Diversity and Distribution of Endemic Stream Insects on a Nationwide Scale, South Korea: Conservation Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ecological Data
2.2. Data Analysis
3. Results
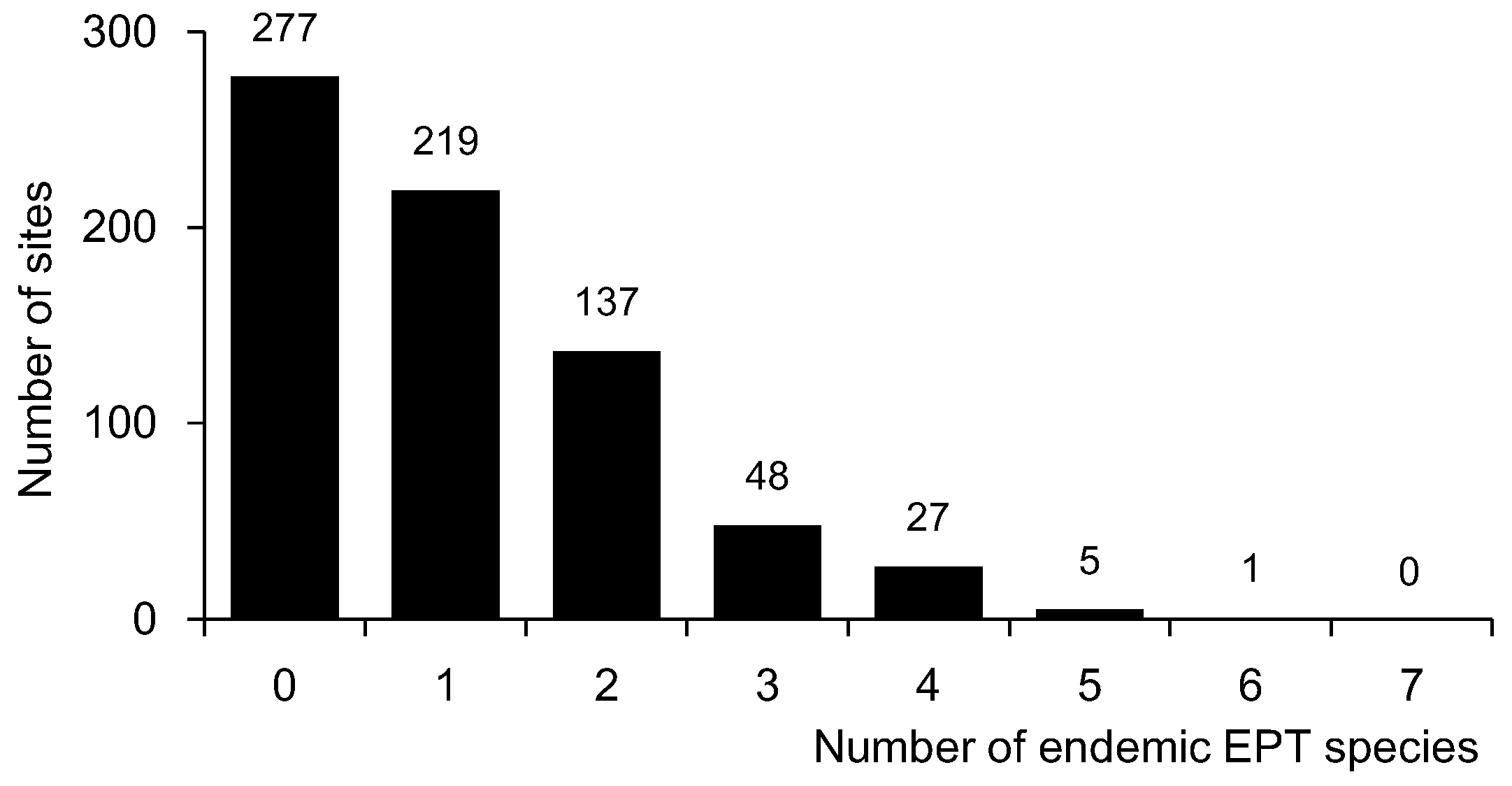
3.1. Characteristics of Endemic EPT Species
3.2. Relationships between Endemic EPT Species and Environmental Factors
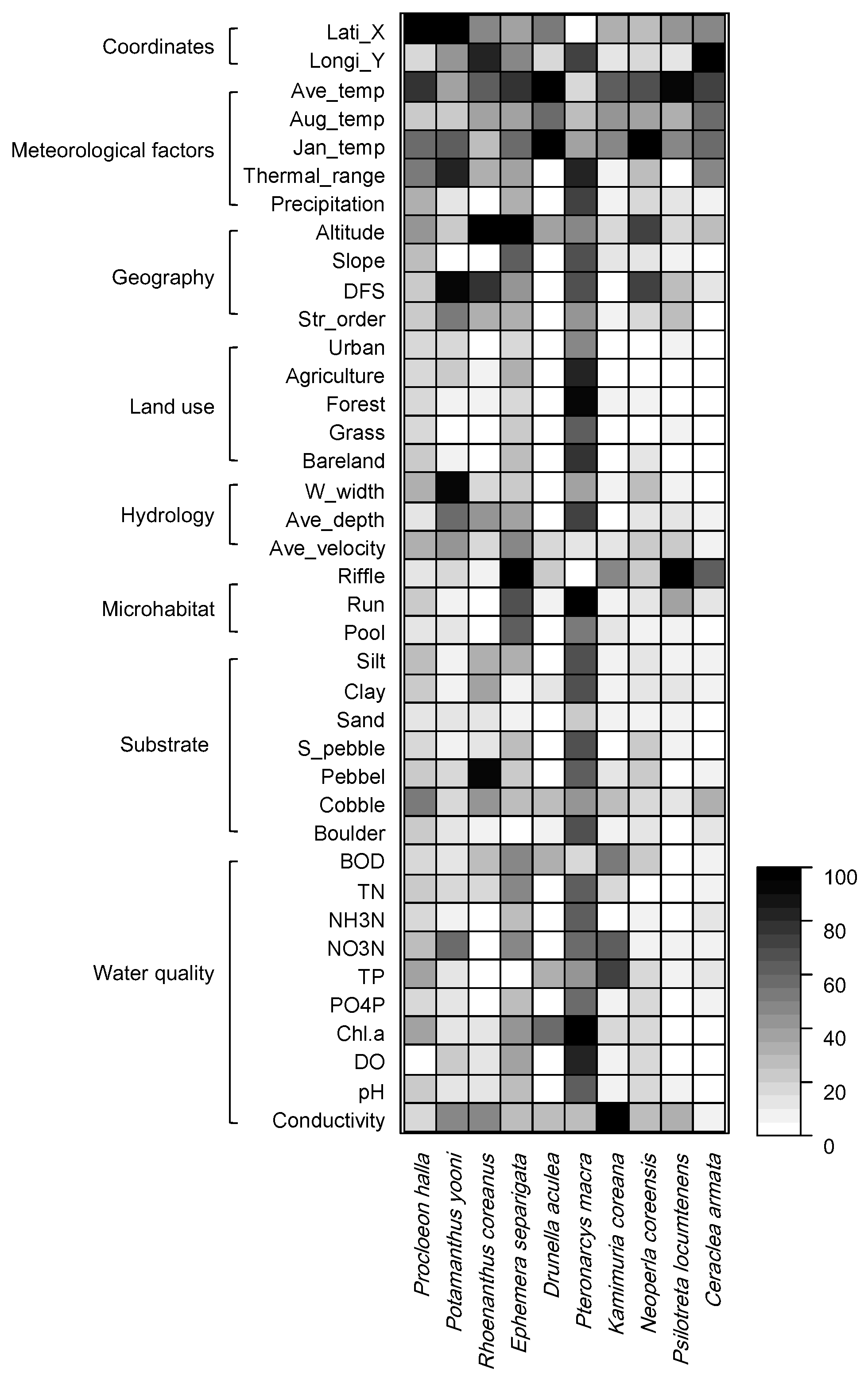
3.3. Influential Environmental Factors on the Occurrences of Endemic Species
4. Discussion
4.1. Priority Species for the Conservation
4.2. Influential Environmental Factors Related to the Existence of Endemic Species: Conservation Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, B.L. Habitat heterogeneity and disturbance influence patterns of community temporal variability in a small temperate stream. Hydrobiologia 2007, 586, 93–106. [Google Scholar] [CrossRef]
- Winterbourn, M.J.; Cadbury, S.; Ilg, C.; Milner, A.M. Mayfly production in a New Zealand glacial stream and the potential effect of climate change. Hydrobiologia 2008, 603, 211–219. [Google Scholar] [CrossRef]
- Gleick, P.H. Water resources. In Encyclopedia of Climate and Weather; Schneider, S.H., Ed.; Oxford University Press: Oxford, UK, 1996; pp. 817–823. [Google Scholar]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Kalin-Arroyo, M.T. Magnitude and distribution of biodiversity. In Global Biodiversity Assessment; Heywood, V.H., Watson, R.T., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 107–192. [Google Scholar]
- Strayer, D.L. Challenges for freshwater invertebrate conservation. J. N. Am. Benthol. Soc. 2006, 25, 271–287. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Cowx, I.G.; Collares-Pereira, M.J.; Coelho, M.M. Freshwater Fish Conservation: Options for the Future; Fishing News: Oxford, UK, 2002; pp. 443–452. [Google Scholar]
- Xu, M.; Wang, Z.; Duan, X.; Pan, B. Effects of pollution on macroinvertebrates and water quality bio-assessment. Hydrobiologia 2014, 729, 247–259. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Koehn, J.D. Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshw. Biol. 2004, 49, 882–894. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennett, D.L.; Cook, M.J. Endemic species: Contribution to community uniqueness, effect of habitat alteration, and conservation priorities. Biol. Conserv. 2011, 144, 155–165. [Google Scholar] [CrossRef]
- Muhlfeld, C.C.; Giersch, J.J.; Hauer, F.R.; Pederson, G.T.; Luikart, G.; Peterson, D.P.; Downs, C.C.; Fagre, D.B. Climate change links fate of glaciers and an endemic alpine invertebrate. Clim. Chang. 2011, 106, 337–345. [Google Scholar] [CrossRef]
- Benstead, J.P.; Pringle, C.M. Deforestation alters the resource base and biomass of endemic stream insects in eastern Madagascar. Freshw. Biol. 2004, 49, 490–501. [Google Scholar] [CrossRef]
- Samways, M.J.; Sharratt, N.J.; Simaika, J.P. Effect of alien riparian vegetation and its removal on a highly endemic river macroinvertebrate community. Biol. Invasions 2011, 13, 1305–1324. [Google Scholar] [CrossRef]
- Flather, C.H.; Sieg, C.H. Species rarity: Definition, causes and classification. In Conservation of Rare or Little-Known Species; Island Press: Washington, DC, USA, 2007; pp. 40–66. [Google Scholar]
- Schmeller, D.S.; Gruber, B.; Budrys, E.; Framsted, E.; Lengyel, S.; Henle, K. National responsibilities in European species conservation: A methodological review. Conserv. Biol. 2008, 22, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Couceiro, S.R.M.; Hamada, N.; Forsberg, B.R.; Pimentel, T.P.; Luz, S.L.B. A macroinvertebrate multimetric index to evaluate the biological condition of streams in the Central Amazon region of Brazil. Ecol. Indic. 2012, 18, 118–125. [Google Scholar] [CrossRef]
- Brown, L.E.; Céréghino, R.; Compin, A. Endemic freshwater invertebrates from southern France: Diversity, distribution and conservation implications. Biol. Conserv. 2009, 142, 2613–2619. [Google Scholar] [CrossRef]
- Céréghino, R.; Lavandier, P. Influence of hypolimnetic hydropeaking on the distribution and population dynamics of Ephemeroptera in a mountain stream. Freshw. Biol. 1998, 40, 385–399. [Google Scholar] [CrossRef]
- Santoul, F.; Figuerola, J.; Mastrorillo, S.; Céréghino, R. Patterns of rare fish and aquatic insects in a southwestern French river catchment in relation to simple physical variables. Ecography 2005, 28, 307–314. [Google Scholar] [CrossRef]
- Li, F.; Chung, N.; Bae, M.J.; Kwon, Y.S.; Park, Y.S. Relationships between stream macroinvertebrates and environmental variables at multiple spatial scales. Freshw. Biol. 2012, 57, 2107–2124. [Google Scholar] [CrossRef]
- Li, F.; Chung, N.; Bae, M.J.; Kwon, Y.S.; Kwon, T.S.; Park, Y.S. Temperature change and macroinvertebrate biodiversity: Assessments of organism vulnerability and potential distributions. Clim. Chang. 2013, 119, 421–434. [Google Scholar] [CrossRef]
- Wagenhoff, A.; Townsend, C.R.; Matthaei, C.D. Macroinvertebrate responses along broad stressor gradients of deposited fine sediment and dissolved nutrients: A stream mesocosm experiment. J. Appl. Ecol. 2012, 49, 892–902. [Google Scholar] [CrossRef]
- Bae, M.J.; Kwon, Y.; Hwang, S.J.; Chon, T.S.; Yang, H.J.; Kwak, I.S.; Park, J.H.; Ham, S.A.; Park, Y.S. Relationships between three major stream assemblages and their environmental factors in multiple spatial scales. Ann. Limnol. Int. J. Limnol. 2011, 47, S91–S105. [Google Scholar] [CrossRef]
- Lee, K.S.; Wenner, D.B.; Lee, I. Using H-and O-isotopic data for estimating the relative contributions of rainy and dry season precipitation to groundwater: Example from Cheju Island, Korea. J. Hydrol. 1999, 222, 65–74. [Google Scholar] [CrossRef]
- MOE; NIER. The Survey and Evaluation of Aquatic Ecosystem Health in Korea; The Ministry of Environment and National Institute of Environmental Research: Incheon, Korea, 2008. (In Korean)
- Quigley, M. Invertebrates of Streams and Rivers: A Key to Identification; Edward Arnold: London, UK, 1977. [Google Scholar]
- Pennak, W. Freshwater Invertebrates of the United States; John Wiley and Sons, Inc.: New York, NY, USA, 1978. [Google Scholar]
- Brighnam, A.R.; Brighnam, W.U.; Gnika, A. Aquatic Insects and Oligochaeta of North and South Carolina; Midwest Aquatic Enterprise: Mahomet, IL, USA, 1982. [Google Scholar]
- Yun, I.B. Illustrated Encyclopedia of Fauna and Flora of Korea. Aquatic Insects; Ministry of Education: Seoul, Korea, 1988.
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America; Hunt Publishing Company: Dubugue, IA, USA, 2006. [Google Scholar]
- WAMIS. Available online: http://www.wamis.go.kr (accessed on 21 August 2014).
- Environmental Systems Research Incorporated (ESRI). ArcGIS 10.0. Environmental Systems Research Incorporated: Redlands, CA, USA, 2011. [Google Scholar]
- Ward JHJ. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 1.15-0. Available online: http://vegan.r-forge.r-project.org (accessed on 26 October 2017).
- R Development Core Team. R: A Language and Environment for Statistical Computing, 3-900051-07-0; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: http://www.R-project.org (accessed on 26 October 2017).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research R Package Version 1.0-9. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 1 April 2017).
- Minchin, P.R. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 1987, 69, 89–107. [Google Scholar] [CrossRef]
- Virtanen, R.; Ilmonen, J.; Paasivirta, L.; Muotka, T. Community concordance between bryophyte and insect assemblages in boreal springs: A broad-scale study in isolated habitats. Freshw. Biol. 2007, 54, 1651–1662. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Vincenzi, S.; Zucchetta, M.; Franzoi, P.; Pellizzato, M.; Pranovi, F.; De Leo, G.A.; Torricelli, P. Application of a Random Forest algorithm to predict spatial distribution of the potential yield of Ruditapes philippinarum in the Venice lagoon, Italy. Ecol. Model. 2011, 222, 1471–1478. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Dobson, F.S.; Yu, J.; Smith, A.T. The importance of evaluating rarity. Conserv. Biol. 1995, 9, 1648–1651. [Google Scholar] [CrossRef]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Ockinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Russell, G.J.; Gittleman, J.L.; Brooks, T.M. The future of biodiversity. Science 1995, 269, 347–350. [Google Scholar] [CrossRef] [PubMed]
- De Bello, F.; Lavorel, S.; Lavergne, S.; Albert, C.H.; Boulangeat, I.; Mazel, F.; Thuiller, W. Hierarchical effects of environmental filters on the functional structure of plant communities: A case study in the French Alps. Ecography 2013, 36, 393–402. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Allan, J.D.; Flecker, A.S. Biodiversity conservation in running waters. BioScience 1993, 43, 32–43. [Google Scholar] [CrossRef]
- Van der Schalie, H. The value of mussel distribution in tracing stream confluence. Mich. Acad. Sci. Arts Lett. 1945, 30, 355–373. [Google Scholar]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Döll, P.; Zhang, J. Impact of climate change on freshwater ecosystems: A global-scale analysis of ecologically relevant river flow alterations. Hydrol. Earth Syst. Sci. 2010, 14, 783–799. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; de Figueroa, J.M.T.; Lek, S.; Park, Y.S. Continental drift and climate change drive instability in insect assemblages. Sci. Rep. 2015, 5, 11343. [Google Scholar] [CrossRef] [PubMed]
- Flather, C.H.; Knowles, M.S.; Kendall, I.A. Threatened and endangered species geography. BioScience 1988, 48, 365–376. [Google Scholar] [CrossRef]
- Deacon, J.E.; Williams, A.E.; Williams, C.D.; Williams, J.E. Fueling population growth in Las Vegas: How large-scale groundwater withdrawal could burn regional biodiversity. BioScience 2007, 57, 688–698. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E. Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. J. Appl. Ecol. 2007, 44, 1089–1094. [Google Scholar] [CrossRef]
- Petts, G.E. Impounded Rivers: Perspectives for Ecological Management; Wiley: Chichester, UK, 1984; p. 285. [Google Scholar]
- Vaughn, C.C.; Taylor, C.M. Impoundments and the decline of freshwater mussels: A case study of an extinction gradient. Conserv. Biol. 1999, 13, 912–920. [Google Scholar] [CrossRef]
- Watters, G.T. Freshwater mussels and water quality: A review of the effects of hydrologic and instream habitat alterations. In Freshwater Mollusk Symposium Proceedings; Tankersley, R.A., Warmolts, D.I., Watters, G.T., Armitage, B.J., Johnson, P.D., Butler, R.S., Eds.; Ohio Biological Survey: Columbus, OH, USA, 2000; pp. 261–274. [Google Scholar]
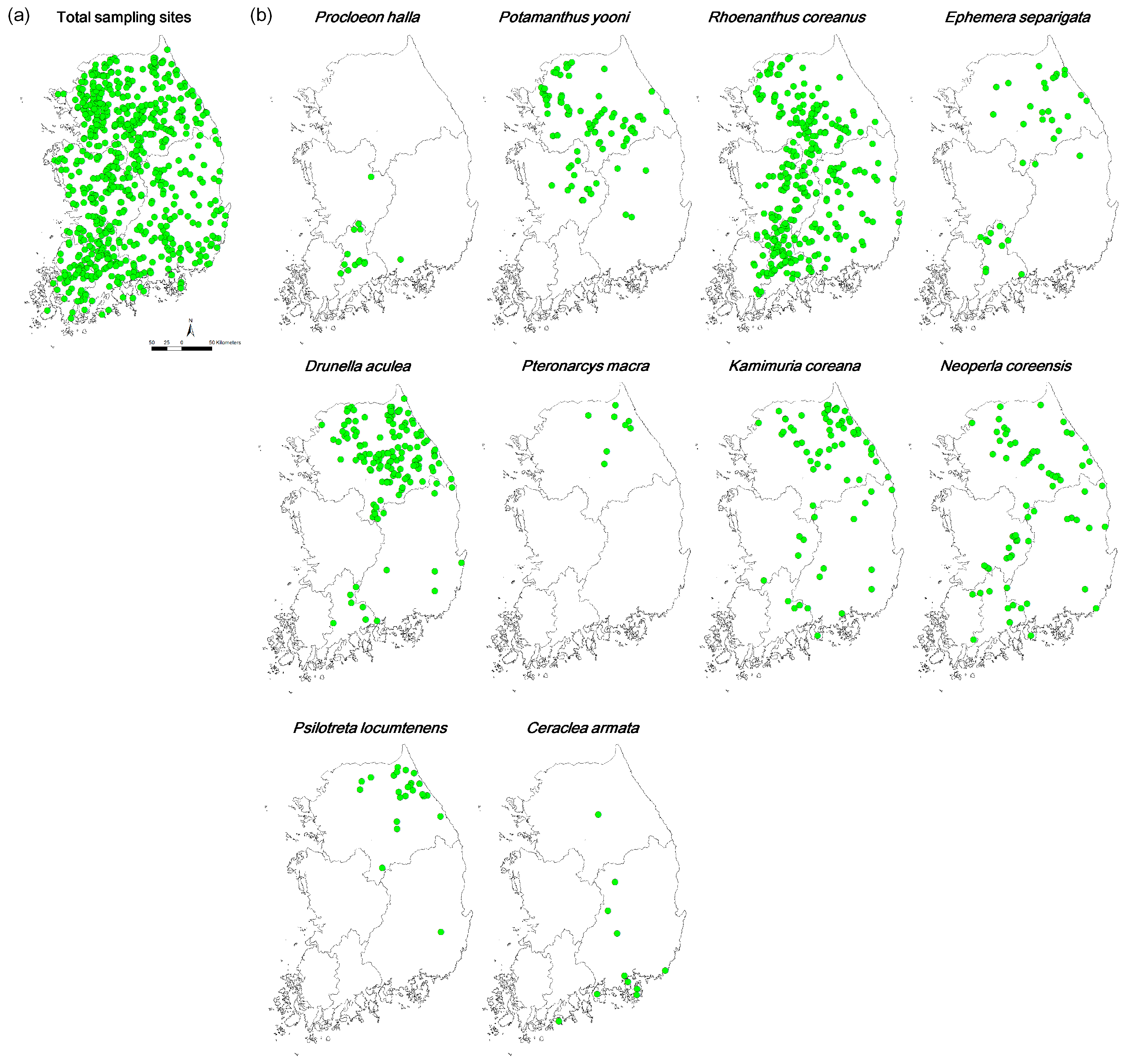

| Order | Species | Abbreviation | River Basin (%) | ||||
|---|---|---|---|---|---|---|---|
| Total | Han | Nakdong | Guem | Yeongseum | |||
| Ephemeroptera | Procloeon halla | Proc | 14 (2.0) | 0 (0.0) | 1 (0.8) | 1 (0.8) | 12 (9.0) |
| Potamanthus yooni | Pota | 86 (12.0) | 67 (20.9) | 4 (3.1) | 15 (11.5) | 0 (0.0) | |
| Rhoenanthus coreanus | Rhoe | 301 (42.2) | 112 (35.0) | 57 (43.8) | 59 (45.4) | 73 (54.5) | |
| Ephemera separigata | Ephe | 36 (5.0) | 23 (7.2) | 2 (1.5) | 0 (0.0) | 11 (8.2) | |
| Drunella aculea | Drun | 151 (21.1) | 135 (42.2) | 10 (7.7) | 0 (0.0) | 6 (4.5) | |
| Plecoptera | Pteronarcys macra | Pter | 8 (1.1) | 8 (2.5) | 0 (0) | 0 (0.0) | 0 (0.0) |
| Kamimuria coreana | Kami | 75 (10.5) | 56 (17.5) | 11 (8.5) | 4 (3.1) | 4 (.03) | |
| Neoperla coreensis | Neop | 69 (9.7) | 33 (10.3) | 13 (10.0) | 14 (10.8) | 9 (6.7) | |
| Trichoptera | Psilotreta locumtenens | Psil | 25 (3.5) | 24 (7.5) | 1 (0.8) | 0 (0.0) | 0 (0.0) |
| Ceraclea armata | Cera | 11 (1.5) | 1 (0.3) | 9 (6.9) | 0 (0.0) | 1 (0.0) | |
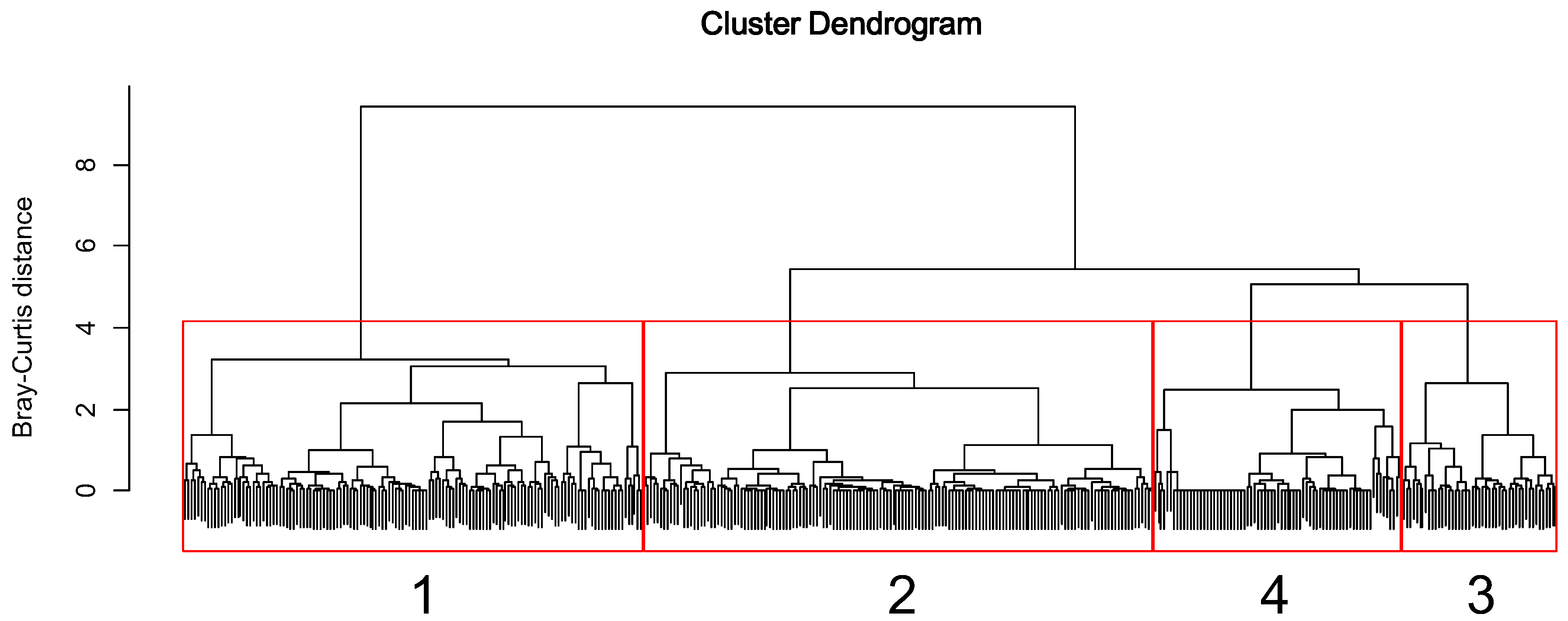
| Order | Species | Cluster | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Ephemeroptera | Procloeon halla | 0.00 (0.00) b | 0.03 (0.10) a | 0.00 (0.00) b | 0.32 (2.26) a |
| Potamanthus yooni | 0.42 (2.52) b | 0.16 (0.49) b | 8.22 (15.28) a | 0.00 (0.00) c | |
| Rhoenanthus coreanus | 0.46 (1.23) c | 25.24 (39.77) a | 9.47 (19.77) b | 0.88 (0.68) b | |
| Ephemera separigata | 0.13 (0.48) a | 0.09 (0.52) ab | 0.02 (0.11) b | 0.04 (0.16) ab | |
| Drunella aculea | 11.83 (16.3) a | 0.06 (0.32) b | 0.03 (0.13) b | 0.04 (0.14) b | |
| Plecoptera | Pteronarcys macra | 0.35 (2.62) a | 0.00 (0.00) b | 0.00 (0.00) b | 0.00 (0.00) b |
| Kamimuria coreana | 2.51 (6.35) a | 0.12 (0.65) b | 0.01 (0.05) b | 0.02 (0.08) b | |
| Neoperla coreensis | 0.19 (0.63) a | 1.23 (3.56) a | 0.02 (0.09) b | 0.03 (0.12) b | |
| Trichoptera | Psilotreta locumtenens | 1.13 (4.57) a | 0.00 (0.00) b | 0.00 (0.00) b | 0.00 (0.00) b |
| Ceraclea armata | 0.09 (0.56) a | 0.00 (0.04) b | 0.00 (0.00) b | 0.00 (0.04) ab | |
| Environmental Factor | Abbreviation | NMS1 | NMS2 | NMS3 | R2 | p |
|---|---|---|---|---|---|---|
| Geology | ||||||
| Latitude | Lati_X | −0.81 | 0.56 | 0.20 | 0.45 | 0.001 |
| Longitude | Longi_Y_ | −0.89 | −0.46 | −0.06 | 0.24 | 0.001 |
| Altitude (m) | −0.85 | −0.08 | 0.51 | 0.37 | 0.001 | |
| Slope (°) | −0.82 | −0.43 | 0.39 | 0.19 | 0.001 | |
| Distance from source | DFS | 0.85 | 0.45 | 0.29 | 0.08 | 0.001 |
| Meteorological factors | ||||||
| Annual average temperature (°C) | Ave_temp | 0.93 | −0.15 | −0.34 | 0.60 | 0.001 |
| August temperature (°C) | Aug_temp | 0.95 | 0.09 | −0.29 | 0.56 | 0.001 |
| January temperature (°C) | Jan_temp | 0.84 | −0.37 | −0.40 | 0.50 | 0.001 |
| Thermal range (°C) | Thermal_range | −0.20 | 0.91 | 0.37 | 0.20 | 0.001 |
| Precipitation (mm) | −0.09 | −0.90 | −0.42 | 0.05 | 0.002 | |
| Land use (%) | ||||||
| Urban | 0.36 | 0.47 | −0.81 | 0.10 | 0.001 | |
| Agriculture | 0.86 | 0.47 | 0.20 | 0.09 | 0.001 | |
| Forest | −0.78 | −0.54 | 0.33 | 0.20 | 0.001 | |
| Grass | 0.48 | 0.25 | −0.84 | 0.01 | 0.281 | |
| Bareland | 0.98 | 0.19 | 0.04 | 0.02 | 0.044 | |
| Hydrology | ||||||
| Stream order | Str_order | 0.87 | 0.46 | 0.20 | 0.23 | 0.001 |
| Water width (m) | W_width | 0.84 | 0.53 | 0.09 | 0.12 | 0.001 |
| Average depth (cm) | Ave_depth | 0.62 | 0.75 | 0.24 | 0.12 | 0.001 |
| Average velocity (cm/s) | Ave_velocity | −0.93 | 0.26 | 0.25 | 0.35 | 0.001 |
| Flow type | ||||||
| Riffle | −0.95 | −0.26 | 0.19 | 0.29 | 0.001 | |
| Run | 0.84 | 0.42 | −0.35 | 0.08 | 0.001 | |
| Pool | 1.00 | −0.02 | 0.10 | 0.07 | 0.001 | |
| Substrate (%) | ||||||
| Silt | 0.67 | 0.31 | −0.68 | 0.02 | 0.037 | |
| Clay | 0.66 | 0.70 | −0.28 | 0.10 | 0.001 | |
| Sand | 0.44 | 0.68 | −0.59 | 0.22 | 0.001 | |
| Small pebble | 0.84 | −0.54 | −0.04 | 0.04 | 0.003 | |
| Pebble | −0.12 | −0.80 | 0.59 | 0.04 | 0.002 | |
| Cobble | −0.61 | −0.46 | 0.64 | 0.22 | 0.001 | |
| Boulder | −0.73 | −0.63 | 0.28 | 0.18 | 0.001 | |
| Physicochemical water quality | ||||||
| Biological oxygen demand (mg/L) | BOD | 0.78 | 0.57 | −0.27 | 0.33 | 0.001 |
| Total nitrogen (mg/L) | TN | −0.03 | 1.00 | −0.09 | 0.15 | 0.001 |
| Ammonia nitrogen (mg/L) | NH3N | 0.55 | 0.82 | −0.16 | 0.05 | 0.002 |
| Nitrate nitrogen (mg/L) | NO3N | 0.23 | 0.97 | 0.12 | 0.13 | 0.001 |
| Total phosphate (mg/L) | TP. | 0.83 | 0.49 | −0.25 | 0.31 | 0.001 |
| Phosphate-phosphorus (mg/L) | PO4P | 0.91 | 0.40 | −0.12 | 0.21 | 0.001 |
| Chlorophyll a (mg/L) | Chl.a | 0.88 | 0.43 | −0.22 | 0.38 | 0.001 |
| Dissolved oxygen (mg/L) | DO | −0.68 | 0.58 | 0.46 | 0.19 | 0.001 |
| pH | pH | 0.80 | 0.46 | 0.39 | 0.10 | 0.001 |
| Conductivity (um/S) | Conductivity | 0.73 | 0.63 | −0.269 | 0.32 | 0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.-J.; Park, Y.-S. Diversity and Distribution of Endemic Stream Insects on a Nationwide Scale, South Korea: Conservation Perspectives. Water 2017, 9, 833. https://doi.org/10.3390/w9110833
Bae M-J, Park Y-S. Diversity and Distribution of Endemic Stream Insects on a Nationwide Scale, South Korea: Conservation Perspectives. Water. 2017; 9(11):833. https://doi.org/10.3390/w9110833
Chicago/Turabian StyleBae, Mi-Jung, and Young-Seuk Park. 2017. "Diversity and Distribution of Endemic Stream Insects on a Nationwide Scale, South Korea: Conservation Perspectives" Water 9, no. 11: 833. https://doi.org/10.3390/w9110833





