Removal of Heavy Metals from Urban Stormwater Runoff Using Bioretention Media Mix
Abstract
:1. Introduction
2. Experiment Design
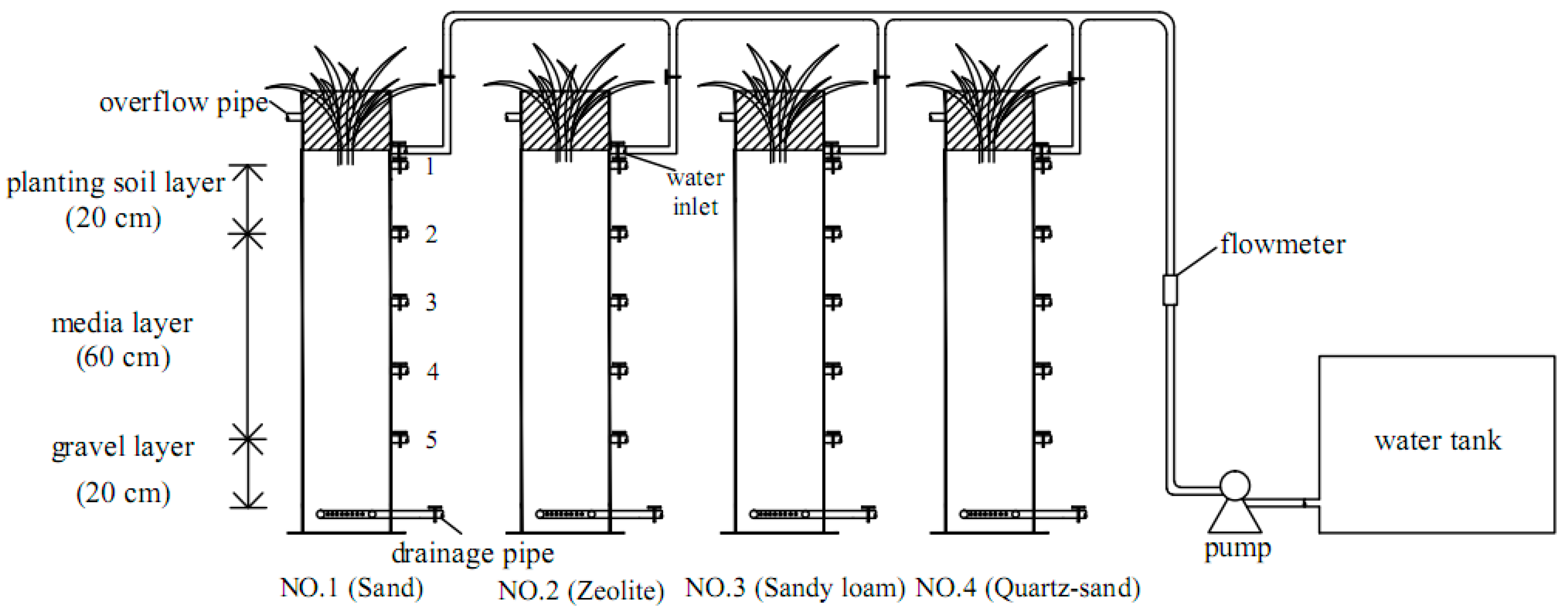
2.1. Experimental Device
2.2. The Composition of Bioretention Media
2.3. Experimental and Analytical Methods
3. Experimental Results and Discussion
3.1. Permeability Coefficients of the Fourreactors
3.2. Removal Efficiency of Heavy Metals via Bioretention Media Mix
3.3. Factors Affecting Heavy Metal Removal
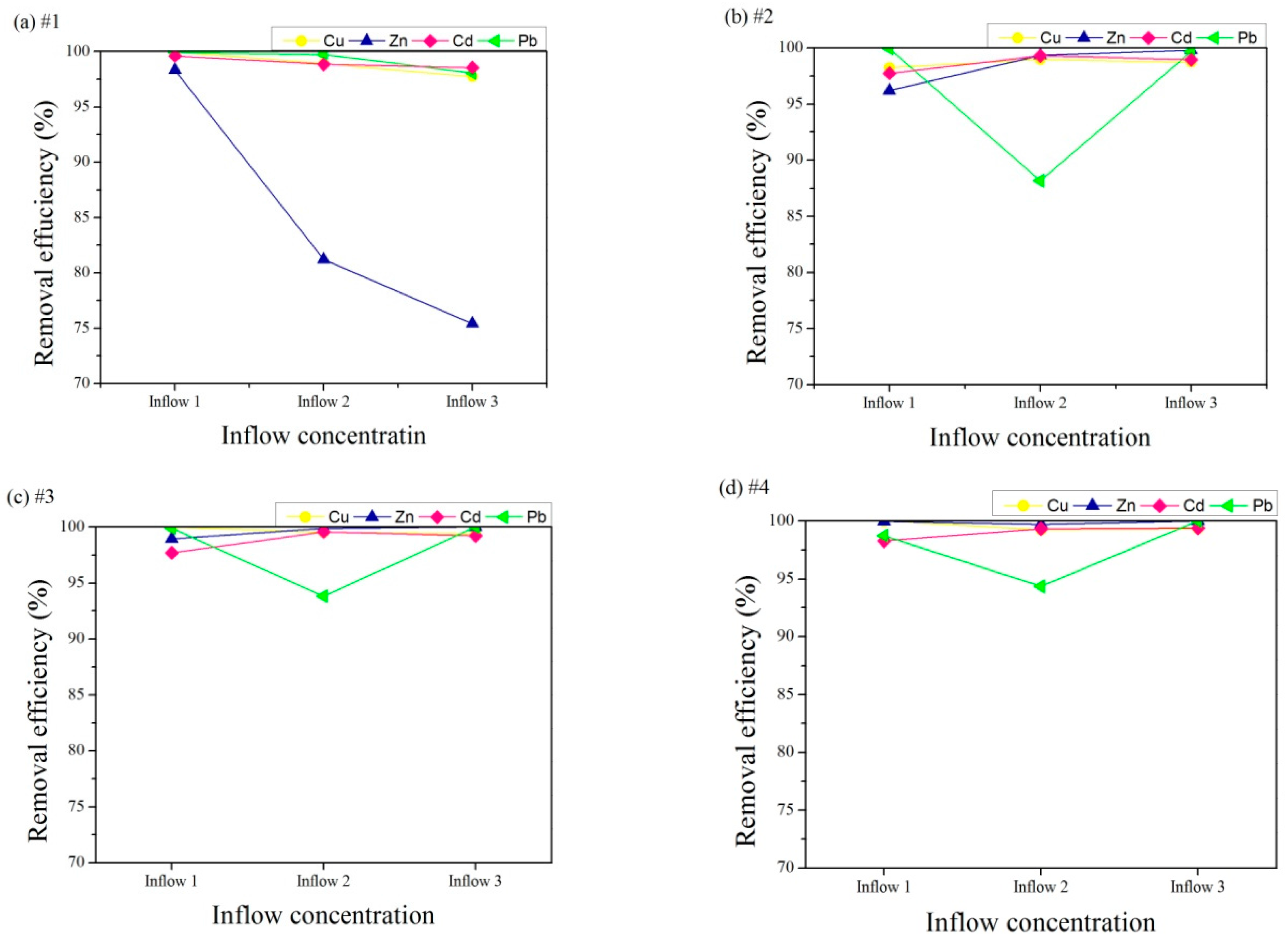
3.3.1. Influent Heavy Metal Concentration
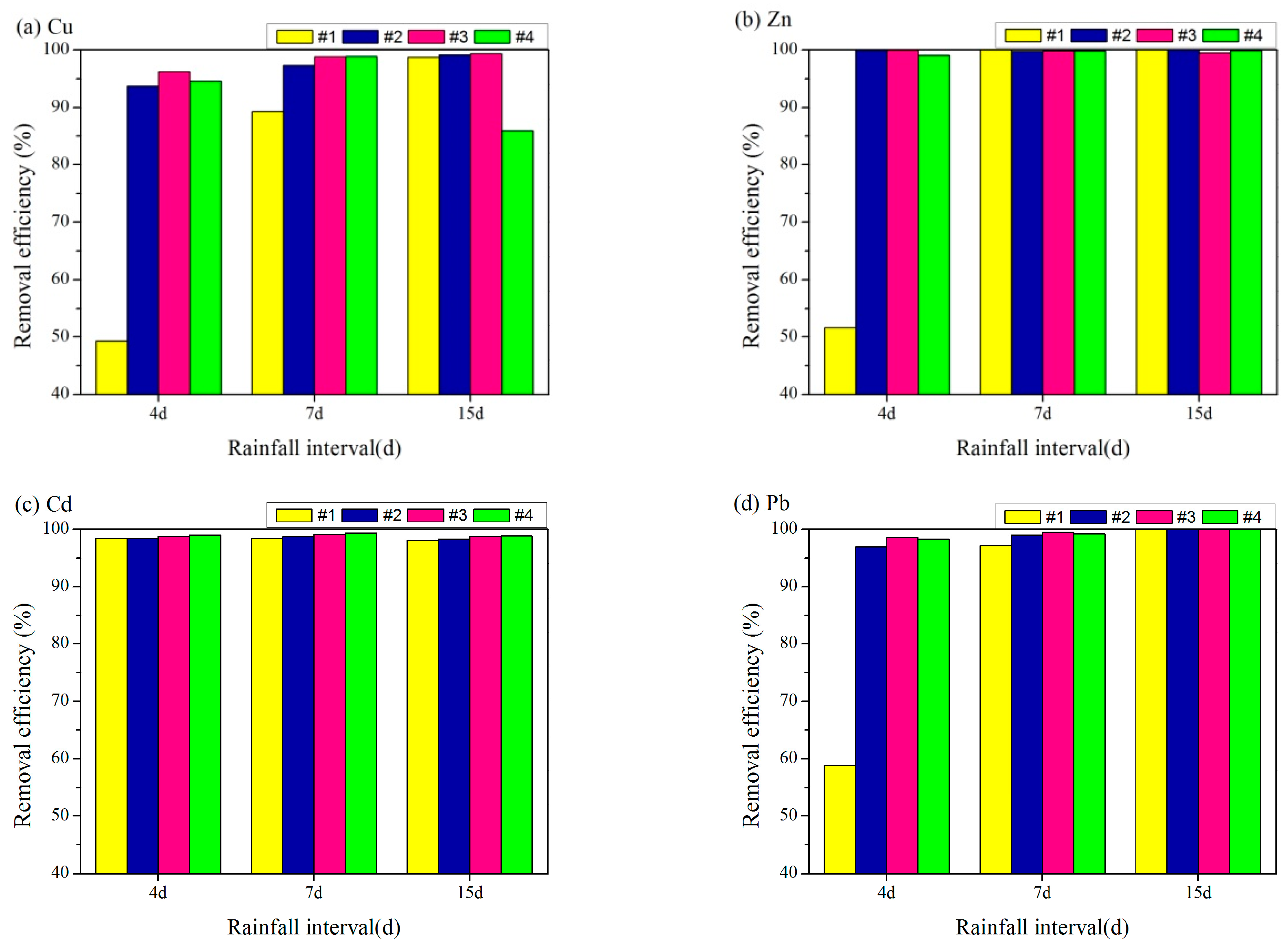
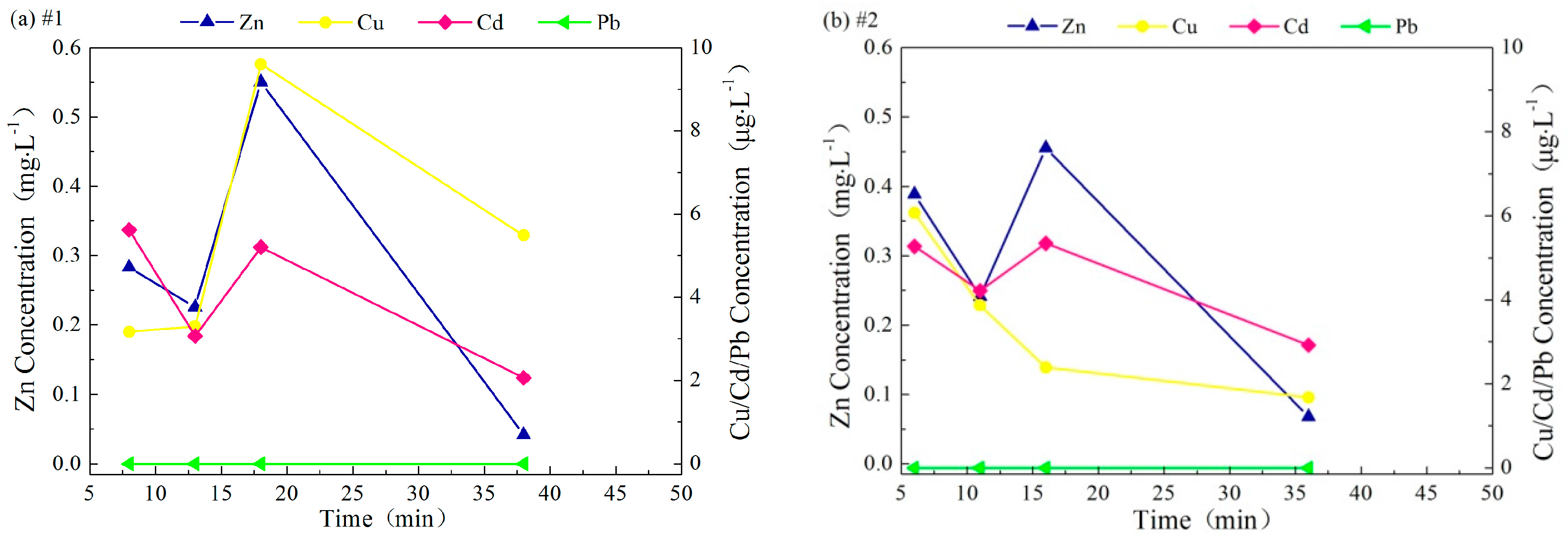
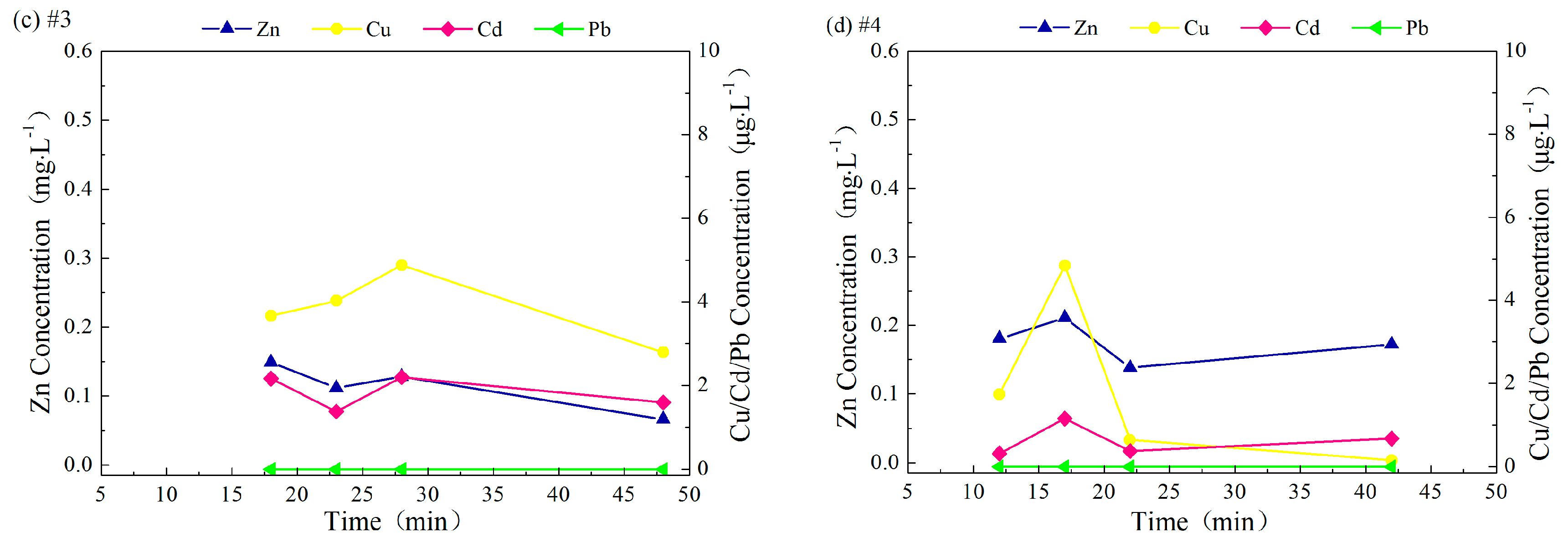
3.3.2. Interval between Rainfall Events
3.3.3. pH
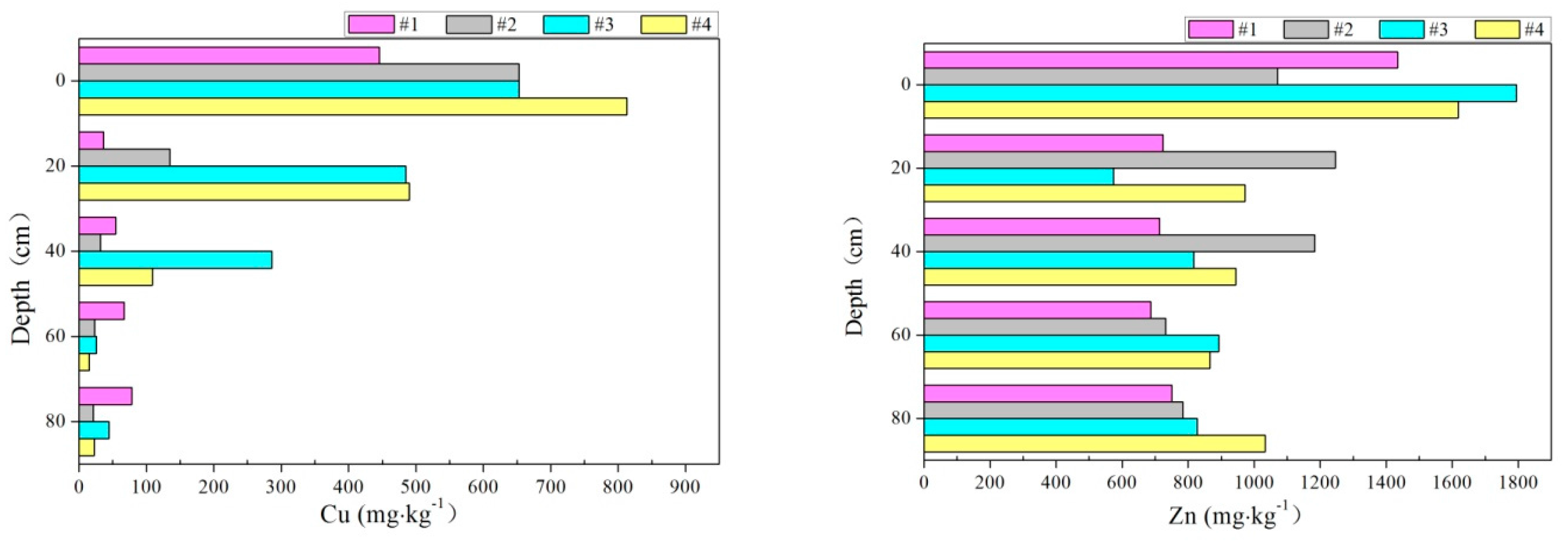
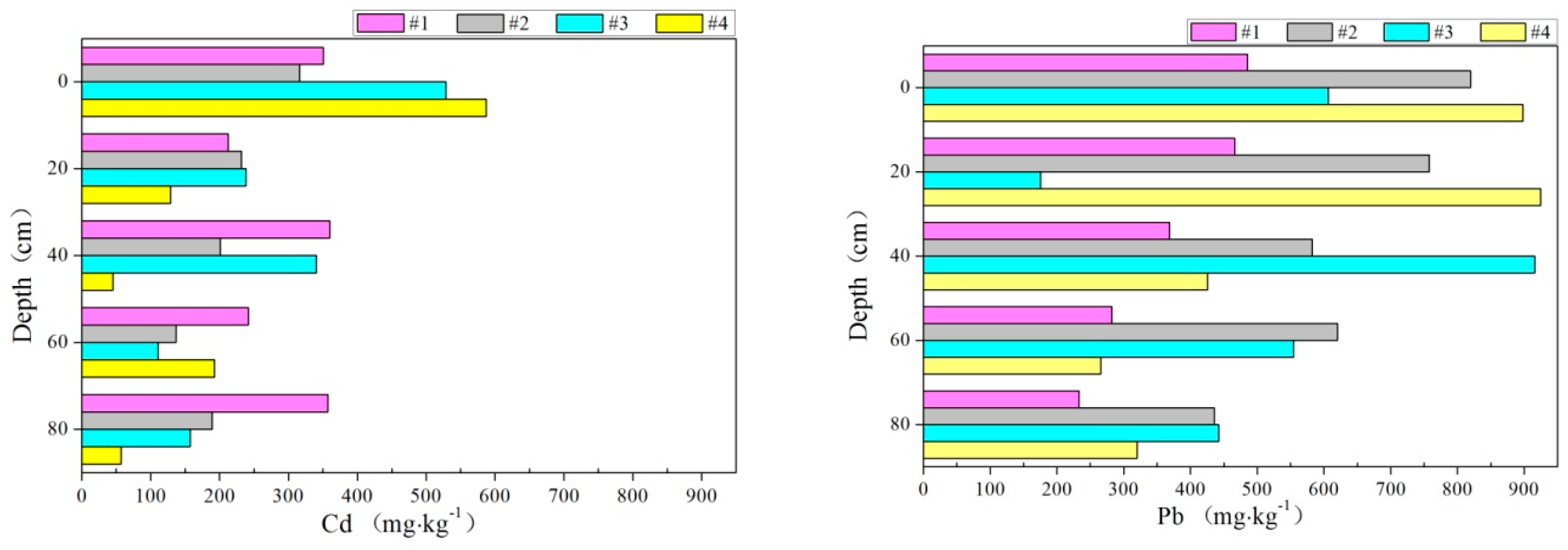
3.4. Accumulation of Heavy Metals
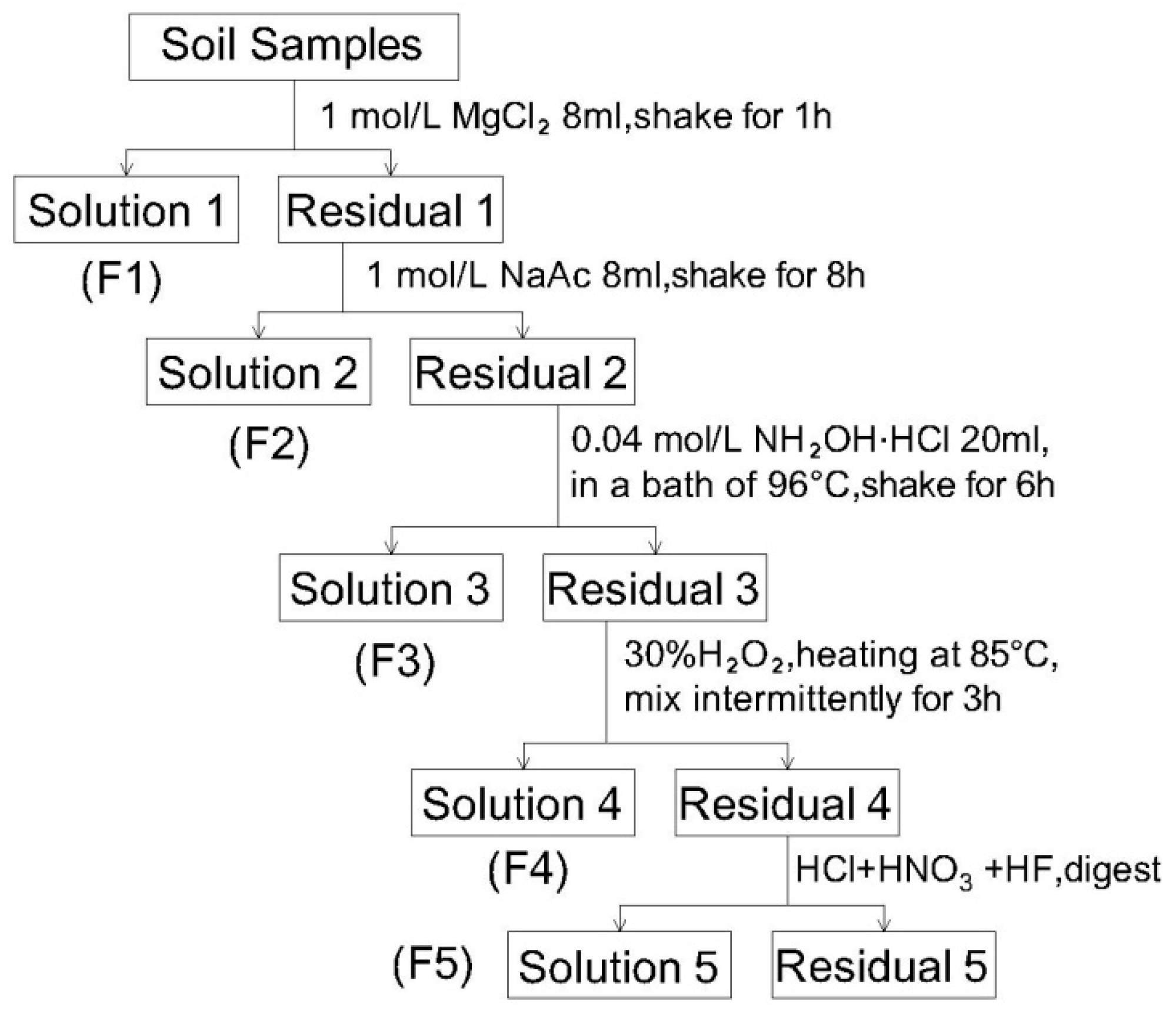
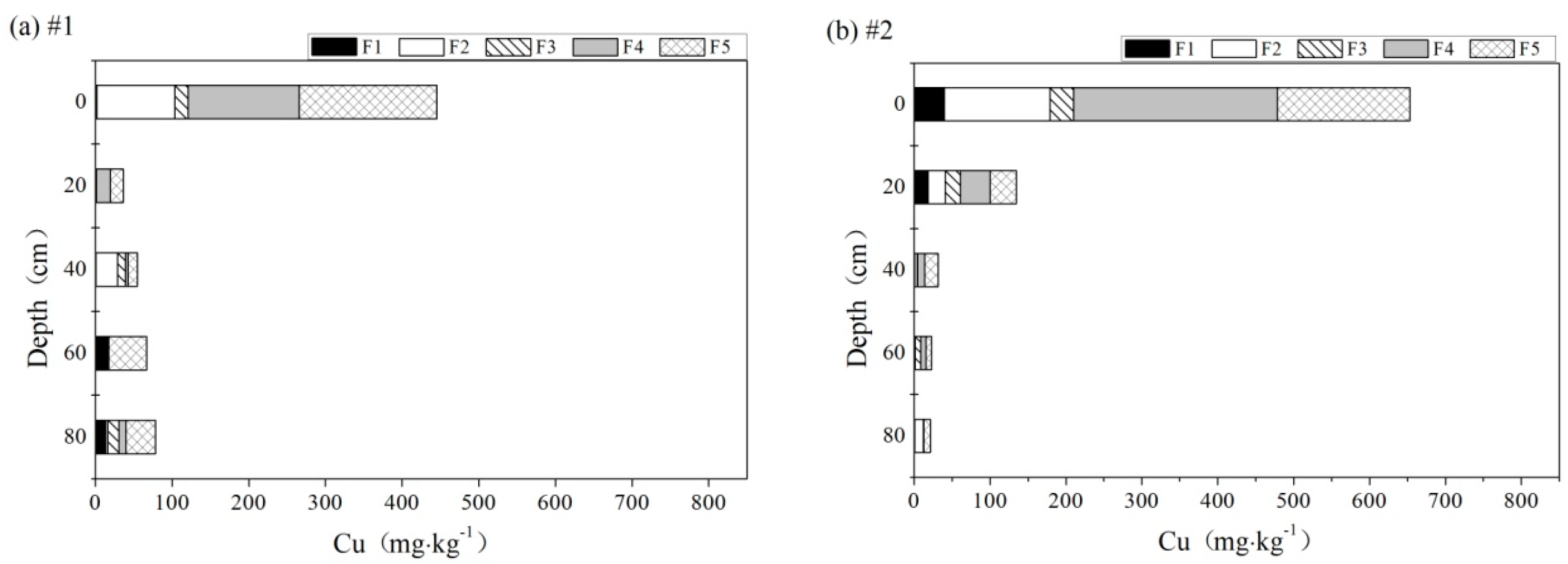
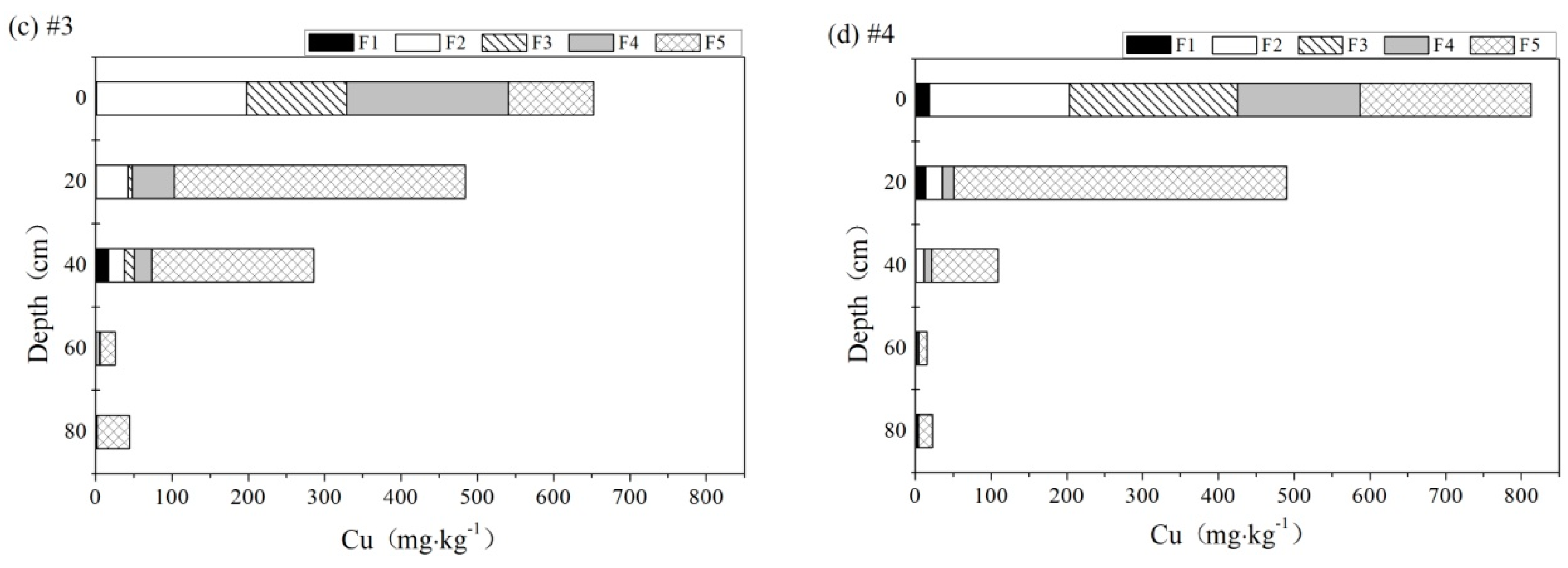
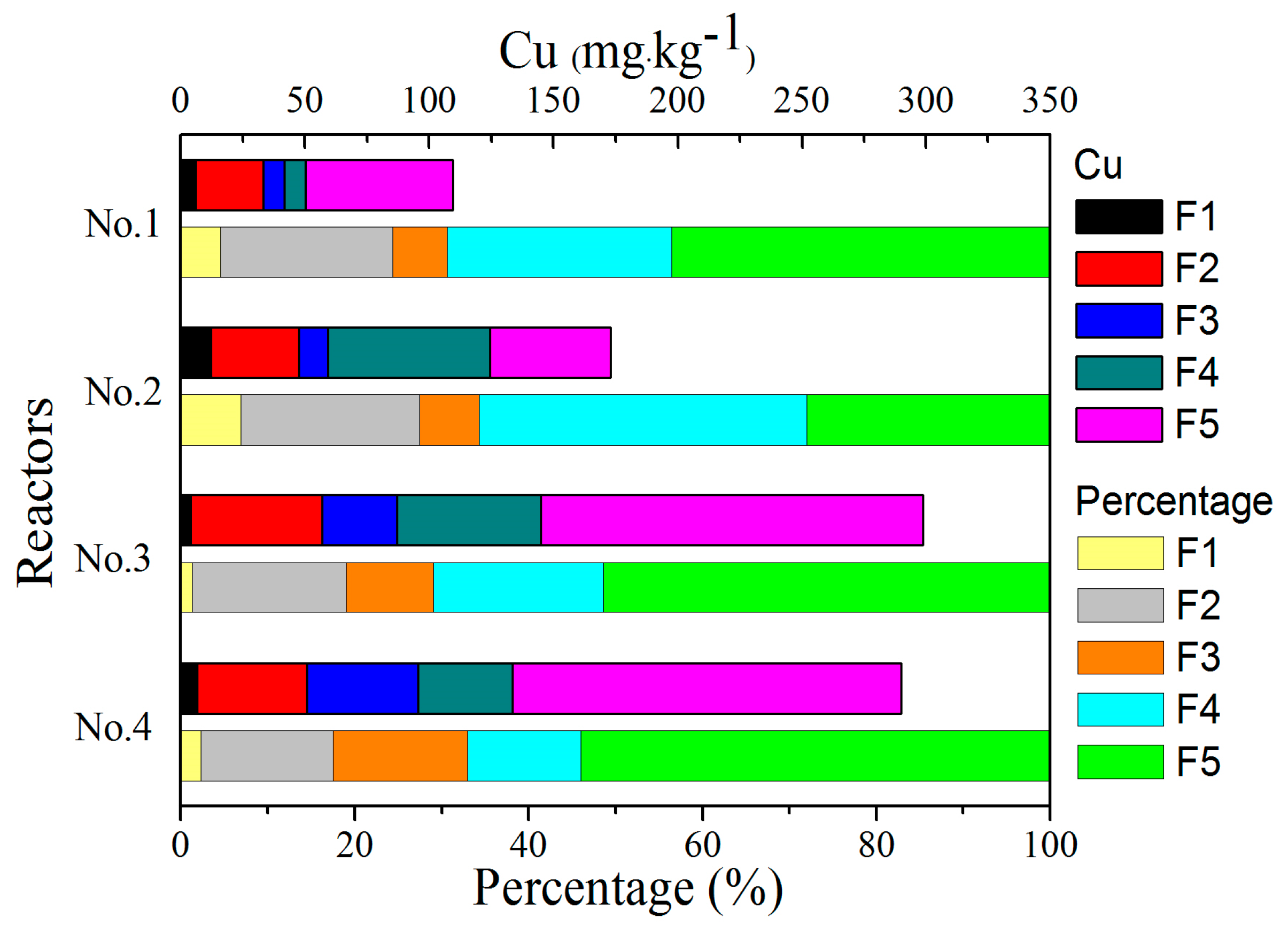
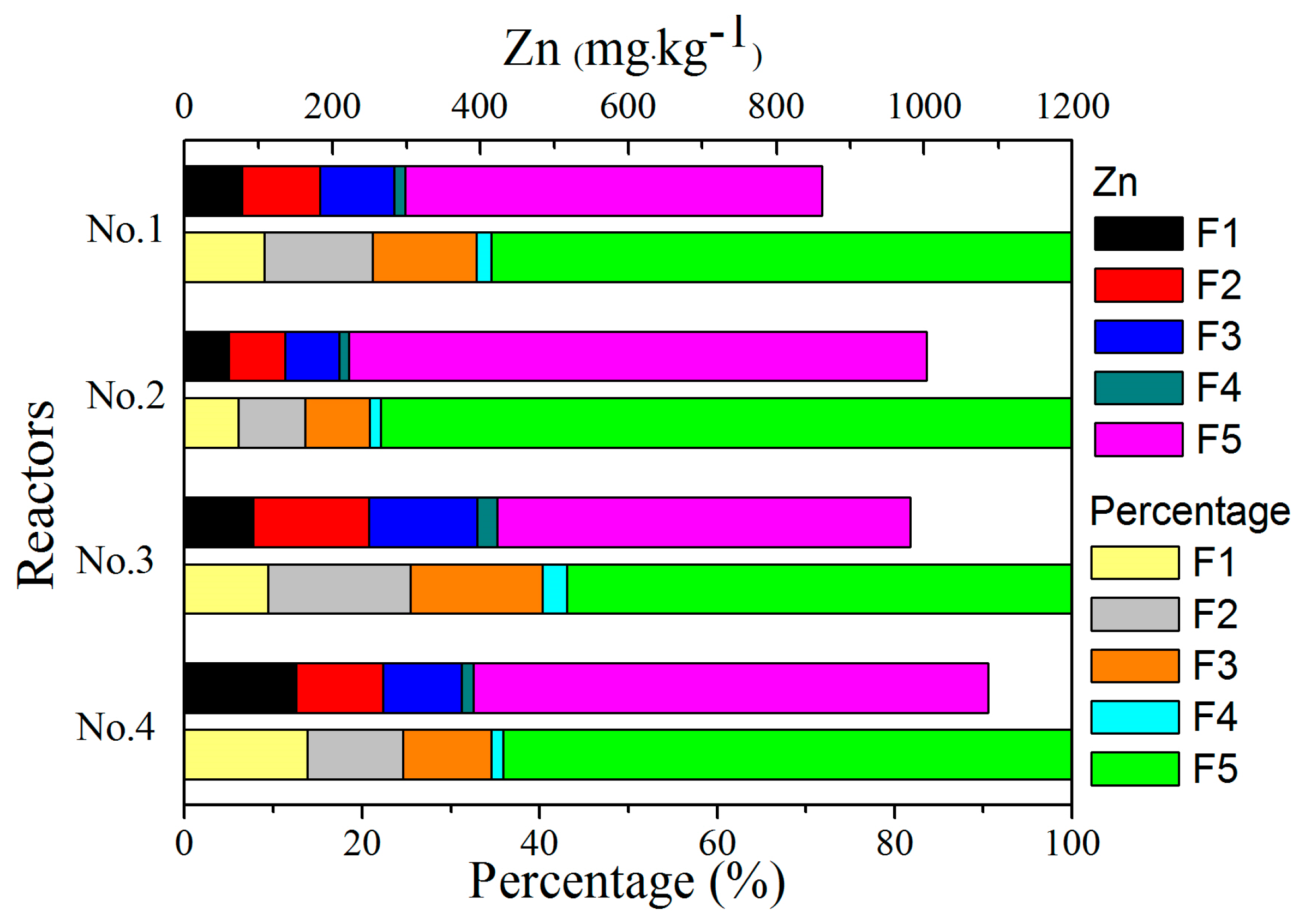
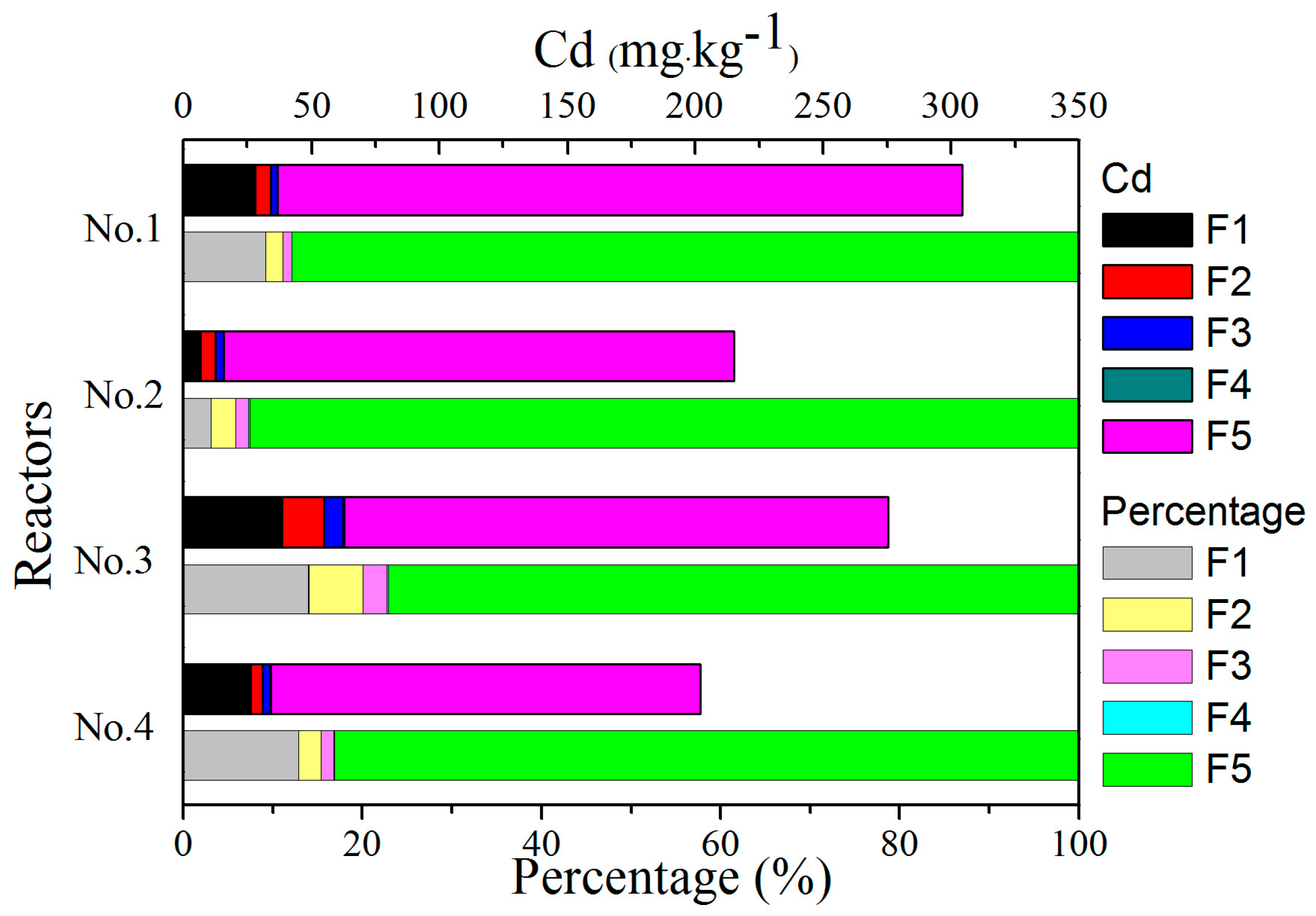
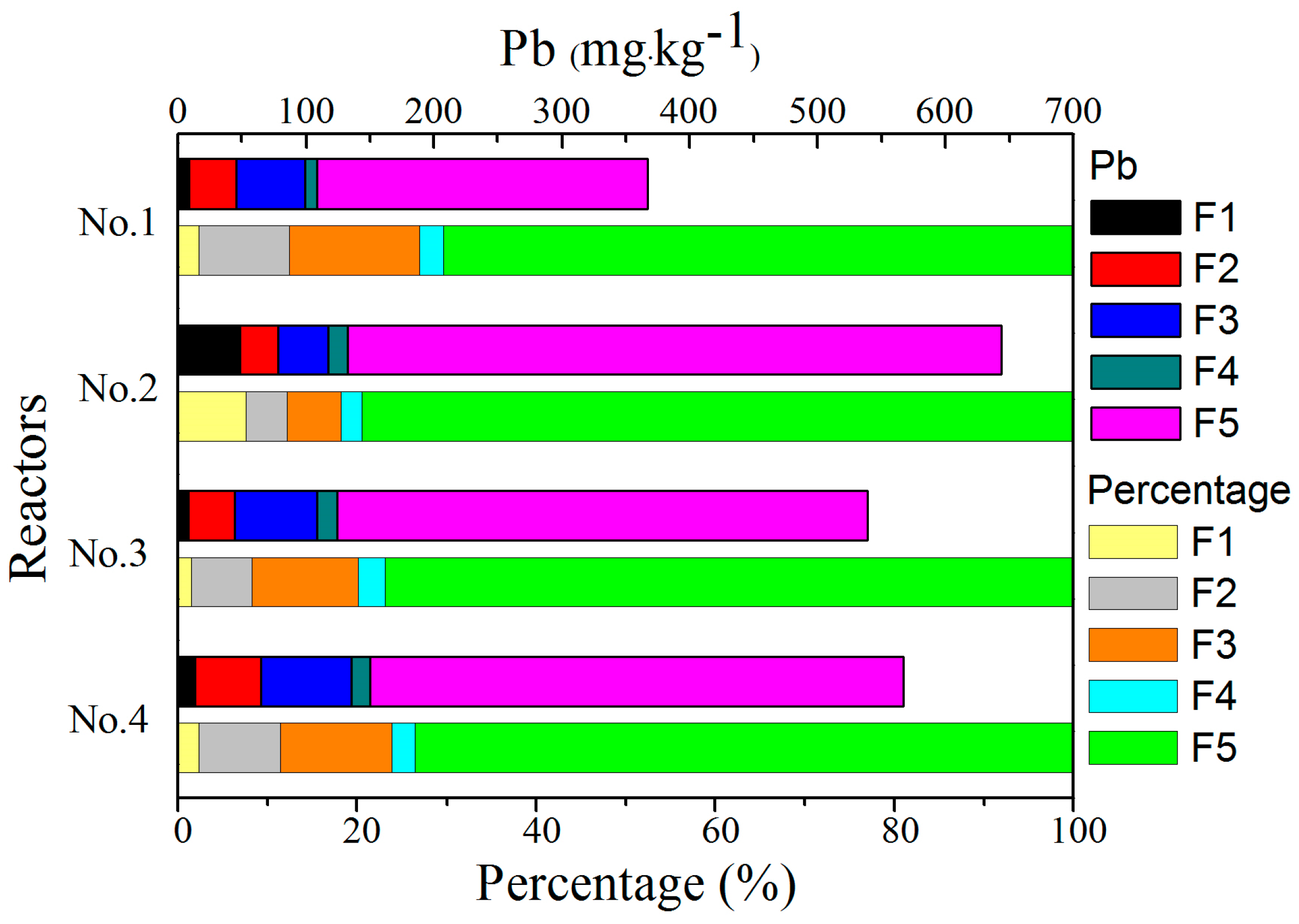
3.5. Speciation of Heavy Metals
3.5.1. Cu
3.5.2. Zn
3.5.3. Cd
3.5.4. Pb
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Heavy Metal (Units) | Reactor (Media Mix) | Influent Concentrations | Effluent Concentrations | Removal Efficiency |
|---|---|---|---|---|
| Cu (μg·L−1) | No.1 (sand) | 303.6 | 0.1 | 100.0% |
| 710.0 | 7.9 | 98.9% | ||
| 1116.4 | 26.4 | 97.7% | ||
| No.2 (zeolite) | 303.6 | 5.4 | 98.2% | |
| 710.0 | 7.4 | 99.0 | ||
| 1116.4 | 14.7 | 98.7 | ||
| No.3 (sandy loam) | 303.6 | 0.0 | 100.0% | |
| 710.0 | 3.2 | 99.6% | ||
| 1116.4 | 7.0 | 99.4% | ||
| No.4 (quartz-sand) | 303.6 | 0.0 | 100.0% | |
| 710.0 | 5.2 | 99.3% | ||
| 1116.4 | 7.6 | 99.3% | ||
| Pb (μg·L−1) | No.1 (sand) | 138.9 | 0.1 | 99.9% |
| 216.6 | 0.5 | 99.8% | ||
| 327.1 | 6.3 | 98.1% | ||
| No.2 (zeolite) | 138.9 | 0.1 | 99.9% | |
| 216.6 | 25.6 | 88.2% | ||
| 327.1 | 1.0 | 99.7% | ||
| No.3 (sandy loam) | 138.9 | 0.1 | 99.9% | |
| 216.6 | 13.4 | 93.8% | ||
| 327.1 | 0.0 | 100.0% | ||
| No.4 (quartz-sand) | 138.9 | 1.8 | 98.3% | |
| 216.6 | 12.3 | 99.3% | ||
| 327.1 | 1.0 | 99.7% | ||
| Cd (μg·L−1) | No.1 (sand) | 557.8 | 2.2 | 99.6% |
| 771.5 | 9.0 | 98.8% | ||
| 1017.2 | 14.8 | 98.5% | ||
| No.2 (zeolite) | 557.8 | 9.8 | 97.7% | |
| 771.5 | 5.3 | 99.3% | ||
| 1017.2 | 10.8 | 98.9% | ||
| No.3 (sandy loam) | 557.8 | 12.9 | 97.7% | |
| 771.5 | 3.3 | 99.6% | ||
| 1017.2 | 7.9% | 99.2% | ||
| No.4 (quartz-sand) | 557.8 | 9.8 | 98.3% | |
| 771.5 | 5.3 | 99.3% | ||
| 1017.2 | 6.0 | 99.4% | ||
| Zn (μg·L−1) | No.1 (sand) | 1808.8 | 30.7 | 98.3% |
| 2457.7 | 462.1 | 81.2% | ||
| 3461.8 | 851.3 | 75.4% | ||
| No.2 (zeolite) | 1808.8 | 69.0 | 96.2% | |
| 2457.7 | 16.7 | 99.3% | ||
| 3461.8 | 7.1 | 99.8% | ||
| No.3 (sandy loam) | 1808.8 | 18.9 | 99.0% | |
| 2457.7 | 3.7 | 99.8% | ||
| 3461.8 | 0.2 | 100% | ||
| No.4 (quartz-sand) | 1808.8 | 0.7 | 100.0% | |
| 2457.7 | 6.9 | 99.7% | ||
| 3461.8 | 0.3 | 100.0% |
References
- Christina, M.C.; Hession, W.C.; Donna, M.R. Watershed imperviousness impacts on stream channel ondition in southeastern pennsylvania. J. Am. Water Resour. Assoc. 2006, 42, 941–956. [Google Scholar]
- Liu, J.; Sample, D.; Bell, C.; Guan, Y. Review and research needs of bioretention used for the treatment of urban stormwater. Water 2014, 6, 1069–1099. [Google Scholar] [CrossRef]
- Davis, B.; Birch, G. Comparison of heavy metal loads in stormwater runoff from major and minor urban roads using pollutant yield rating curves. Environ. Pollut. 2010, 158, 2541–2545. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Cadmium removal from urban stormwater runoff via bioretention technology and effluent risk assessment for discharge to surface water. J. Contam. Hydrol. 2016, 185–186, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Yin, C.Q.; He, Q.C.; Kong, L.L. Contribution of pollution load of storm runoff in urban areas of Hanyang, Wuhan City on the receiving water. China Environ. Sci. 2007, 3, 312–316. (In Chinese) [Google Scholar]
- Pan, G.Q.; Che, W.; Li, J.-Q.; Li, H.-Y. Urban runoff pollution control quantity and Its design rainfall in China. China Water Wastewater 2008, 22, 25–29. (In Chinese) [Google Scholar]
- Cederkvist, K.; Jensen, M.; Ingvertsen, S.; Holm, P. Controlling stormwater quality with filter soil—event and dry weather testing. Water 2016, 8, 349. [Google Scholar] [CrossRef]
- Helmreich, B.; Hilliges, R.; Schriewer, A.; Horn, H. Runoff pollutants of a highly trafficked urban road—Correlation analysis and seasonal influences. Chemosphere 2010, 80, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.B.; Revitt, D.M.; Harrop, D.O.; Beckwith, P.R. The contribution of highway surfaces to urban stormwater sediments and metal loadings. Sci. Total Environ. 1987, 59, 339–349. [Google Scholar] [CrossRef]
- Gao, J.J.; Zhang, L.P.; Huang, S.B.; Ma, M.; Wang, Z.J. Preliminary health risk assessment of heavy metals in drinking waters in Beijing. Environ. Sci. 2004, 25, 47–50. (In Chinese) [Google Scholar]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption characteristics of construction waste for heavy metals from urban stormwater runoff. Chin. J. Chem. Eng. 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Murakami, M.; Nakajima, F.; Furumai, H. The sorption of heavy metal species by sediments in soakaways receiving urban road runoff. Chemosphere 2008, 70, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Hu, L.; Wang, C.C. The measuring of heavy metal content in road runoff. Environ. Chem. 2009, 28, 145–146. (In Chinese) [Google Scholar]
- Li, Q.Q.; Li, T.L.; Zhao, Q.; Liu, D.; Jin, Z. Characteristics of heavy metal pollution in road rainfall-runoff of Tianjin city. Ecol. Environ. Sci. 2011, 1, 143–148. (In Chinese) [Google Scholar]
- Zhang, K.; He, L.I.; Dafang, F.U.; Fang, Z. Characteristics of heavy metal pollution in runoff from three different types of roofs. Acta Sci. Circumst. 2011, 31, 724–730. (In Chinese) [Google Scholar]
- Chinese Environmental Quality Standard for Surface Water(GB 3838–2002); China Environmental Science Press: Beijing, China, 2002.
- Ouyang, W.; Wang, W.; Haom, F.; Song, K.; Wang, Y. Pollution characterization of urban stormwater runoff on different underlying surface conditions. China Environ. Sci. 2010, 30, 1249–1256. (In Chinese) [Google Scholar]
- Davis, A.P. Green Engineering Principles Promote Low-impact Development. Environ. Sci. Technol. 2005, 39, 338A–344A. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Cao, Y.; Rippy, M.; Afrooz, A.; Grant, S. Indicator and pathogen removal by low impact development best management practices. Water 2016, 8, 600. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Winogradoff, D. Water quality improvement through bioretention: Lead, copper, and zinc removal. Water Environ. Res. 2003, 75, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Valeo, C.; Chu, A.; He, J. A data driven approach to bioretention cell performance: Prediction and design. Water 2013, 5, 13–28. [Google Scholar] [CrossRef]
- Lucke, T.; Nichols, P.W.B. The pollution removal and stormwater reduction performance of street-side bioretention basins after ten years in operation. Sci. Total Environ. 2015, 536, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Mohammad, S.; Himanshu, S.; Christie, M. Laboratory study of biological retention for urban stormwater management. Water Environ. Res. 2001, 71, 5–14. [Google Scholar] [CrossRef]
- Sun, X.; Davis, A.P. Heavy metal fates in laboratory bioretention systems. Chemosphere 2007, 66, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Davis, A.P. Heavy metal capture and accumulation in bioretention media. Environ. Sci. Technol. 2008, 42, 5247–5253. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.E. Lowimpact development practices: A reviewof current 17 research and recommendations for future directions. Water Air Soil Pollut. 2007, 186, 351–363. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Xu, H.; Ni, F.J.; Liu, Q.Q.; Shen, H.; Chen, R.S. Research on hydraulic conductivity of porous asphalt mixture. China J. Highw. Transp. 2004, 17, 1–5. (In Chinese) [Google Scholar]
- Wu, C.; Junqi, L. Urban Stormwater Utilization Technology and Management; China Architecture & Building Press: Beijing, China, 2006. [Google Scholar]
- Deletic, A.; McCarthy, D.; Chandresena, G.; Li, Y.; Hatt, B.; Payne, E.; Zhang, K.; Henry, R.; Kolotelo, P.; Randjelovic, A.; et al. Biofilters and Wetlands for Stormwater Treatment and Harvesting; Cooperative Research Centre for Water Sensitive Cities, Monash University: Melbourne, Australia, 2014; p. 67. [Google Scholar]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Removal of heavy metals from urban stormwater runoff using different filter materials. J. Environ. Chem. Eng. 2014, 2, 282–292. [Google Scholar] [CrossRef]
- Rios, C.A.; Williams, C.D.; Roberts, C.L. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, Y.S. Simultaneous removal of coexistent heavy metals from simulated urban stormwater using four sorbents: A porous iron sorbent and its mixtures with zeolite and crystal gravel. J. Hazard. Mater. 2009, 168, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Genç-Fuhrman, H.; Mikkelsen, P.S.; Ledin, A. Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater: Experimental comparison of 11 different sorbents. Water Res. 2007, 41, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Lim, W.; Hu, J.Y.; Ziegler, A.; Ong, S.L. Comparison of filter media materials for heavy metal removal from urban stormwater runoff using biofiltration systems. J. Environ. Manag. 2015, 147, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Marsalek, J.; Rochfort, Q. Use of a biofilter for treatment of heavy metals in highway runoff. Water Qual. Res. J. Can. 2000, 35, 563–580. [Google Scholar]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydraulic and pollutant removal performance of stormwater filters under variable wetting and drying regimes. Water Sci. Technol. 2007, 56, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.; Greenway, M.; Phillips, I. Removal of dissolved nitrogen, phosphorus and carbon from stormwater by biofiltration mesocosms. Water Sci. Technol. 2007, 55, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shan, B.; Tang, W.; Dong, L.; Zhang, W.; Pei, Y. Heavy metal concentrations and speciation in riverine sediments and the risks posed in three urban belts in the Haihe Basin. Ecotoxicol. Environ. Saf. 2017, 139, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Sreekumari, S.S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z. Cadmium adsorption in soil influenced by dissolved organic matter derived from rice straw and sediment. Chin. J. Appl. Ecol. 2002, 13, 183–186. [Google Scholar]
- Neyestani, M.R.; Bastami, K.D.; Esmaeilzadeh, M.; Shemirani, F.; Khazaali, A.; Molamohyeddin, N.; Afkhami, M.; Nourbakhsh, S.; Dehghani, M.; Aghaei, S.; et al. Geochemical speciation and ecological risk assessment of selected metals in the surface sediments of the northern Persian Gulf. Marine Pollut. Bull. 2016, 109, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Anju, M.; Banerjee, D.K. Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 2010, 78, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Wang, C.X.; Chen, Q. Form distribution and transformation of heavy metals of Cu, Pb, Zn, Cr and Cd in soils. Agro-Environ. Prot. 2002, 21, 9–12. (In Chinese) [Google Scholar]
- Wang, Z.W.; Liu, X.; Yao, X.S.; Hou, X. Effects of soluble inorganic salts on the distribution and bioavailability of cadmium in soil. J. Agric. Environ. Sci. 2008, 27, 884–888. (In Chinese) [Google Scholar]
- Xu, L.; Wang, T.; Wang, J.; Lu, A. Occurrence, speciation and transportation of heavy metals in 9 coastal rivers from watershed of Laizhou Bay, China. Chemosphere 2017, 173, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, K.; Noguera, D.R.; Jiang, J.; Oyserman, B.; Zhao, N.; Zhao, Q.; Cui, F. Transformation and speciation of typical heavy metals in soil aquifer treatment system during long time recharging with secondary effluent: Depth distribution and combination. Chemosphere 2016, 165, 100–109. [Google Scholar] [CrossRef] [PubMed]
| Sources | Cu | Pb | Cd | Zn |
|---|---|---|---|---|
| Level I [16] | 10.0 | 10.0 | 1.0 | 50.0 |
| Level II [16] | 1000.0 | 10.0 | 5.0 | 1000.0 |
| Level III [16] | 1000.0 | 50.0 | 50.0 | 1000.0 |
| Beijing [13] 1 | 53.0–987.0 | 56.0–774.0 | 20.0–162.0 | 1421.0–59,855.0 |
| Tianjin [14] 1 | 2.0–19.0 | -- | 1.0–19.0 | -- |
| Nanjing [15] 2 | 8.0–34.0 | 25.0–121.0 | 540.0–2340.0 | 240.0–1030.0 |
| Beijing [17] 3 | 500.0 | 80.0 | 40.0 | 2000.0 |
| Media Mix | Main Chemical Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| CO2 | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | K2O | |
| Sand + lignin | 36.6 | 42.6 | 9.73 | 3.02 | 1.29 | 1.64 | 2.06 |
| Zeolite + lignin | 40.2 | 43.6 | 9.15 | 2.17 | 0.89 | 1.13 | 1.4 |
| Sandy-loam + lignin | 16.5 | 53.2 | 13.1 | 5.87 | 2.57 | 3.69 | 2.47 |
| Quartz-sand+ lignin | 9.32 | 81.7 | 4.15 | 0.78 | 0.44 | 0.86 | 2.18 |
| Planting soil | 43.6 | 34.8 | 10.5 | 1.75 | 1.9 | 2.53 | 1.42 |
| Heavy Metal | Source | Analysis Method | Testing Equipment | Detection Limit (μg·L−1) |
|---|---|---|---|---|
| Cu | CuCl2·2H2O | Graphite furnace-AAS 1 | Hitachi Z-2010 Polarization Zeeman AAS 2 | 1.0 |
| Pb | Pb(NO3)2 | Graphite furnace-AAS | 1.0 | |
| Cd | CdCl2·2.5H2O | Graphite furnace-AAS | 0.2 | |
| Zn | ZnSO4·7H2O | Flame method | 0.1 |
| Heavy Metals | Base 1 | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| Cu | 500.0 | 303.6 | 710.0 | 1116.4 |
| Pb | 80.0 | 138.9 | 216.6 | 327.1 |
| Cd | 40.0 | 557.8 | 771.5 | 1017.2 |
| Zn | 2000.0 | 1808.8 | 2457.7 | 3461.8 |
| Heavy Metals | Reactor No. 1 (Sand) | Reactor No. 2 (Zeolite) | ||||||
| Cu | Pb | Cd | Zn | Cu | Pb | Cd | Zn | |
| Minimum | 98.0 | 100.0 | 94.1 | 95.7 | 98.2 | 99.1 | 100.0 | 95.1 |
| Maximum | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Mean 1 | 99.5 ± 1.0 | 100.0 ± 0.0 | 98.4 ± 2.9 | 98.5 ± 2.0 | 99.1 ± 0.9 | 99.8 ± 0.4 | 100.0 ± 0.0 | 98.6 ± 2.4 |
| Reactor No. 3 (Sandy Loam) | Reactor No. 4 (Quartz Sand) | |||||||
| Minimum | 94.6 | 100.0 | 99.5 | 99.2 | 92.3 | 100.0 | 100.0 | 100.0 |
| Maximum | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Mean 1 | 97.6 ± 2.8 | 100.0 ± 0.0 | 99.8 ± 0.3 | 99.5 ± 0.5 | 97.1 ± 3.3 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, Y.; Yang, L.; Tu, N.; Xi, G.; Fang, X. Removal of Heavy Metals from Urban Stormwater Runoff Using Bioretention Media Mix. Water 2017, 9, 854. https://doi.org/10.3390/w9110854
Wang J, Zhao Y, Yang L, Tu N, Xi G, Fang X. Removal of Heavy Metals from Urban Stormwater Runoff Using Bioretention Media Mix. Water. 2017; 9(11):854. https://doi.org/10.3390/w9110854
Chicago/Turabian StyleWang, Jianlong, Yuanling Zhao, Liqiong Yang, Nannan Tu, Guangpeng Xi, and Xing Fang. 2017. "Removal of Heavy Metals from Urban Stormwater Runoff Using Bioretention Media Mix" Water 9, no. 11: 854. https://doi.org/10.3390/w9110854






