, X.; Liu, W.; Yang, D. Characterization of a Microbial Community in an Anammox Process Using Stored Anammox Sludge. Water 2017, 9, 829. https://doi.org/10.3390/w9110829
Chen W, Dai X, Cao D, Hu X, Liu W, Yang D. Characterization of a Microbial Community in an Anammox Process Using Stored Anammox Sludge. Water. 2017; 9(11):829. https://doi.org/10.3390/w9110829
Chicago/Turabian StyleChen, Wenjing, Xiaohu Dai, Dawen Cao, Xiaona Hu, Wenru Liu, and Dianhai Yang. 2017. "Characterization of a Microbial Community in an Anammox Process Using Stored Anammox Sludge" Water 9, no. 11: 829. https://doi.org/10.3390/w9110829




