Survey of Heavy Metal Contamination in Water Sources in the Municipality of Torola, El Salvador, through In Situ Sorbent Extraction
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Solutions
2.2. Instruments and Apparatus
2.3. Evaluation of the Different Sorbents
2.4. Field Study
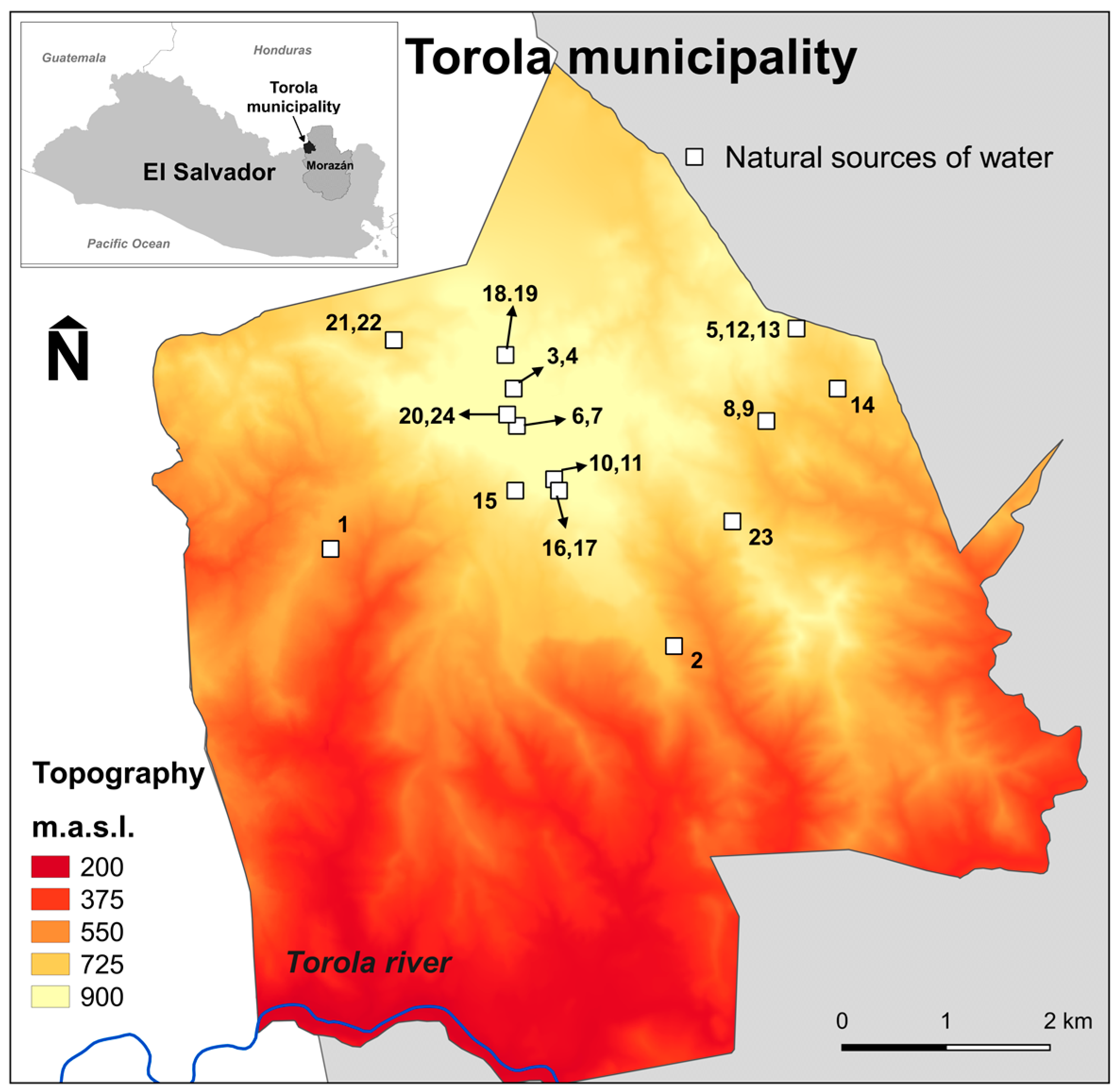
2.4.1. Description of the Studied Area and Sampling Sites
2.4.2. In Situ Metal Extraction Procedure
2.4.3. Data Treatment
3. Results and Discussion
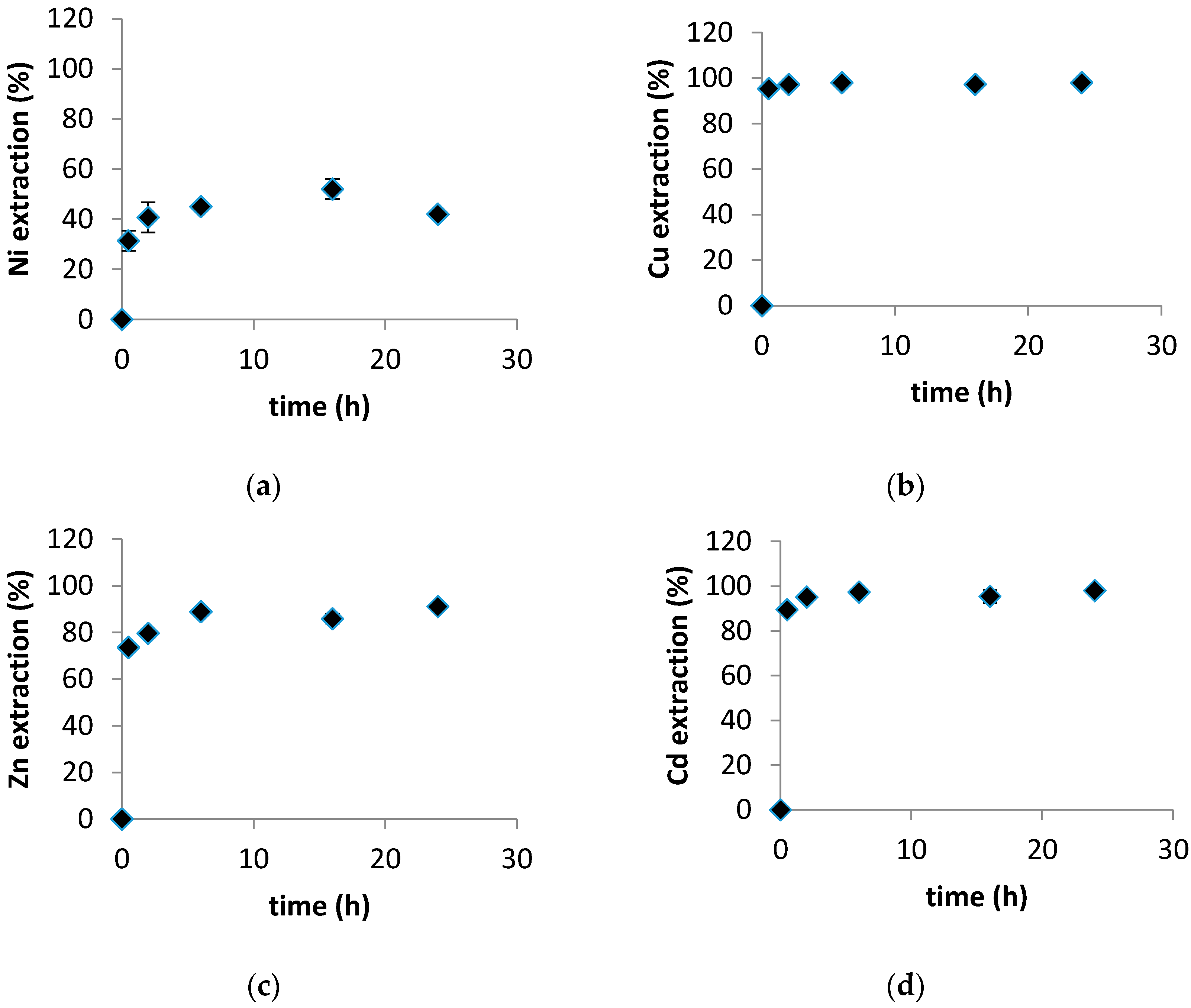
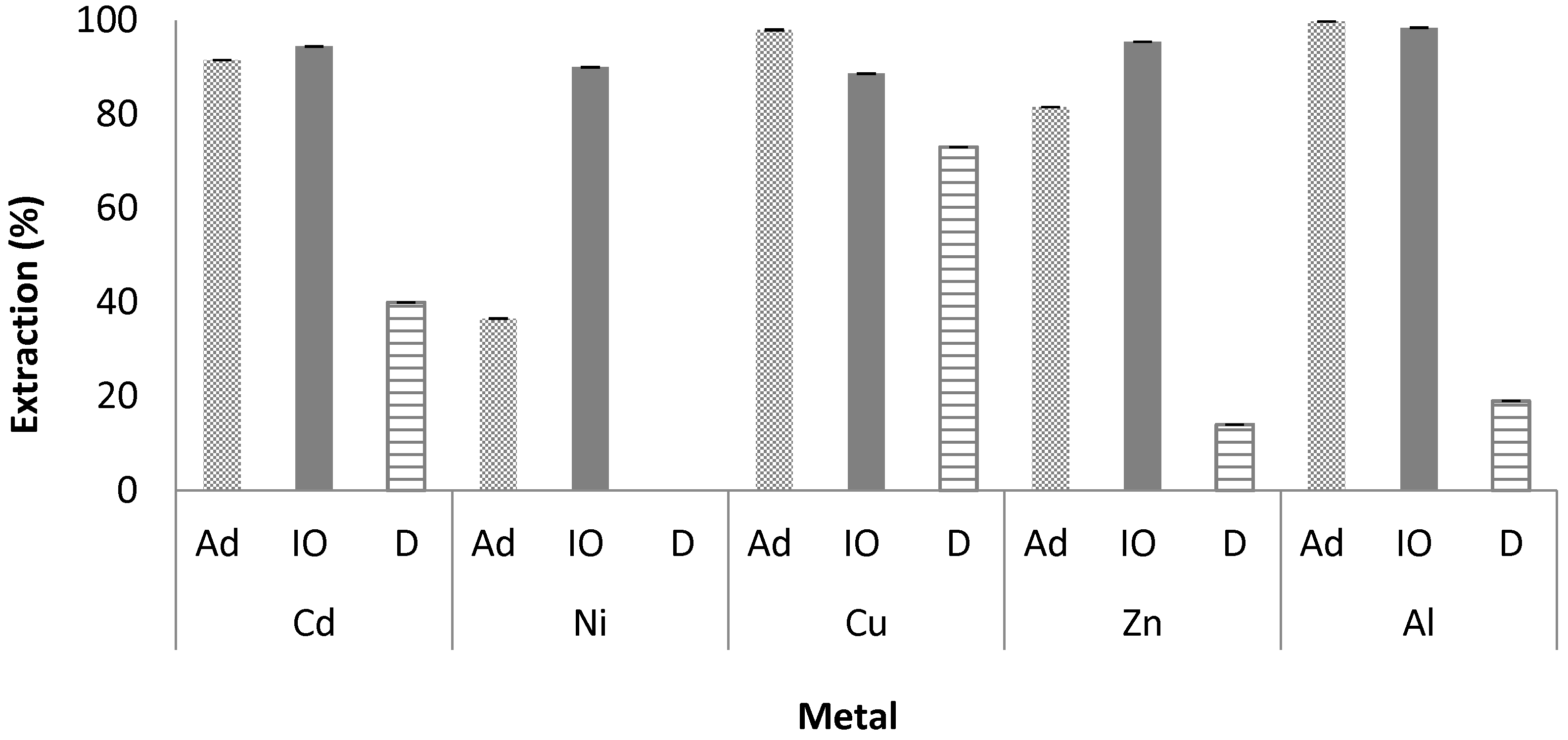
3.1. Evaluation of the Sorbents
3.2. Results Obtained in the Field Study
3.2.1. General Physicochemical Parameters
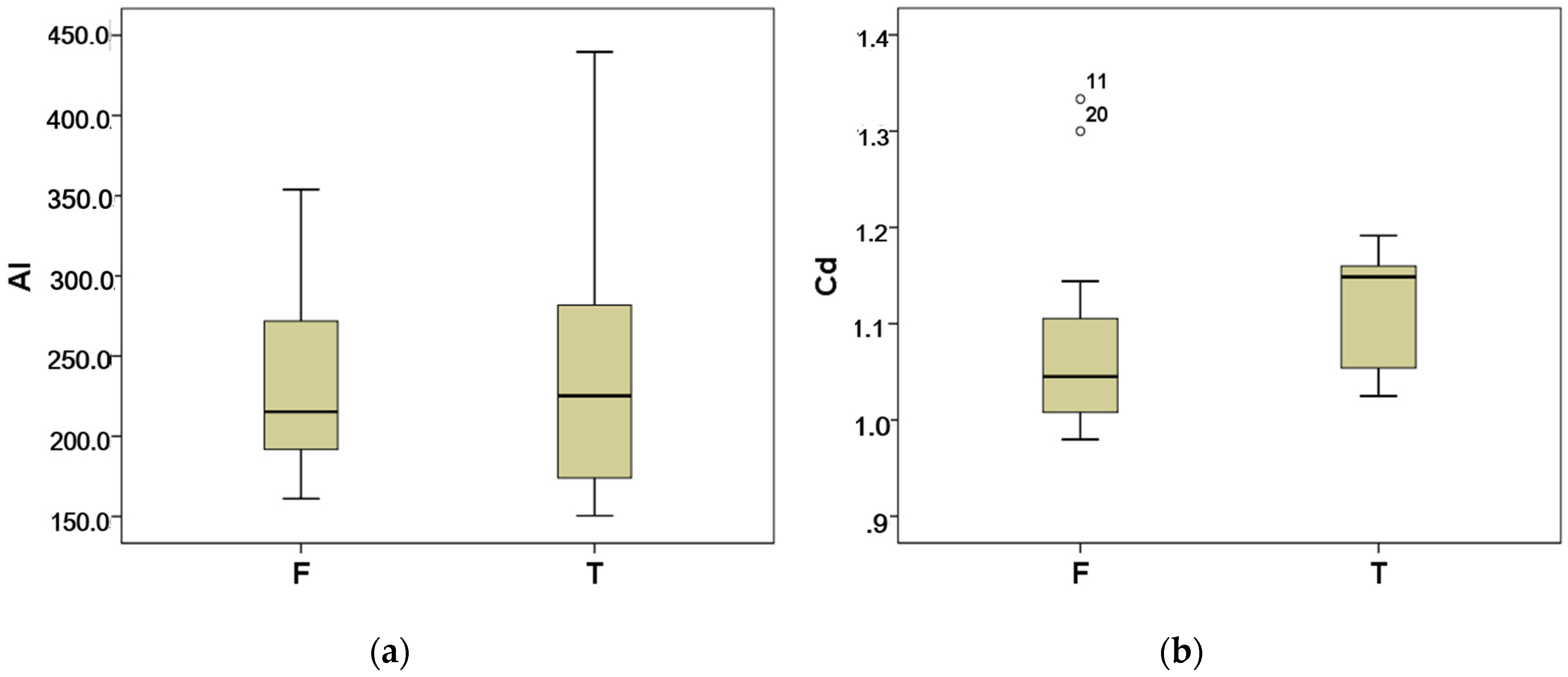
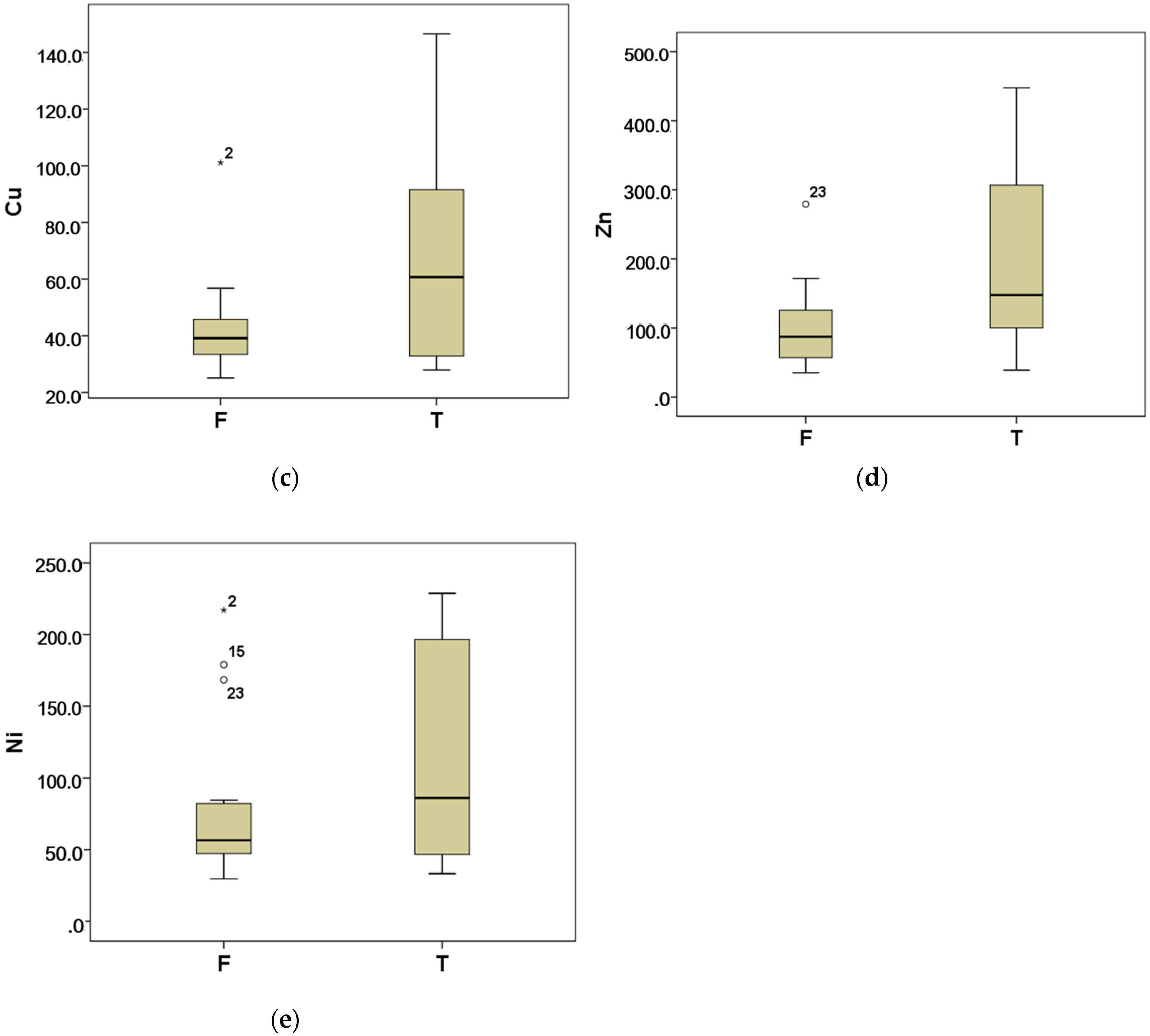
3.2.2. Heavy Metals
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 3, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2014, 36, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Frías-Espericueta, M.G.; Osuna-López, J.I.; Izaguirre-Fierro, G.; Aguilar-Juárez, M.; Voltolina, D. Cadmium and lead in commercial importance organisms in the coastal zone of Sinaloa, México: 20 years of studies. Oceánides 2010, 25, 27–39. [Google Scholar]
- Farcas, A.; Matei, A.V.; Florian, C.; Badea, M.; Coman, G. Health Effects Associated with Acute and Chronic Exposure to Pesticides. In Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe; Simeonov, L.I., Macaev, F.Z., Simeonova, B.G., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 103–110. ISBN 978-94-007-6460-6. [Google Scholar]
- Bundschuh, J.; Pérez Carrera, A.; Litter, M. Introducción: Distribución del arsénico en las regions Ibérica e Iberamericana (Iberoarsen). In Distribución Del Arsénico en Las Regions Ibérica e Iberamericana; Bundschuh, J., Carrera, A.P., Litter, M., Eds.; CYTED: Madrid, Spain, 2008; pp. 1–3. [Google Scholar]
- Ministerio de Economía de El Salvador (MINEC). Encuesta de Hogares de Propósitos Múltiples EHPM-2014; Gobierno de la República de el Salvador: Ciudad Delgado, El Salvador, 2015.
- Quinteros, E.; Ribó, A.; Mejía, R.; López, A.; Belteton, W.; Comandari, A.; Orantes, C.M.; Pleites, E.B.; Hernández, C.E.; López, D.L. Heavy metals and pesticide exposure from agricultural activities and former agrochemical factory in a Salvadoran rural community. Environ. Sci. Pollut. Res. 2017, 24, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Ribó, A.; Quinteros, E.; Mejía, R.; Alfaro, D.; Beltetón, W.; Orantes, C.M.; Hernández, C.E.; Pleités, E.; López, D.L. Riesgo de Exposición a Contaminantes Nefrotóxicos en Las Comunidades Las Brisas, El Salvador; CONIA 2015; Universidad Centroamericana “José Simeón Cañas” UCA: La Libertad, El Salvador, 2016; pp. 1–15. ISSN 2308-409X. [Google Scholar]
- López, D.L.; Bundschuh, J.; Birkle, P.; Armienta, M.A.; Cumbal, L.; Sracek, O.; Ormachea, M. Arsenic in volcanic geothermal fluids of Latin America. Sci. Total Environ. 2012, 429, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Medio Ambiente y Recursos Naturales (MARN). National Assessment of Sanitary Quality of Surface Waters in El Salvador; Gobierno de la República de el Salvador: San Salvador, El Salvador, 2006.
- García-Trabanino, R.; Aguilar, R.; Reyes Silva, C.; Ortiz Mercado, M.; Leiva Merino, R. End-stage renal disease among patients in a referral hospital in El Salvador. Rev. Panam. Salud Pública 2002, 12, 202–206. [Google Scholar]
- Orantes, C.M.; Herrera, R.; Almaguer, M.; Brizuela, E.G.; Hernández, C.E.; Bayarre, H.; Amaya, J.C.; Calero, D.J.; Orellana, P.; Colindres, R.M.; et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev. 2011, 13, 14–22. [Google Scholar] [PubMed]
- Herrera, R.; Orantes, C.M.; Almaguer, M.; Alfonso, P.; Bayarre, H.D.; Leiva, I.M.; Smith, M.J.; Cubias, R.A.; Torres, C.G.; Almendárez, W.O.; et al. Clinical characteristics of chronic kidney disease of nontraditional causes in Salvadoran farming communities. MEDICC Rev. 2014, 16, 39–48. [Google Scholar] [PubMed]
- Orantes, C.M.; Herrera, R.; Almaguer, M.; Brizuela, E.G.; Núñez, L.; Alvarado, N.P.; Fuentes, E.J.; Bayarre, H.D.; Amaya, J.C.; Calero, D.J.; et al. Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities. MEDICC Rev. 2014, 16, 23–30. [Google Scholar] [PubMed]
- Orantes, C.M.; Herrera, R.; Almaguer, M.; López, L.; Vela, X.F.; Hernández, M.; Barba, L. Toward a Comprehensive Hypothesis of Chronic Interstitial Nephritis in Agricultural Communities. Adv. Chronic Kidney Dis. 2017, 24, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Orantes, C.M.; Herrera, R.; Almaguer, M.; López, L.; Silva, L.C.; Orduñez, P.; Siribaddana, S.; Gunatilake, S.; De Broe, M.E. Chronic interstitial nephritis in agricultural communities: A worldwide epidemic with social, occupational and environmental determinants. Nephrol. Dial. Transplant. 2016, 32, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, M.; Hosseinzadeh-Khanmiri, R.; Abolhasani, J.; Ghorbani-Kalhor, E.; Hassanpour, A. Solid phase extraction of heavy metal ions from agricultural samples with the aid of a novel functionalized magnetic metal-organic framework. RSC Adv. 2015, 5, 19884–19892. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S. Solid-phase-extraction procedures for atomic spectrometry determination of copper. TrAC-Trend Anal. Chem. 2014, 62, 106–122. [Google Scholar] [CrossRef]
- Mirabi, A.; Rad, A.S.; Nourani, S. Application of modified nanoparticles as a sorbent for preconcentration and determination of nickel ions in food and environmental water samples. TrAC-Trend Anal. Chem. 2015, 74, 146–151. [Google Scholar] [CrossRef]
- Tuzen, M.; Saygi, K.O.; Soylak, M. Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 152, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Anticó, E.; Salvadó, V. Recovery of palladium(II) and gold(III) from diluted liquors using the resin duolite GT-73. Anal. Chim. Acta 1999, 381, 61–67. [Google Scholar] [CrossRef]
- Matúš, P.; Kubová, J. Complexation of labile aluminum species by chelating resins Iontosorb—A new method for Al environmental risk assessment. J. Inorg. Biochem. 2005, 99, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; Fontàs, C.; Anticó, E. Titanium dioxide solid phase for inorganic species adsorption and determination: The case of arsenic. Environ. Sci. Pollut. Res. 2017, 24, 10939–10948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40–44. [Google Scholar] [PubMed]
- Ministerio de Salud de El Salvado. Salvadoran Mandatory Standard_Water, Drinking Water, 2nd ed.; No. NSO 13.07.01:08; Ministerio de Salud de El Salvado: San Salvador, República de El Salvador, 2009.
- Dodds, W.K.; Jones, J.R.; Welch, E.B. Suggested classification of stream trophic state: Distribution of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res. 1998, 32, 1455–1462. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Driever, S. The role in lateritic Ni mining in Latin American countries with special reference to Guatemala. GeoJournal 1985, 11, 29–42. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Ganaderia (MAG). Clasificación de Suelos por División Política de El Salvador, C.A.; Ministerio de Agricultura y Ganaderia: Soyapango, El Salvador, 2012.
- Mortvedt, J.J. Heavy metal contaminants in inorganic and organic fertilizers. Fertil. Res. 1996, 43, 56–61. [Google Scholar] [CrossRef]
- Frankowski, M.; Ziola-Frankowska, A.; Kurzyca, I.; Novotný, K.; Vaculovic, T.; Kanický, V.; Siepak, M.; Siepak, J. Determination of aluminum in groundwater samples by GF-ASS, ICP-AES, ICP-MS and modelling of inorganic aluminum complexes. Environm. Monit. Assess. 2011, 182, 71–84. [Google Scholar] [CrossRef] [PubMed]
| Sorbent | Matrix | Functional Group | Particle Size | Specific Surface Area | Swelling | Exchange Capacity |
|---|---|---|---|---|---|---|
| Adsorbsia As600 | Titanium dioxide (anatase) | Titanium dioxide (anatase) | 250–1180 µm | 250 m2 g−1 | a | a |
| Iontosorb Oxin 100 | Cellulose | 8-hydroxiquinoline | 30–50 µm | b | 1.4558 g H2O/ g dry sorbent | 0.77 mmol Cu/g dry |
| Duolite GT-73 | Polystyrene | Thiol | 300–1180 µm | b | b | 0.25 mmol Cu/g dry c |
| Eluent | Cd | Ni | Cu | Zn | Al | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad | IO | Ad | IO | Ad | IO | Ad | IO | Ad | IO | |
| 3 M HCl | 41 (1) | 69 | 31 (8) | 86 | 62 (5) | 74 | 62 (15) | 78 | 58 (19) | 55 |
| 0.1 M EDTA | 67 (5) | 36 | 47 (13) | 45 | 71 (5) | 43 | 61 (10) | 52 | 12 (1) | 12 |
| 0.5 M NaSCN | 52 (2) | 53 | 23 (2) | 12 | 60 (8) | 13 | 38 (2) | 20 | 44 (2) | 25 |
| Code | Sampling Point (W = Wells) (T = Tanks) | pH | T (°C) | Conductivity (µS·cm−1) | Chloride (mg L−1) | Sulphate (mg L−1 SO4) | Hardness (mg L−1 CaCO3) | Nitrate (mg L−1 N-NO3) | Ammonium (mg L−1 N-NH4) | Phosphate (mg L−1 PO4) | Fluoride (mg L−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T. La Caída | 7.2 | - | 179 | >25 | <5 | <50 | 0.61 | 0.26 | 0.98 | <0.05 |
| 2 | W. Cerritos | 8.2 | 26 | 195 | 3.4 | 10 | <50 | 1.53 | 0.14 | 0.55 | 0.55 |
| 3 | T. El Picacho | 6.8 | 26.6 | 37.9 | 1.7 | 21 | <50 | 0.11 | 0.12 | 0.36 | <0.05 |
| 4 | W. El Picacho | 6.8 | 24.7 | 40.5 | 4.2 | <5 | <50 | - | 0.27 | 0.38 | 0.13 |
| 5 | W. Peña Hueca | 6.6 | 24.4 | 112.9 | 13.7 | <5 | <50 | 0.17 | 0.8 | 1.06 | <0.05 |
| 6 | T. Maragua | 8.2 | 26 | 110 | 11.6 | <5 | <50 | <0.3 | 0.36 | 1.62 | <0.05 |
| 7 | W. Maragua | 7.6 | 25 | 96.7 | 22.5 | <5 | <50 | <0.3 | 0.51 | 0.83 | <0.05 |
| 8 | T. Ojos Agua Amates | 7.6 | 26.2 | 170 | 9.2 | 12 | <50 | >1 | 0.62 | <0.05 | |
| 9 | W. Ojos Agua Amates | 7.6 | 24.5 | 175 | 9 | <5 | <50 | 0.1 | 0.28 | 0.08 | 0.29 |
| 10 | T. El Calvario | 7.2 | 26 | 136 | 19.2 | <5 | <50 | 0.15 | 0.09 | 0.8 | 0.18 |
| 11 | W. El Calvario | 6.8 | 25.9 | 94 | 11.7 | 29 | <50 | <0.3 | 0.14 | 1.11 | 0.07 |
| 12 | T. Aguazarca | 8.3 | 26.3 | 102.3 | >25 | 13 | <50 | 0.18 | >1 | 0.65 | <0.05 |
| 13 | W. Aguazarca | 6.8 | 25.8 | 110.8 | 5.1 | 23 | <50 | 0.54 | 0.27 | >4 | <0.05 |
| 14 | W. La Ceiba | 7.2 | 26.5 | 208 | 5.9 | <5 | <50 | 0.1 | 0.61 | >4 | 0.96 |
| 15 | W. Joya del Chongue | 6.8 | 28 | 106 | - | - | - | <0.3 | 0.36 | 1.02 | 0.06 |
| 16 | W. Barrio Ctro. Nuevo | 6.8 | - | 132.8 | 9.6 | <5 | 117 | <0.3 | 0.37 | 0.6 | <0.05 |
| 17 | T. Barrio Ctro. Nuevo | 7.2 | 26.5 | 135.2 | 4 | <5 | <50 | <0.3 | 0.03 | 0.97 | <0.05 |
| 18 | W. El Portillo | 6.8 | 24.8 | 143.3 | 4.6 | <5 | <50 | <0.3 | 0.38 | 1.84 | 0.11 |
| 19 | T. El Portillo | 7.6 | 36 | 90.5 | 3.8 | 43 | <50 | <0.3 | >1 | 1.34 | 0.15 |
| 20 | W. Sicahuites | 6.5 | - | 75.2 | <0.5 | <5 | <50 | <0.3 | 0.21 | 0.39 | 0.12 |
| 21 | W. La Joya de Tijeretas | 6.8 | 28 | 110 | - | - | - | - | - | - | - |
| 22 | T. La Joya de Tijeretas | 6.8 | 32 | 106 | 5.9 | <5 | <50 | <0.3 | 0.18 | 1.16 | 0.15 |
| 23 | W. El Pedrero | 7.6 | 25.2 | 106 | 24.8 | <5 | <50 | <0.3 | 0.18 | 1.9 | <0.05 |
| 24 | W. El Borbollón | 7.6 | - | 146 | >25 | <5 | <50 | 0.41 | 0.17 | >4 | 0.17 |
| Metal | Well (n = 15) | Tank (n = 9) |
|---|---|---|
| Al | 215.2 (191.0, 284.6) | 225.2 (166.5, 317.5) |
| Cd | 1.04 (1.00, 1.12) | 1.2 (1.05, 1.16) |
| Cu | 39.1 (33.1, 48.4) | 60.7 (32.2, 96.3) |
| Zn | 87.3 (54.9, 126.8) | 147.7 (98.0, 316.2) |
| Ni | 56.5 (46.2, 84.6) | 86.1 (45.0, 196.6) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anticó, E.; Cot, S.; Ribó, A.; Rodríguez-Roda, I.; Fontàs, C. Survey of Heavy Metal Contamination in Water Sources in the Municipality of Torola, El Salvador, through In Situ Sorbent Extraction. Water 2017, 9, 877. https://doi.org/10.3390/w9110877
Anticó E, Cot S, Ribó A, Rodríguez-Roda I, Fontàs C. Survey of Heavy Metal Contamination in Water Sources in the Municipality of Torola, El Salvador, through In Situ Sorbent Extraction. Water. 2017; 9(11):877. https://doi.org/10.3390/w9110877
Chicago/Turabian StyleAnticó, Enriqueta, Sergi Cot, Alexandre Ribó, Ignasi Rodríguez-Roda, and Clàudia Fontàs. 2017. "Survey of Heavy Metal Contamination in Water Sources in the Municipality of Torola, El Salvador, through In Situ Sorbent Extraction" Water 9, no. 11: 877. https://doi.org/10.3390/w9110877





