Unraveling the Long-Term Effects of Cr(VI) on the Performance and Microbial Community of Nitrifying Activated Sludge System
Abstract
:1. Introduction
2. Materials and Methods
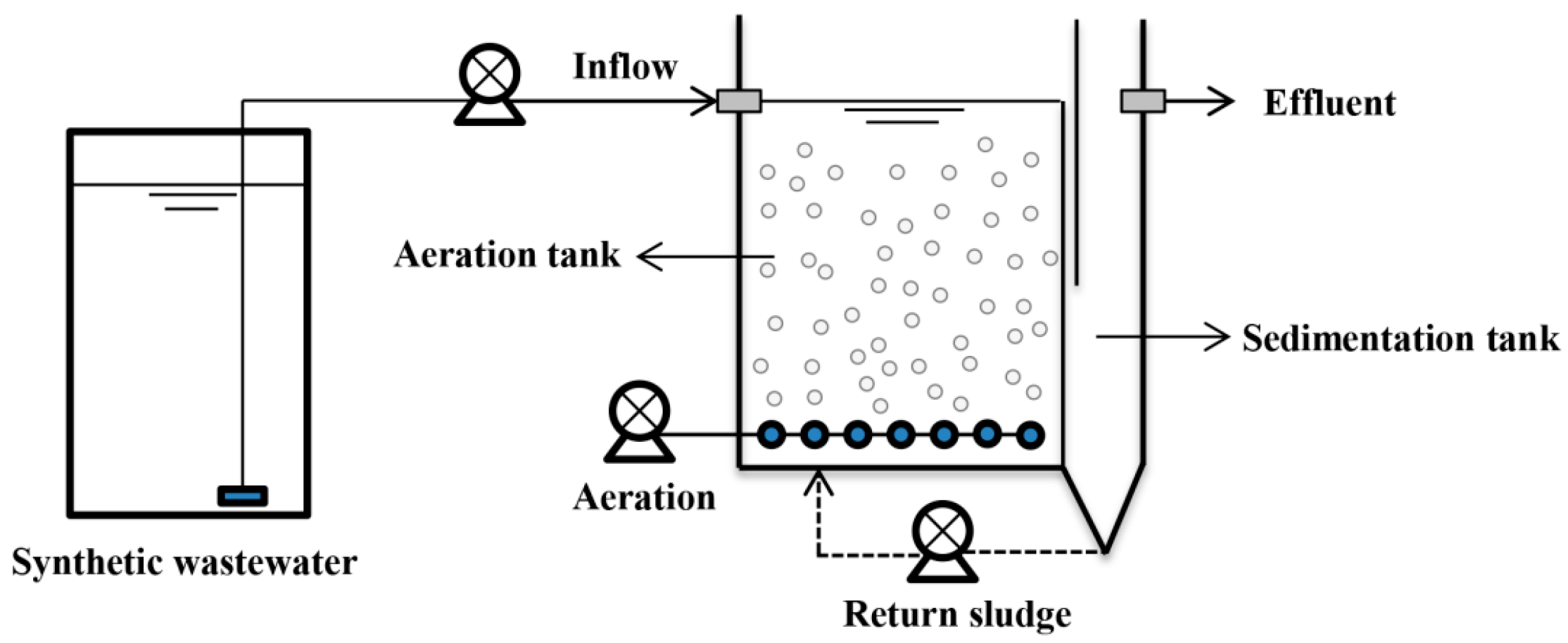
2.1. Experimental Apparatus and Wastewater Composition
2.2. Experimental Design
2.3. Experimental Data and Analytical Methods
2.4. Microbial Community Analysis Based on 16S rRNA High-Throughput Sequencing
3. Results and Discussion
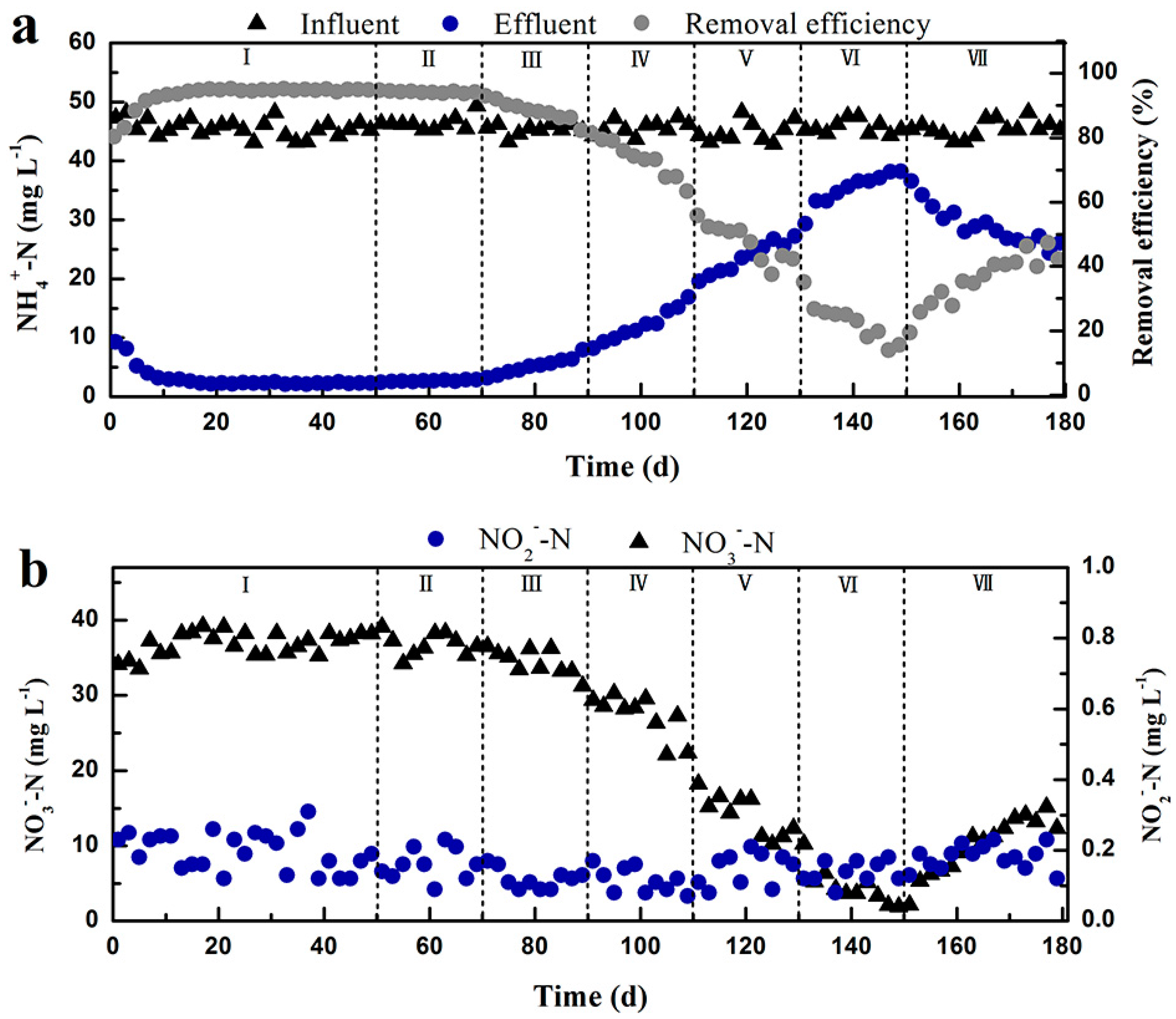
3.1. Performance of the Nitrifying Activated Sludge System at Different Influent Cr(VI) Concentrations
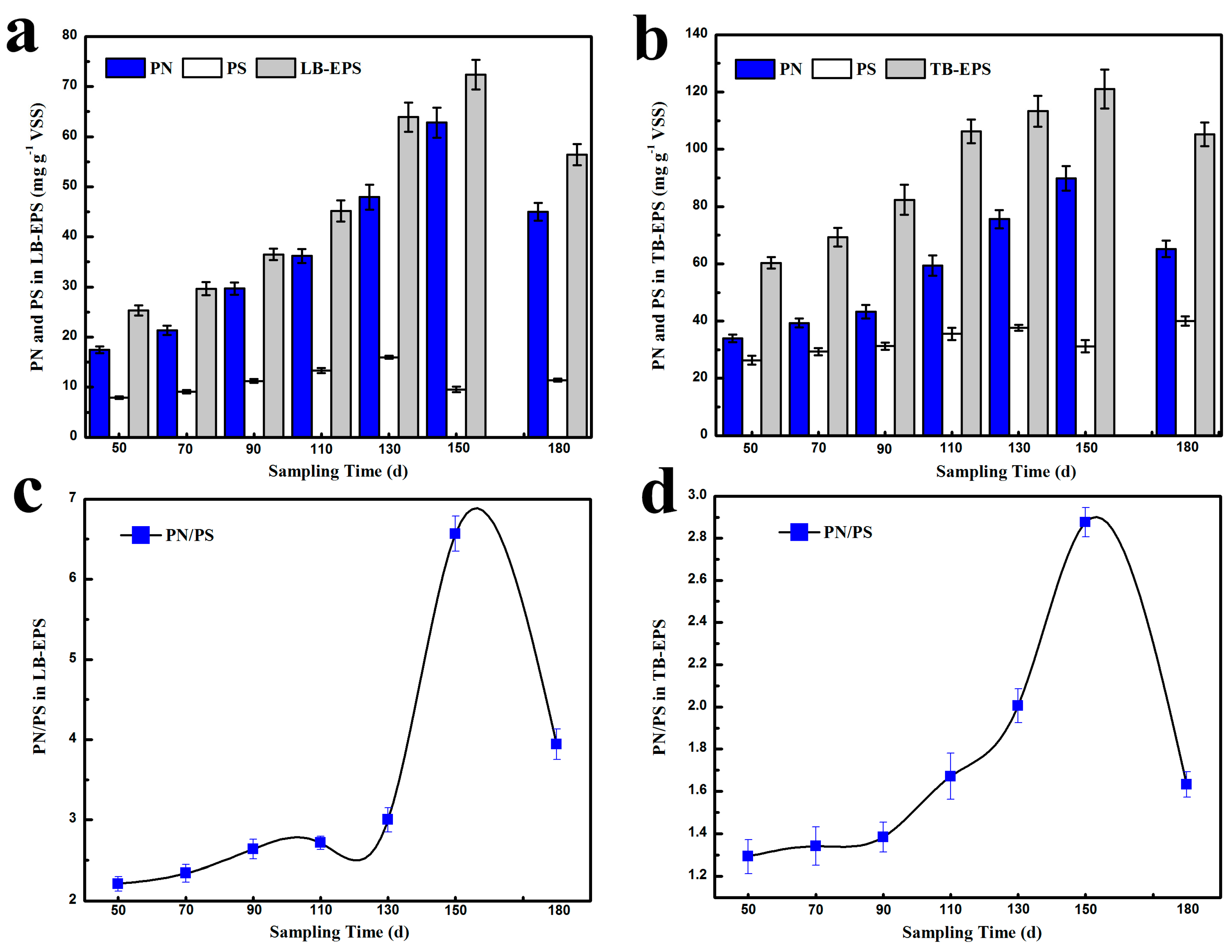
3.2. Characteristics of Nitrifying Activated Sludge at Different Influent Cr(VI) Concentrations
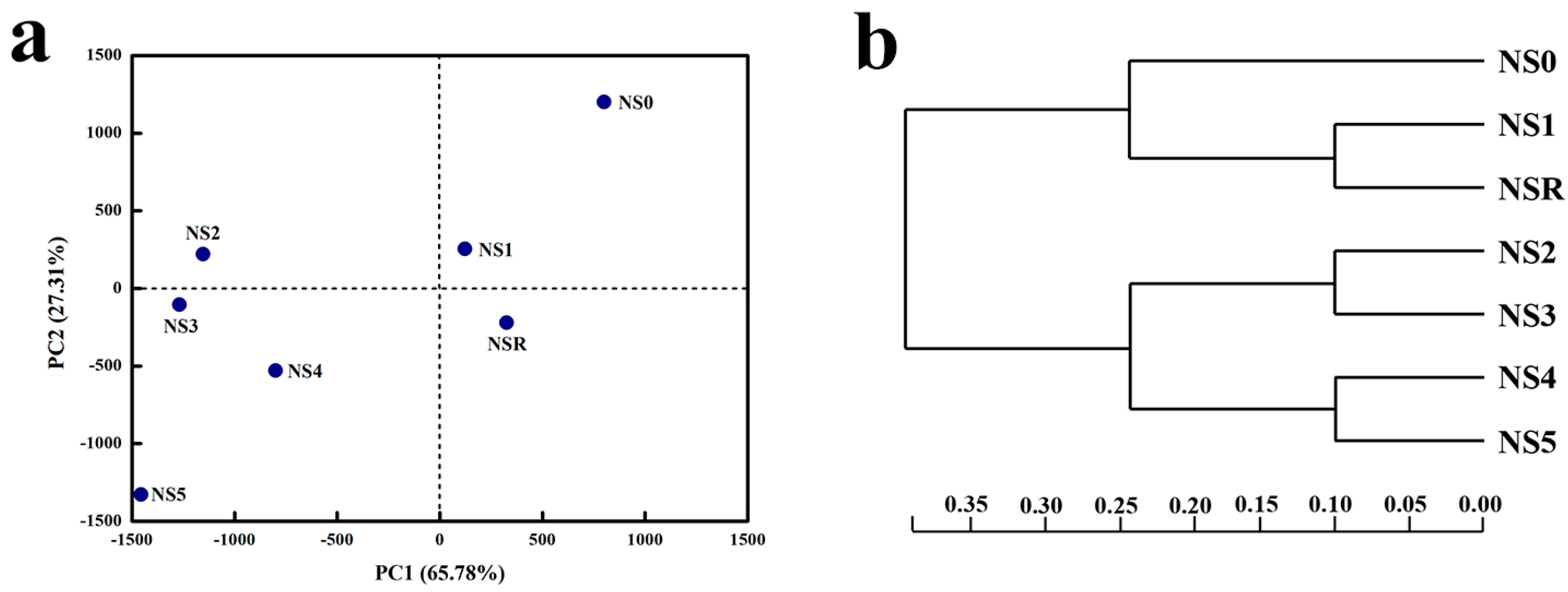
3.3. Richness, Diversity, and Similarity of Microbial Community at Different Cr(VI) Concentrations
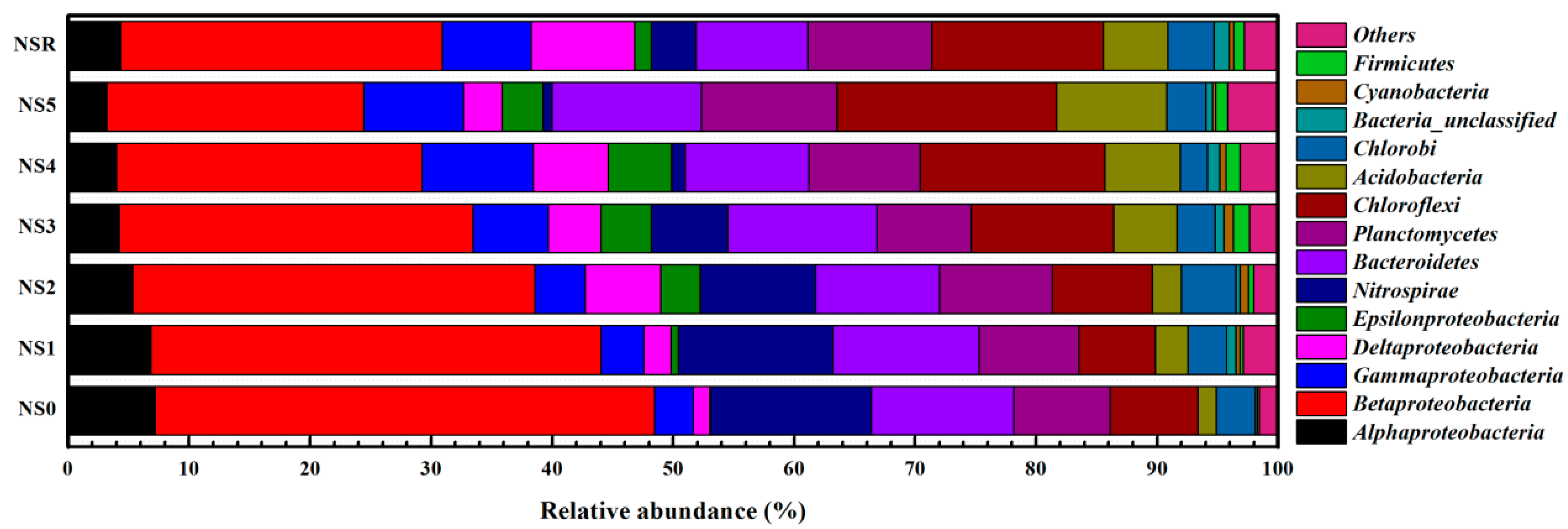
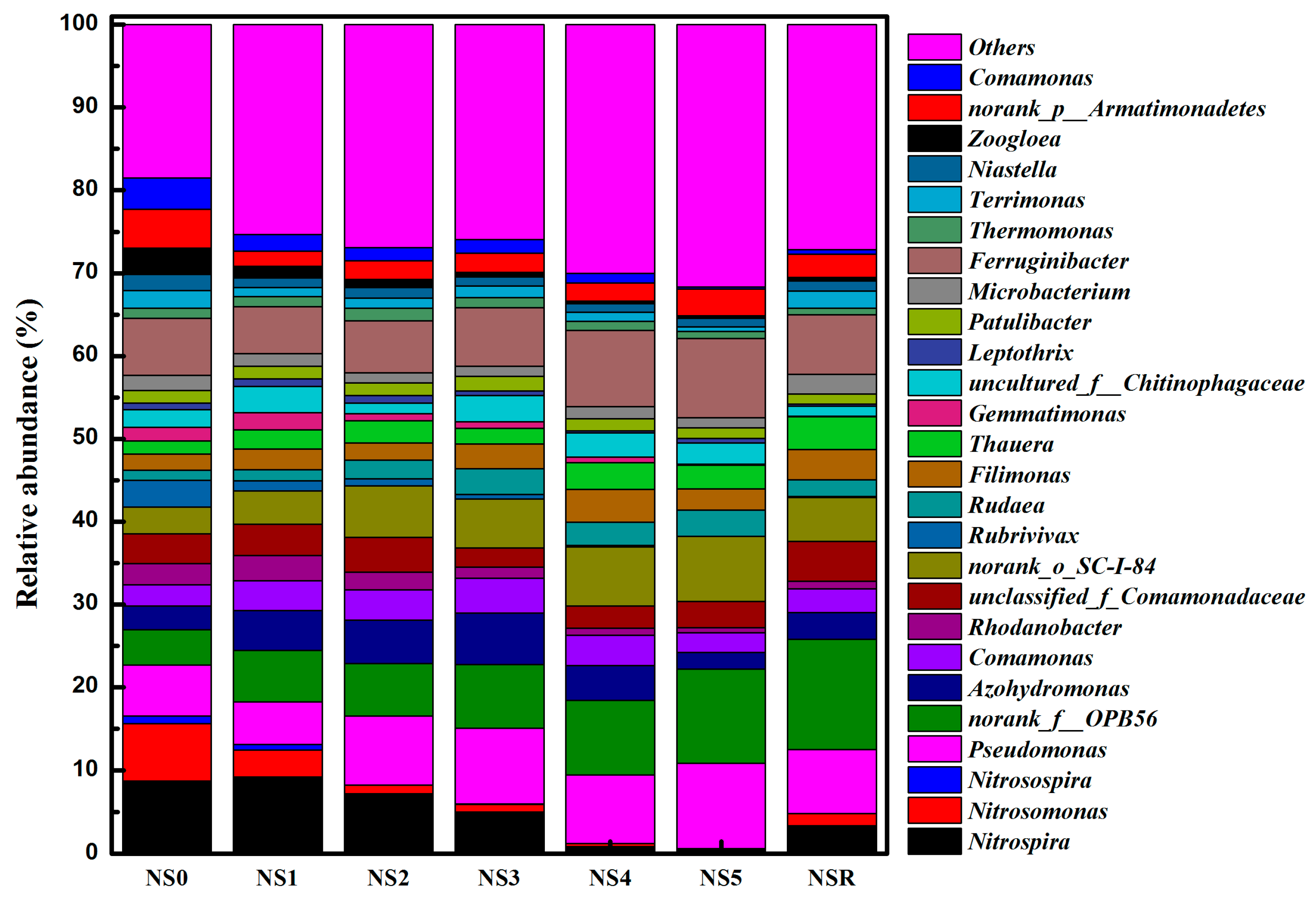
3.4. Variations of Microbial Communities and Functional Bacterial Populations at Different Influent Cr(VI) Concentrations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, H.B.; Wang, D.B.; Li, X.M.; Yang, Q.; Luo, K.; Zeng, G.M.; Tang, M.L. Effects of Cd(II) on wastewater biological nitrogen and phosphorus removal. Chemosphere 2014, 117, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Su, B.; Sun, P.D.; Lou, J.; Han, J. Long-term effect of low concentration Cr(VI) on P removal in granule-based enhanced biological phosphorus removal (EBPR) system. Chemosphere 2015, 121, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, M.; Wei, J.; Ma, K.; Zhang, J.; Yang, Y.; Yu, S. Extracellular polymeric substances, microbial activity and microbial community of biofilm and suspended sludge at different divalent cadmium concentrations. Bioresour. Technol. 2016, 205, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Domingo, J.W.S. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhi, W.; Liu, Y.; Karyala, S.; Vikesland, P.J.; Chen, X.; Zhang, H. Lead toxicity to the performance, viability, and community composition of activated sludge microorganisms. Environ. Sci. Technol. 2015, 49, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Patra, H.K. Attenuation of Chromium Toxicity by Bioremediation Technology. In Reviews of Environmental Contamination and Toxicology; Springer: Amsterdam, The Netherlands, 2011; Volume 210, pp. 1–34. [Google Scholar]
- Vaiopoulou, E.; Gikas, P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: A review. Water Res. 2012, 46, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Ferro Orozco, A.M.; Contreras, E.M.; Zaritzky, N.E. Cr(VI) reduction capacity of activated sludge as affected by nitrogen and carbon sources, microbial acclimation and cell multiplication. J. Hazard. Mater. 2010, 176, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S.; Thomaidis, N.S.; Mamais, D.; Papanikolaou, E.C.; Tsakon, A.; Lekkas, T.D. Effects of chromium (VI) addition on the activated sludge process. Water Res. 2003, 37, 2140–2148. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Jiang, R.; Wang, C.; Yin, H.B. Effects of Cr(VI) on the performance and kinetics of the activated sludge process. Bioresour. Technol. 2011, 102, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Sun, P.D.; Xu, S.J.; Luo, T.; Lou, J.Q.; Han, J.Y.; Song, Y.Q. Impact of Cr(VI) on P removal performance in enhanced biological phosphorus removal (EBPR) system based on the anaerobic and aerobic metabolism. Bioresour. Technol. 2012, 121, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Cecen, F.; Semerci, N.; Geyik, A.G. Inhibition of respiration and distribution of Cd, Pb, Hg, Ag and Cr species in a nitrifying sludge. J. Hazard. Mater. 2010, 178, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Dai, Z.; Peng, L.; Wu, Y.; Zou, H.; Lu, X. Metagenomic and metabolomic analysis reveals the effects of chemical phosphorus recovery on biological nutrient removal system. Chem. Eng. J. 2017, 328, 1087–1097. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water & Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Wang, Z.; Gao, M.; Wang, Z.; She, Z.; Chang, Q.; Sun, C.; Zhang, J.; Ren, Y.; Yang, N. Effect of salinity on extracellular polymeric substances of activated sludge from an anoxic–aerobic sequencing batch reactor. Chemosphere 2013, 93, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, M.; She, Z.; Jin, C.; Zhao, Y.; Yang, S.; Guo, L.; Wang, S. Effects of hexavalent chromium on performance and microbial community of an aerobic granular sequencing batch reactor. Environ. Sci. Pollut. Res. 2015, 22, 4575–4586. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, Y.; Peng, L.; Dai, Z.; Li, X.; Lu, X. Effects of calcium on the performance, bacterial population and microbial metabolism of a denitrifying phosphorus removal system. Bioresour. Technol. 2017, 243, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, X.; Peng, L.; Li, X.; Dai, Z. Enrichment culture of denitrifying phosphorus removal sludge and its microbial community analysis. Environ. Technol. 2017, 38, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Doan, T.V.; Yoo, K.; Choi, S.; Kim, C.; Park, J. Discovery of commonly existing anode biofilm microbes in two different wastewater treatment MFCs using FLX titanium pyrosequencing. Appl. Microbiol. Biotechnol. 2010, 87, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shao, M.F.; Ye, L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, P.; Lou, J.; Fang, Z.; Guo, M.; Song, Y.; Tang, X.; Jiang, T. The long-term effect of nitrite on the granule-based enhanced biological phosphorus removal system and the reversibility. Bioresour. Technol. 2013, 132, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chandran, K.; Grasso, D.; Smets, B.F. Effect of nickel and cadmium speciation on nitrification inhibition. Environ. Sci. Technol. 2004, 36, 3074–3078. [Google Scholar] [CrossRef]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of extracellular polymeric substances (EPS) production in bioaggregation: Application to wastewater treatment. Appl. Microbiol. Biot. 2015, 99, 9883–9905. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Xia, J.; Chen, Y.; Liu, Z.; Guo, J.; Shen, Y.; Zhang, C.; Wang, J. Thermodynamics of binding interactions between extracellular polymeric substances and heavy metals by isothermal titration microcalorimetry. Bioresour. Technol. 2017, 232, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Ma, Z.; Sun, L.; Han, M.; Lu, J.; Li, Z.; Mohamad, O.A.; Wei, G. Extracellular polymeric substances from copper-tolerance Sinorhizobium meliloti immobilize Cu2+. J. Hazard. Mater. 2013, 261, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, D.; Loffhagen, N.; Babel, W. Protein synthesis patterns in Acinetobacter calcoaceticus induced by phenol and catechol show specificities of responses to chemostress. FEMS Microbiol. Lett. 2001, 200, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wen, X.; Yan, H.; Ding, K.; Zhao, F.; Hu, M. Bacterial community dynamics in a functionally stable pilot-scale wastewater treatment plant. Bioresour. Technol. 2011, 102, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Oshima, K.; Suda, W.; Ranasinghe, P.; Li, N.; Gedara, E.; Gunawardana, W.; Hattori, M.; Mino, T. Bacterial population dynamics in a laboratory activated sludge reactor monitored by pyrosequencing of 16S rRNA. Microbes Environ. 2013, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.M.; Shao, M.F.; Li, C.L.; Gao, X.L.; Sun, F.Y. A comparative study of the bacterial community in denitrifying and traditional enhanced biological phosphorus removal processes. Microbes Environ. 2014, 29, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.M.; Shao, M.F.; Li, C.L.; Li, J.; Xia, X. Bacterial diversity and community structure of denitrifying phosphorus removal sludge instrict anaerobic/anoxic systems operated with different carbon sources. J. Chem. Technol. Biotechnol. 2014, 89, 1842–1849. [Google Scholar] [CrossRef]
- Nogueira, R.; Melo, L.F. Competition between Nitrospira spp. and Nitrobacter spp. in nitrite-oxidizing bioreactors. Biotechnol. Bioeng. 2010, 95, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujitani, H.; Aoi, Y.; Tsuneda, S. Selective enrichment of two different types of Nitrospira-like nitrite-oxidizing bacteria from a wastewater treatment plant. Microbes Environ. 2013, 28, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, Z.; Rong, Q.; Zhang, H. Detailed comparison of bacterial communities during seasonal sludge bulking in a municipal wastewater treatment plant. Water Res. 2016, 105, 157–166. [Google Scholar] [CrossRef] [PubMed]
| Cr(VI) (mg L−1) | Sampling Time (d) | SVI (mL g−1 MLSS) | MLVSS/MLSS (mg mg−1) | SOUR (mg N g−1 VSS h−1) | SAOR (mg N g−1 VSS h−1) | SNOR (mg N g−1 VSS h−1) |
|---|---|---|---|---|---|---|
| 0 | 50 | 72.35 ± 2.78 | 0.72 ± 0.05 | 53.24 ± 3.29 | 6.31 ± 0.56 | 7.33 ± 0.45 |
| 0.1 | 70 | 83.25 ± 3.69 | 0.75 ± 0.07 | 45.36 ± 3.36 | 5.28 ± 0.22 | 4.21 ± 0.29 |
| 0.2 | 90 | 92.77 ± 3.25 | 0.81 ± 0.02 | 32.55 ± 2.98 | 3.47 ± 0.18 | 3.54 ± 0.23 |
| 0.3 | 110 | 123.55 ± 5.21 | 0.85 ± 0.03 | 28.79 ± 2.15 | 2.25 ± 0.21 | 1.56 ± 0.18 |
| 0.4 | 130 | 156.78 ± 5.79 | 0.92 ± 0.03 | 22.39 ± 3.12 | 1.79 ± 0.17 | 1.12 ± 0.16 |
| 0.5 | 150 | 187.36 ± 7.12 | 0.96 ± 0.04 | 18.17 ± 2.36 | 1.68 ± 0.09 | 0.88 ± 0.11 |
| 0 | 180 | 136.77 ± 4.65 | 0.83 ± 0.02 | 30.15 ± 1.02 | 2.97 ± 0.13 | 1.13 ± 0.19 |
| Sample ID | Effective Reads | Normalization No. | OTUs No. | Good’s Coverage | Richness | Diversity | ||
|---|---|---|---|---|---|---|---|---|
| Ace | Chao 1 | Shannon | Simpson | |||||
| NS0 | 39,527 | 30,265 | 893 | 0.9974 | 941.87 | 925.57 | 4.37 | 0.029 |
| NS1 | 37,564 | 30,265 | 882 | 0.9947 | 823.60 | 811.61 | 4.34 | 0.032 |
| NS2 | 36,117 | 30,265 | 856 | 0.9968 | 724.52 | 699.23 | 4.24 | 0.047 |
| NS3 | 36,129 | 30,265 | 821 | 0.9902 | 494.56 | 525.07 | 3.83 | 0.041 |
| NS4 | 31,580 | 30,265 | 815 | 0.9968 | 418.51 | 422.32 | 3.35 | 0.091 |
| NS5 | 30,265 | 30,265 | 802 | 0.9946 | 377.26 | 378.77 | 3.23 | 0.135 |
| NSR | 32,896 | 30,265 | 833 | 0.9956 | 526.39 | 465.38 | 3.97 | 0.105 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Dai, H.; Zhang, J.; Yang, T.; Chen, F. Unraveling the Long-Term Effects of Cr(VI) on the Performance and Microbial Community of Nitrifying Activated Sludge System. Water 2017, 9, 909. https://doi.org/10.3390/w9120909
Wang X, Dai H, Zhang J, Yang T, Chen F. Unraveling the Long-Term Effects of Cr(VI) on the Performance and Microbial Community of Nitrifying Activated Sludge System. Water. 2017; 9(12):909. https://doi.org/10.3390/w9120909
Chicago/Turabian StyleWang, Xingang, Hongliang Dai, Jie Zhang, Tongyi Yang, and Fangyan Chen. 2017. "Unraveling the Long-Term Effects of Cr(VI) on the Performance and Microbial Community of Nitrifying Activated Sludge System" Water 9, no. 12: 909. https://doi.org/10.3390/w9120909





