The Source, Flow Rates, and Hydrochemical Evolution of Groundwater in an Alluvial Fan of Qilian Mountain, Northwest China
Abstract
:1. Introduction
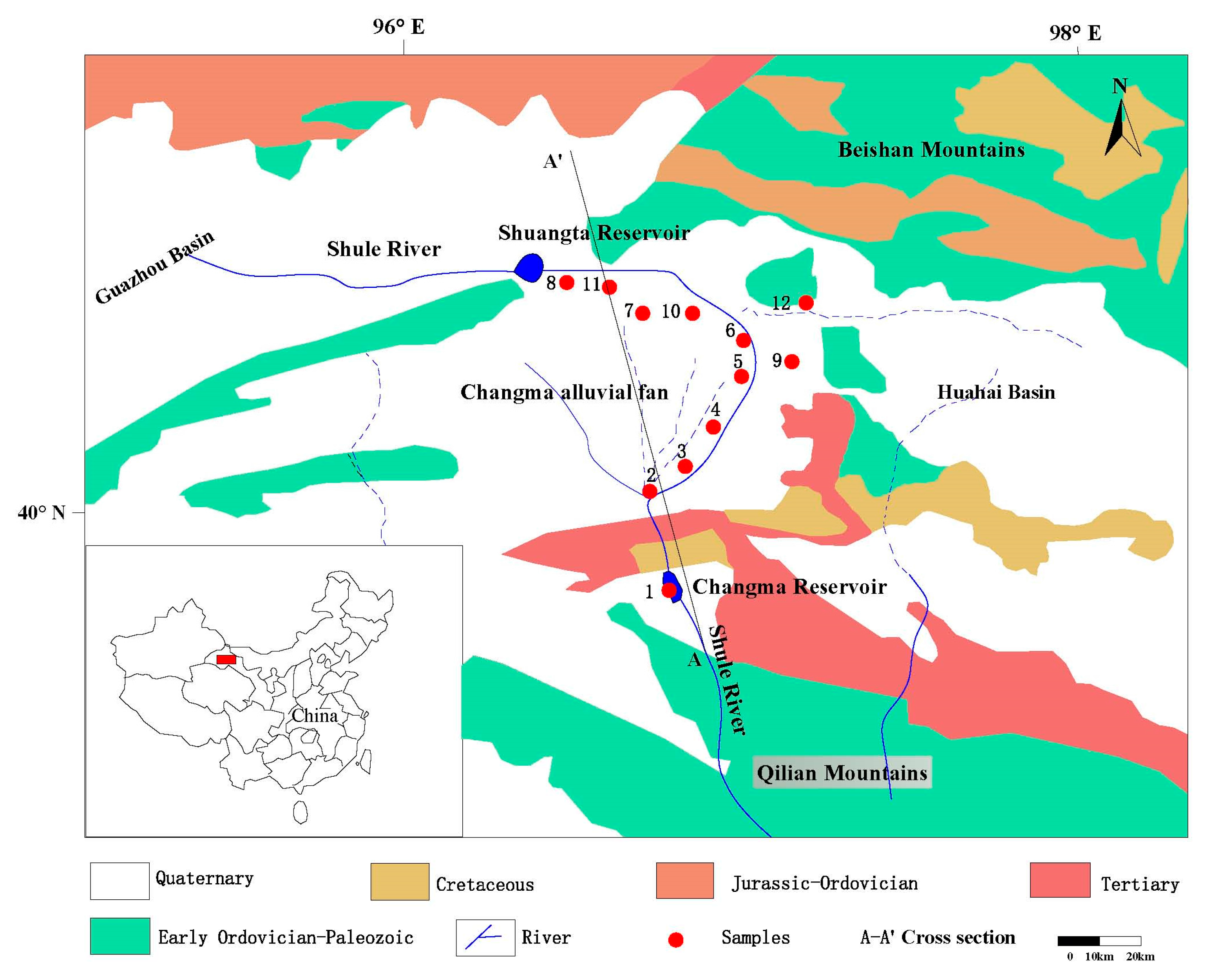
2. Study Area
2.1. General Setting
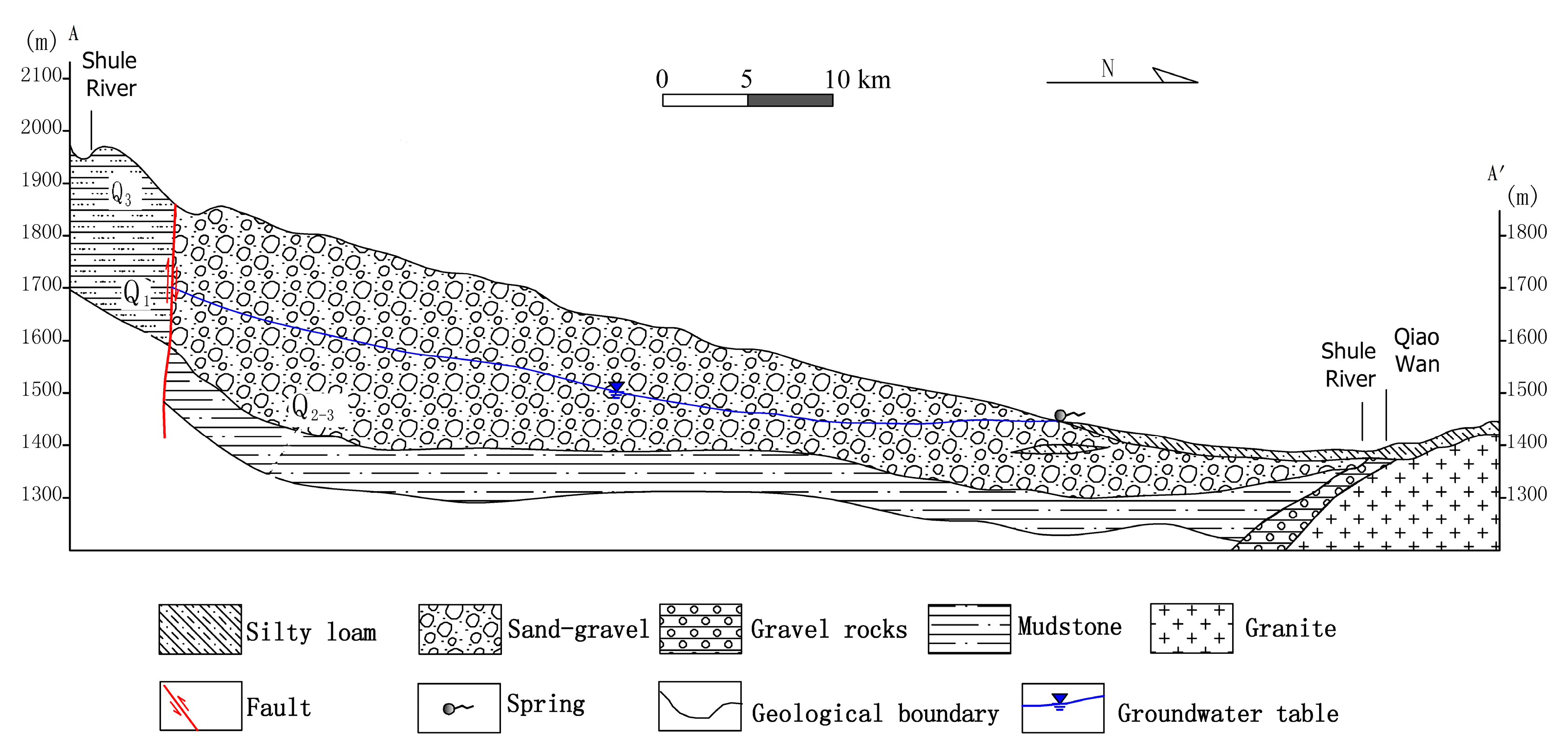
2.2. Geology and Hydrogeology
3. Materials and Methods
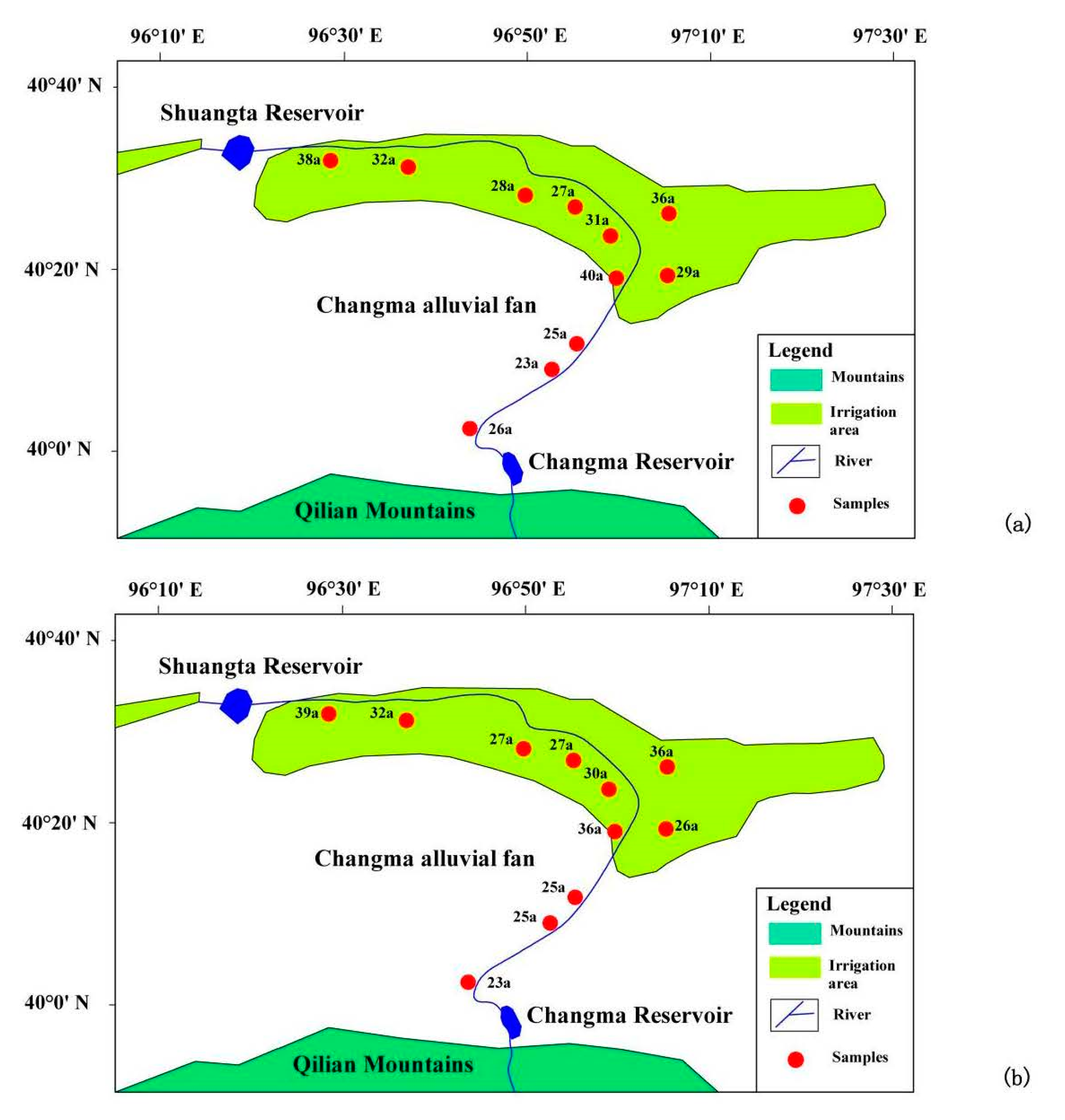
3.1. Sampling Profiles
3.2. Chemical and Isotopic Analysis
4. Results and Discussion
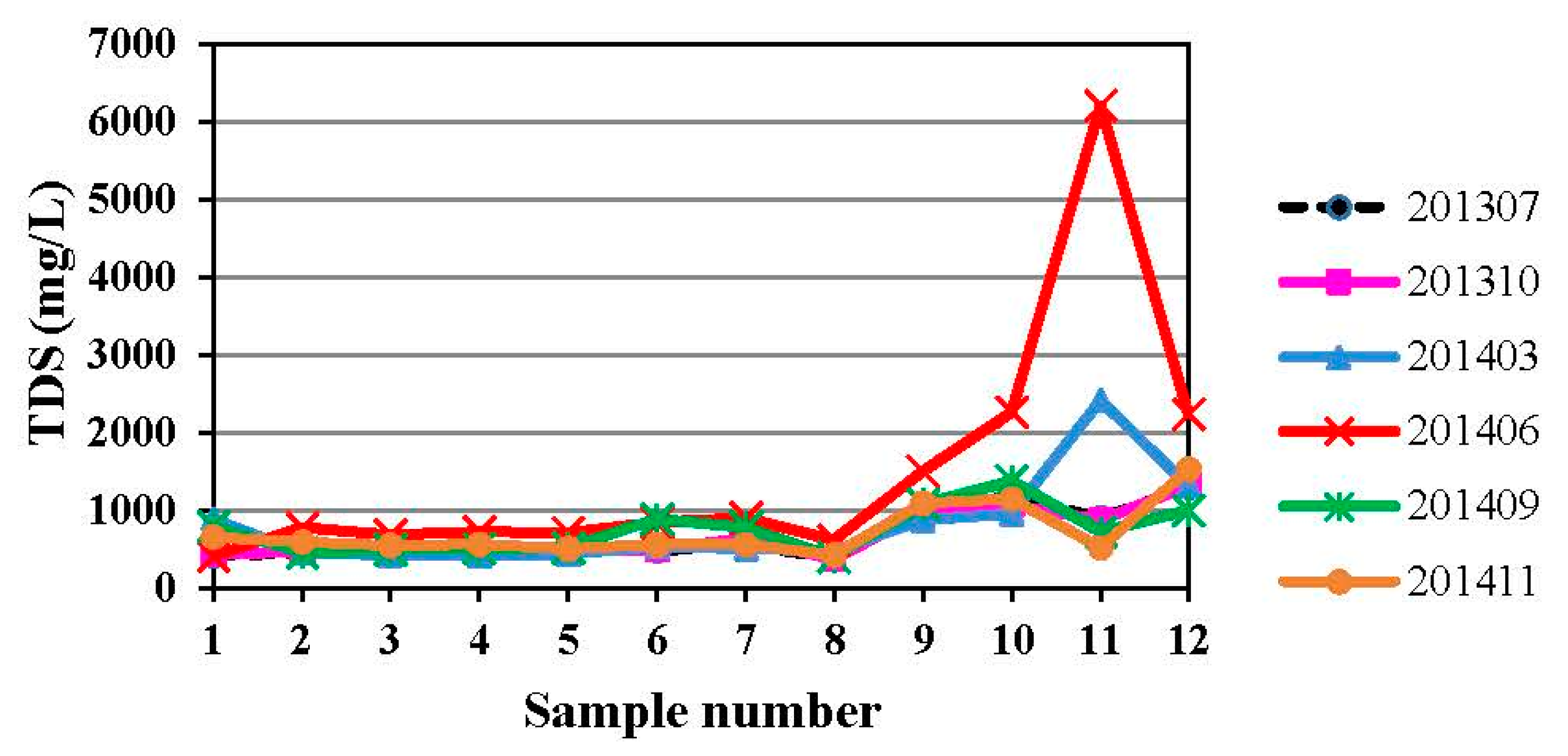
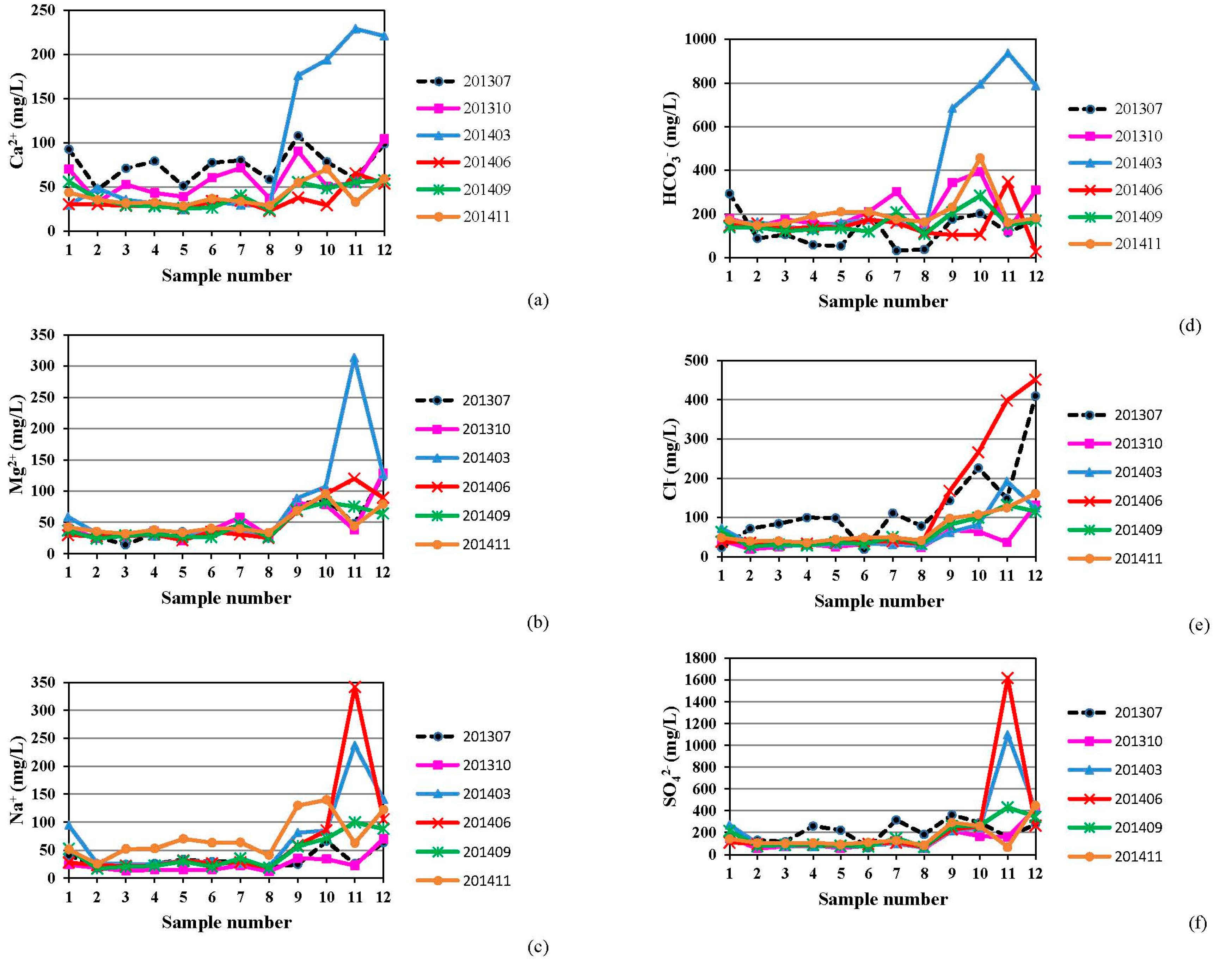
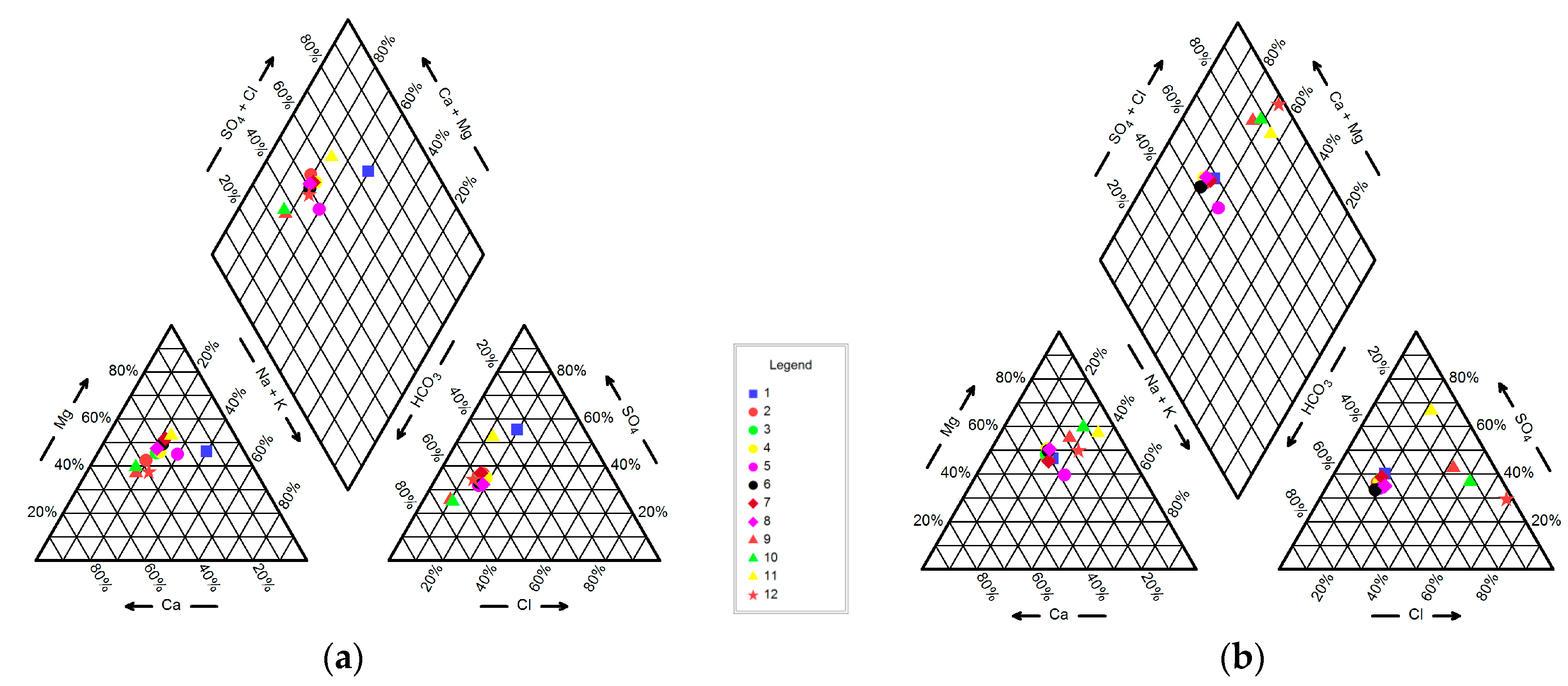
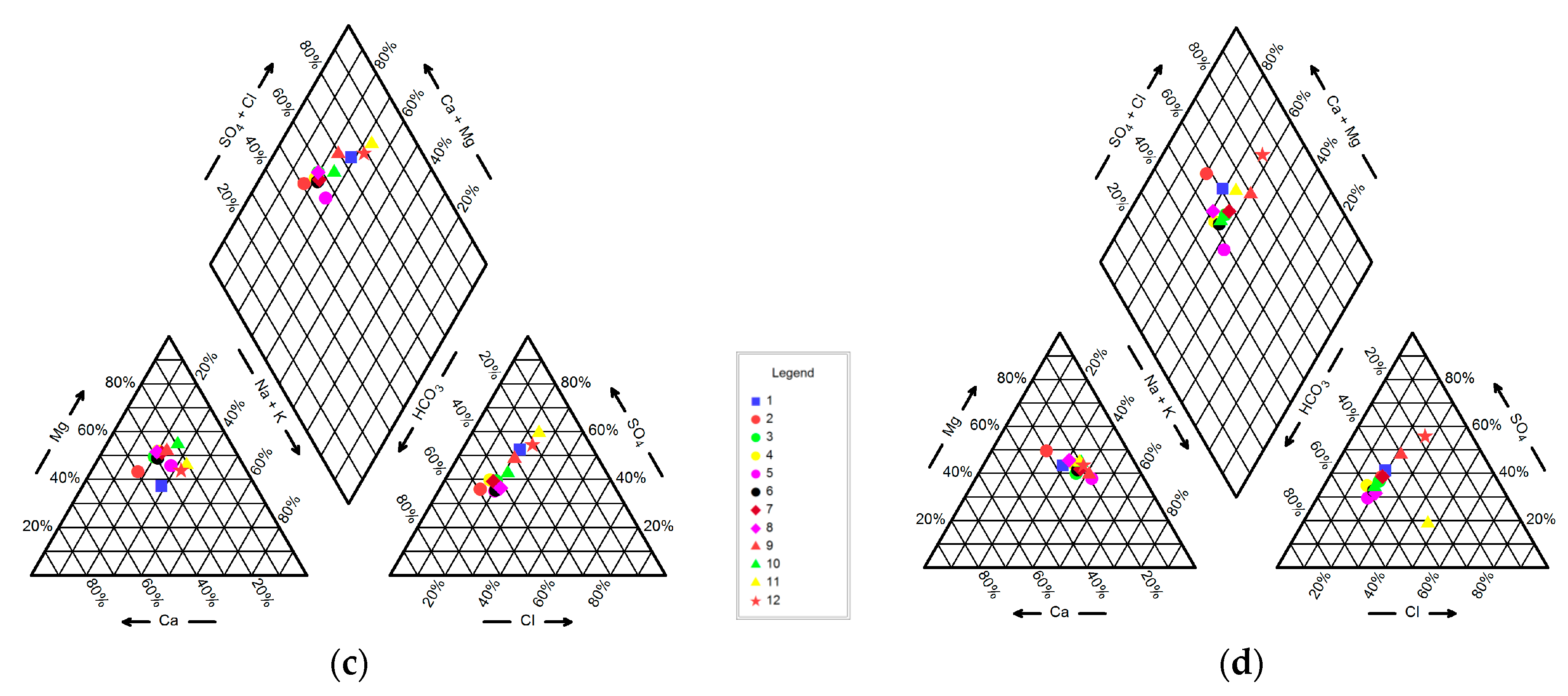
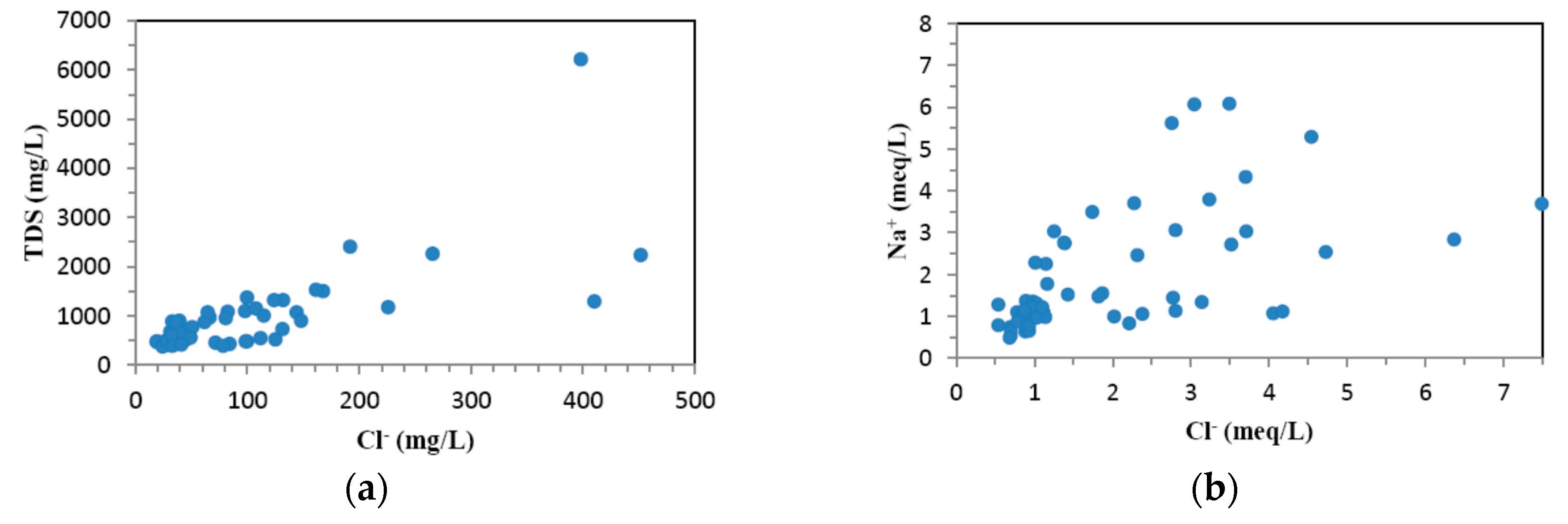
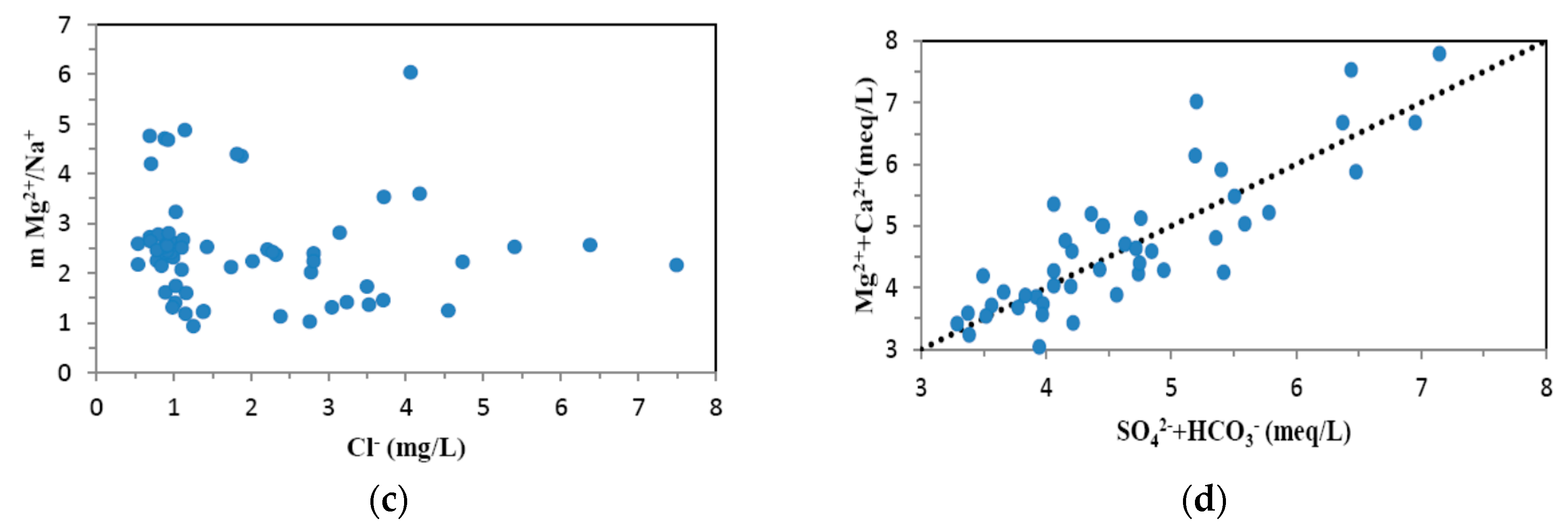
4.1. Hydrogeochemistry of Groundwater
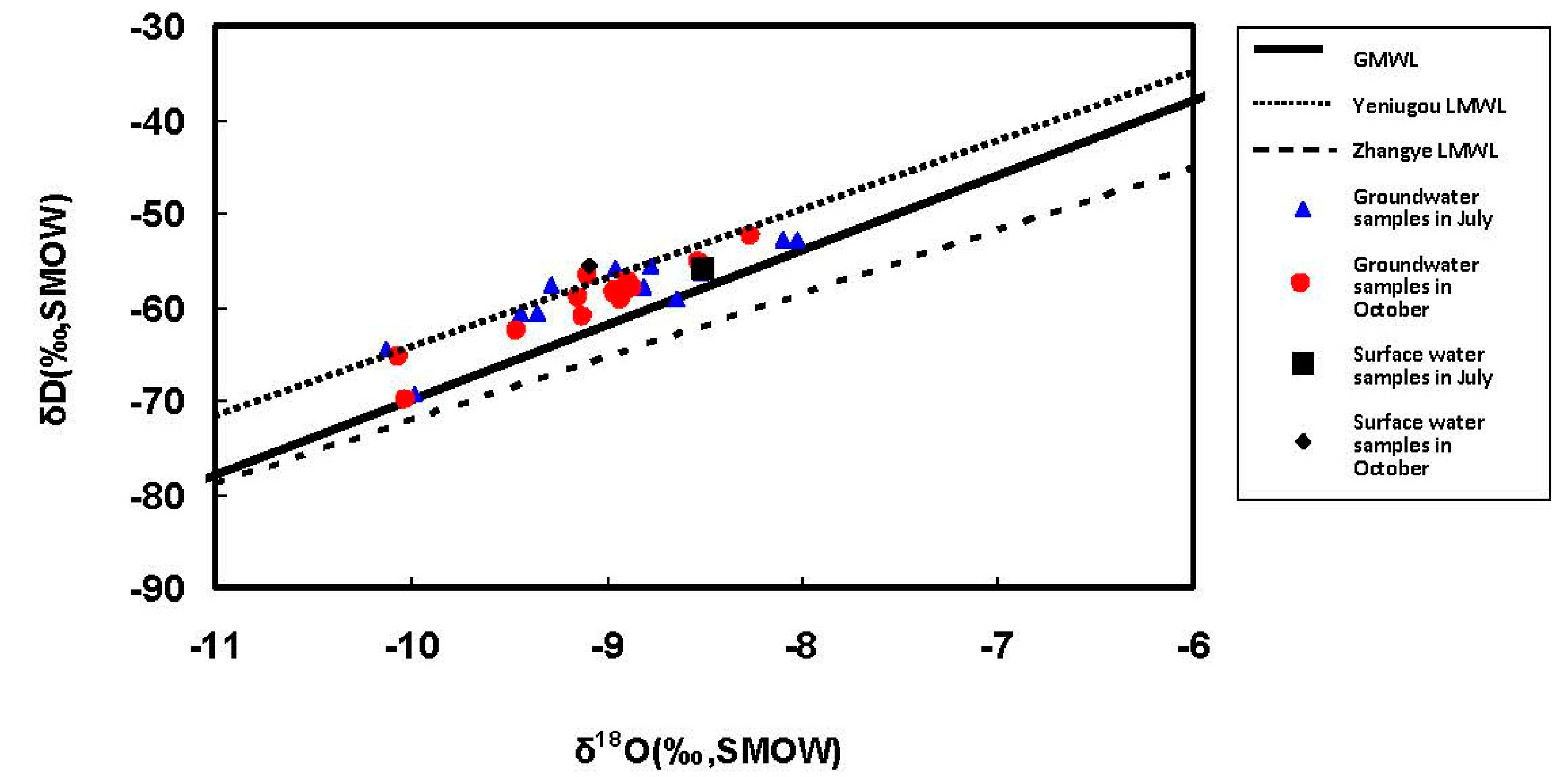
4.2. Isotope Analysis
4.3. Tritium Content in Groundwater
4.4. CFC Apparent Ages
4.5. Evolution of Groundwater in the Alluvial Fan
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, G.F.; Li, Z.Z.; Su, Y.H.; Ma, J.Z.; Zhang, Y.Y. Hydrogeochemical and isotope evidence of groundwater evolution and recharge in Minqin Basin, Northwest China. J. Hydrol. 2007, 333, 239–251. [Google Scholar] [CrossRef]
- Ma, J.Z.; He, J.H.; Qi, S.; Zhu, G.F.; Zhao, W.; Edmunds, W.M.; Zhao, Y.P. Groundwater recharge and evolution in the Dunhuang Basin. Appl. Geochem. 2013, 28, 19–31. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Keese, K.E.; Flint, A.L.; Flint, L.E.; Gaye, C.B.; Edmunds, W.M.; Simmers, I. Global synthesis of groundwater recharge insemiarid and arid regions. Hydrol. Process. 2006, 20, 3335–3370. [Google Scholar] [CrossRef]
- Vairavamoorthy, K.; Gorantiwar, S.D.; Pathirana, A. Managing urban water supplies in developing countries-climate change and water scarcity scenarios. Phys. Chem. Earth Parts A/B/C 2008, 33, 330–339. [Google Scholar] [CrossRef]
- Vanderzalm, J.L.; Jeuken, B.M.; Wischusen, J.D.H.; Pavelic, P.; La Salle, C.L.; Knapton, A.; Dillon, P.J. Recharge sources and hydrogeochemical evolution of groundwater in alluvial basins in arid central Australia. J. Hydrol. 2011, 397, 71–82. [Google Scholar] [CrossRef]
- Keesari, T.; Kulkarni, U.P.; Deodhar, A.; Ramanjaneyulu, P.S.; Sanjukta, A.K.; Kumar, U.S. Geochemical characterization of groundwater from an arid region in India. Environ. Earth Sci. 2014, 71, 4869–4888. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Ma, J.; Aeschbach-Hertig, W.; Kipfer, R.; Darbyshire, D.P.F. Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl. Geochem. 2006, 21, 2148–2170. [Google Scholar] [CrossRef]
- Qi, S.Z.; Luo, F. Hydrological indicators of desertification in the Heihe River Basin of arid northwest China. J. Hum. Environ. 2006, 35, 319–321. [Google Scholar]
- Aeschbach-Hertig, W.; Gleeson, T. Regional strategies for the accelerating global problem of groundwater depletion. Nat. Geosci. 2012, 5, 853–861. [Google Scholar] [CrossRef]
- Lapworth, D.J.; MacDonald, A.M.; Tijani, M.N.; Darling, W.G.; Gooddy, D.C.; Bonsor, H.C.; Araguas-Araguas, L.J. Residence times of shallow groundwater in West Africa: implications for hydrogeology and resilience to future changes in climate. Hydrogeol. J. 2013, 21, 673–686. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.L.; Zhu, W.H.; Zhong, Z.S. Basis of Hydrogeochemistry; Geology Press: Beijing, China, 1993. (In Chinese) [Google Scholar]
- He, J.H.; Ma, J.Z.; Zhang, P.; Tian, L.M.; Zhu, G.F.; Edmunds, W.M.; Zhang, Q.H. Groundwater recharge environments and hydrogeochemical evolution in the Jiuquan Basin, Northwest China. Appl. Geochem. 2012, 27, 866–878. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.J.; Zhang, Y.C.; Liu, C.M. Groundwater recharge and hydrogeochemical evolution in the Ejina Basin, northwest China. J. Hydrol. 2013, 476, 72–86. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.; Yang, L.; Zhang, Y.; Han, D.; Ma, Y.; Bu, H. Identifying the origin and geochemical evolution of groundwater using hydrochemistry and stable isotopes in the Subei Lake basin, Ordos energy base, Northwestern China. Hydrol. Earth Syst. Sc. 2015, 19, 551–565. [Google Scholar] [CrossRef]
- Kamtchueng, B.T.; Fantong, W.Y.; Ueda, A.; Tiodjio, E.R.; Anazawa, K.; Wirmvem, M.J.; Mvondo, J.O.; Nkamdjou, L.S.; Kusakabe, M.; Ohba, T.; et al. Assessment of shallow groundwater in Lake Nyos catchment (Cameroon, Central-Africa): implications for hydrogeochemical controls and uses. Environ. Earth Sci. 2014, 72, 3663–3678. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Z.F.; Wang, J.G.; Dou, Z.; Guo, Q.N. Spatial and temporal variability of the chemistry of the shallow groundwater in the alluvial fan area of the Luanhe river, North China. Environ. Earth Sci. 2014, 72, 5123–5137. [Google Scholar] [CrossRef]
- Ledesma-Ruiz, R.; Pasten-Zapata, E.; Parra, R. ; Harter, T; Mahlknecht, J. Investigation of the geochemical evolution of groundwater under agricultural land: A case study in northeastern Mexico. J. Hydrol. 2015, 521, 410–423. [Google Scholar] [CrossRef]
- Peng, T.R.; Huang, C.C.; Wang, C.H.; Liu, T.K.; Lu, W.C.; Chen, K.Y. Using oxygen, hydrogen, and tritium isotopes to assess pond water’s contribution to groundwater and local precipitation in the pediment tableland areas of northwestern Taiwan. J. Hydrol. 2012, 450, 105–116. [Google Scholar] [CrossRef]
- Peng, T.R.; Lu, W.C.; Chen, K.Y.; Zhan, W.J.; Liu, T.K. Groundwater-recharge connectivity between a hills-and plains’ area of western Taiwan using water isotopes and electrical conductivity. J. Hydrol. 2014, 517, 226–235. [Google Scholar] [CrossRef]
- Liu, Y.; Yamanaka, T.; Zhou, X.; Tian, F.; Ma, W. Combined use of tracer approach and numerical simulation to estimate groundwater recharge in an alluvial aquifer system: A case study of Nasunogahara area, central Japan. J. Hydrol. 2014, 519, 833–847. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Walton, N.R.C. A geochemical and isotopic approach to recharge evaluation in semi-arid zones; past and present. In Arid-Zone Hydrology, Investigation with Isotope Techniques; International Atomic Energy Agency (IAEA): Vienna, Austria, 1980; pp. 47–68. [Google Scholar]
- Lee, E.S.; Krothe, N.C. A four-component mixing model for water in a karst terrain in south-central Indiana, USA. Using solute concentration and stable isotopes as tracers. Chem. Geol. 2001, 179, 129–143. [Google Scholar] [CrossRef]
- Oster, H.; Sonntag, C.; Munnich, K.O. Groundwater age dating with chlorofluorocarbons. Water Resour. Res. 1996, 32, 2989–3001. [Google Scholar] [CrossRef]
- Happell, J.D.; Opsahlb, S.; Top, Z.; Chanton, J.P. Apparent CFC and 3H/3He age differences in water from Floridan Aquifers springs. J. Hydrol. 2006, 319, 410–426. [Google Scholar] [CrossRef]
- Qin, D.J.; Qian, Y.P.; Han, L.F.; Wang, Z.M.; Li, C. , Zhao, Z.F. Assessing impact of irrigation water on groundwater recharge and quality in arid environment using CFCs, tritium and stable isotopes, in the Zhangye Basin, Northwest China. J. Hydrol. 2011, 405, 194–208. [Google Scholar] [CrossRef]
- Qin, D.J.; Zhao, Z.F.; Han, L.F.; Qian, Y.P.; Ou, L.; Wu, Z.Q.; Wang, M.C. Determination of groundwater recharge regime and flow path in the Lower Heihe River basin in an arid area of Northwest China by using environmental tracers: Implications for vegetation degradation in the Ejina Oasis. Appl. Geochem. 2012, 27, 1133–1145. [Google Scholar] [CrossRef]
- Prinn, R.G.; Weiss, R.F.; Fraser, P.J.; Simmonds, P.G.; Cunnold, D.M.; Alyea, F.N.; O’Doherty, S.; Salameh, P.; Miller, BH.R.; Huang, J.; et al. A history of chemically and radiatively important gases in air deduced from ALE/GAGE/AGAGE. J. Geophys. Res. 2000, 105, 17751–17792. [Google Scholar] [CrossRef]
- Parisi, S.; Paternoster, M.; Kohfahl, C.; Pekdeger, A.; Meyer, H.; Hubberten, H.W.; Spilotro, G.; Mongelli, G. Groundwater recharge areas of a volcanic aquifer system inferred from hydraulic, hydrogeochemical and stable isotope data: Mount Vulture, southern Italy. Hydrogeol. J. 2011, 19, 133–153. [Google Scholar] [CrossRef]
- Diaw, M.; Faye, S.; Stichler, W.; Maloszewski, P. Isotopic and geochemical characteristics of groundwater in the Senegal River delta aquifer: Implication of recharge and flow regime. Environ. Earth Sci. 2012, 66, 1011–1020. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, G.M.; Dong, Y.H.; Han, D.M.; Zhang, J.Y. Using hydrochemical and isotopic data to determine sources of recharge and groundwater evolution in an arid region: A case study in the upper-middle reaches of the Shule River basin, northwestern China. Environ. Earth Sci. 2015, 73, 1901–1915. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, L.; Su, Y.H.; Zhang, Y.W.; Si, J.H. Distribution and evolution of water chemistry in Heihe River Basin. Environ. Geol. 2005, 45, 947–956. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Nie, Z.L.; Zhang, G.H.; Wan, L.; Shen, J.M. Environmental isotopic study on the recharge and residence time of groundwater in the Heihe River Basin, northwestern China. Hydrogeol. J. 2006, 14, 1635–1651. [Google Scholar] [CrossRef]
- Chen, L.J.; Feng, Q. Geostatistical analysis of temporal and spatial variations in groundwater levels and quality in the Minqin oasis, Northwest China. Environ. Earth Sci. 2013, 70, 1367–1378. [Google Scholar] [CrossRef]
- Ma, J.Z.; Wang, X.S.; Edmunds, W.M. The characteristics of ground-water resources and their changes under the impacts of human activity in the arid northwest China-a case study of the Shiyang River Basin. J. Arid Environ. 2005, 61, 277–295. [Google Scholar] [CrossRef]
- Jiao, J.J. Crescent Moon Spring: A disappearing natural wonder in the Gobidesert, China. Ground Water 2010, 48, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Feng, Q.; Liu, W.; Li, Z.X.; Wen, X.H.; Si, J.H.; Xi, H.Y.; Guo, R.; Jia, B. Stable isotopic and geochemical identification of groundwater evolution and recharge sources in the arid Shule River Basin of Northwestern China. Hydrol. Process. 2015, 29, 4703–4718. [Google Scholar] [CrossRef]
- He, J.H.; Ma, J.Z.; Zhao, W.; Sun, S. Groundwater evolution and recharge determination of the Quaternary aquifer in the Shule River basin, Northwest China. Hydrogeol. J. 2015, 23, 1745–1759. [Google Scholar] [CrossRef]
- Yan, C.Y. Study on the change of groundwater recharge and environment change caused by surface water redistribution. Arid Zone Res. 2007, 24, 428–433, (In Chinese with English abstract). [Google Scholar]
- Ma, C.F. Analysis on the variation of groundwater recharges and levels in the Shulehe River basin after the Changma reservoir running. J. Arid Land Resour. Environ. 2008, 22, 49–55, (In Chinese with English abstract). [Google Scholar]
- Gansu Geology Survey. The Report and Map for Hydrogeological Survey in the Shule River Basin (1:200,000), Gansu Science and Technology Press: Lanzhou, China, 1978.
- Han, W.; Hu, L.T.; Chen, C.X.; Zhang, H.S.; Cheng, X.X. Problems about numerical simulation of groundwater flow in Yumen-Tashi Basin, Shulehe River, China. Site Investig. Sci. Technol. 2005, 1, 15–18, (In Chinese with English abstract). [Google Scholar]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice Hall: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Epstein, S.; Mayeda, T. Variation of 18O content of waters from natural sources. Geochim. Cosmochim. Acta 1953, 4, 213–224. [Google Scholar] [CrossRef]
- Coleman, M.L.; Shepherd, T.J.; Durham, J.J.; Rouse, J.E.; Moore, G.R. Reduction of water with zinc for hydrogen isotope analysis. Anal. Chem. 1982, 54, 993–995. [Google Scholar] [CrossRef]
- Joussaume, S.; Sadourny, R.; Jouzel, J. A general-circulation model of water isotope cycles in the atmosphere. Nature 1984, 311, 24–29. [Google Scholar] [CrossRef]
- Yamanaka, T.; Tsujimura, M.; Oyunbaatar, D.; Davaa, G. Isotopic variation of precipitation over eastern Mongolia and its implication for the atmospheric water cycle. J. Hydrol. 2007, 333, 21–34. [Google Scholar] [CrossRef]
- Adomako, D.; Osae, S.; AkitiT, T.; Faye, S.; Maloszewski, P. Geochemical and isotopic studies of groundwater conditions in the Densu River Basin of Ghana. Environ. Earth Sci. 2011, 62, 1071–1084. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.N.; Li, W.H. Temporal and spatial variation of water stable isotopes(18O and 2H) in the Kaidu River basin, Northwestern China. Hydrol. Process. 2014, 28, 653–661. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press/Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- Dansgaard, W. Stable isotopes in precipitation. Tellus B 1964, 16, 436–468. [Google Scholar]
- International Atomic Energy Agency/World Meteorological Organization (IAEA/WMO). Global Network of Isotopes in Precipitation: The GNIP Database. 2006. Available online: http://www.iaea.org/water (accessed on 20 July 2011).
- Mook, W.G. Environmental Isotopes in the Hydrological Cycle: Principles and Applications; UNESCO and the International Atomic Energy Agency (IAEA): Paris, France, 2001. [Google Scholar]
- Busenberg, E.; Plummer, L.N. Dating young groundwater with sulfur hexafluoride: Natural and anthropogenic sources of sulfur hexafluoride. Water Resour. Res. 2000, 36, 3011–3030. [Google Scholar] [CrossRef]
| No. | Longitude | Latitude | Elevation (m) | Well Depth (m) | Water Table Depth (m) |
|---|---|---|---|---|---|
| 1 | 96°48′52.14″ | 39°57′36.33″ | 1960 | Shule River water | - |
| 2 | 96°45′4.71″ | 40°2′28.74″ | 1872 | 70 | 27 |
| 3 | 96°54′0.52″ | 40°7′48.37″ | 1698 | 180 | 120 |
| 4 | 96°56′52.05″ | 40°11′4.11″ | 1627 | 100 | 55 |
| 5 | 97°0′36.99″ | 40°17′10.49″ | 1519 | 100 | 7 |
| 6 | 97°1′29.43″ | 40°20′52.69″ | 1453 | 66 | 8.3 |
| 7 | 96°52′8.47″ | 40°26′11.37″ | 1427 | 50 | 10 |
| 8 | 96°32′24.48″ | 40°31′59.90″ | 1340 | 10 | 2 |
| 9 | 97°8′37.21″ | 40°19′24.84″ | 1441 | Spring water | - |
| 10 | 96°57′19.72″ | 40°24′55.43″ | 1422 | Spring water | - |
| 11 | 96°38′13.6″ | 40°31′31.10″ | 1363 | Spring water | - |
| 12 | 97°7′32.99″ | 40°25′34.24″ | 1390 | 90 | 30 |
| No. | Sampling Date | pH | Temp °C | EC (μs/cm) | TDS (mg/L) | Na+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) | Cl− (mg/L) | HCO3− (mg/L) | SO42− (mg/L) | NO3− (mg/L) | Ionic Balance Error (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 July 2013 | 7.5 | 18.2 | 825 | 412 | 40.06 | 3.46 | 32.64 | 92.8 | 24.29 | 291.69 | 131.84 | 0.42 | 5.0 |
| 16 October 2013 | 7.6 | 16.2 | 900 | 446 | 23.83 | 1.81 | 41.28 | 70.4 | 42.7 | 178.09 | 149.84 | 2.76 | 4.9 | |
| 22 March 2014 | 7.8 | 10.6 | 1413 | 886 | 93.52 | 4.57 | 58.84 | 29.5 | 72.32 | 155.43 | 272.4 | 1.87 | 1.3 | |
| 28 June 2014 | 6.9 | 12 | 830 | 413 | 27.98 | 2.44 | 29.48 | 30.47 | 38.13 | 146.06 | 111.67 | 2.86 | −5.0 | |
| 23 September 2014 | 7.7 | 12.9 | 1586 | 794 | 52.16 | 3.16 | 36.84 | 56.11 | 63.81 | 139.03 | 215.55 | 2.97 | −2.3 | |
| 5 November 2014 | 7.9 | 11 | 1338 | 664 | 51.62 | 4.34 | 42.14 | 43.67 | 49.3 | 171.22 | 141.31 | 3.93 | 5.5 | |
| 2 | 18 July 2013 | 7.5 | 17.3 | 920 | 460 | 23.04 | 2.87 | 26.88 | 47.2 | 71.66 | 87.47 | 133 | 2.24 | −4.9 |
| 16 October 2013 | 7.3 | 15.1 | 952 | 474 | 18.4 | 1.16 | 24.88 | 32.8 | 19.02 | 142.15 | 59.08 | 1.67 | 4.8 | |
| 22 March 2014 | 7.3 | 9.7 | 912 | 456 | 26.71 | 2.52 | 32.59 | 48.35 | 33.91 | 162.46 | 100.29 | 4.11 | 4.8 | |
| 28 June 2014 | 6.6 | 11 | 1240 | 788 | 23.53 | 2.08 | 28.42 | 30.38 | 35.1 | 155.43 | 96.6 | 2.87 | −5.9 | |
| 23 September 2014 | 7.6 | 13.6 | 904 | 452 | 17.09 | 1.79 | 24.23 | 36.59 | 24.51 | 138.25 | 79.22 | 2.64 | −0.1 | |
| 5 November 2014 | 7.4 | 10.9 | 1170 | 586 | 25.35 | 3.29 | 35.3 | 35.4 | 39.51 | 147.62 | 105.94 | 4.56 | 0.7 | |
| 3 | 18 July 2013 | 7.4 | 15 | 858 | 428 | 24.46 | 2.91 | 14.4 | 71.2 | 84.38 | 104.64 | 116.85 | 2.51 | −5.3 |
| 16 October 2013 | 8 | 13.9 | 860 | 430 | 12.92 | 1.49 | 28.32 | 52.8 | 24.9 | 174.97 | 75.65 | 2.84 | 3.8 | |
| 22 March 2014 | 7.1 | 15.2 | 856 | 426 | 25.56 | 2.39 | 29.97 | 35.46 | 27.75 | 142.63 | 82.56 | 3.67 | 5.3 | |
| 28 June 2014 | 6.8 | 12 | 1342 | 684 | 21.86 | 2.03 | 27.59 | 28.79 | 31.17 | 130.44 | 88.01 | 3.12 | −1.6 | |
| 23 September 2014 | 7.7 | 16.8 | 970 | 494 | 20.75 | 2.11 | 28.83 | 29.47 | 31.79 | 120.28 | 89.21 | 3.08 | 0.6 | |
| 5 November 2014 | 7.6 | 11.8 | 1082 | 548 | 52 | 4.39 | 31.91 | 31.52 | 40.74 | 157.16 | 103.69 | 4.89 | 5.1 | |
| 4 | 18 July 2013 | 7.4 | 15.1 | 962 | 480 | 26.09 | 2.95 | 32.64 | 79.2 | 99.66 | 57.49 | 260.49 | 1.61 | −7.6 |
| 16 October 2013 | 7.9 | 11.1 | 906 | 452 | 14.84 | 1.75 | 36.48 | 43.2 | 31.38 | 156.69 | 85.8 | 2.8 | 5.0 | |
| 22 March 2014 | 7.7 | 13.7 | 840 | 418 | 25.28 | 2.43 | 28.32 | 31.51 | 29.76 | 127.31 | 75.38 | 2.4 | 5.8 | |
| 28 June 2014 | 6.9 | 15 | 1162 | 726 | 22.32 | 2.23 | 30.89 | 28.97 | 33.64 | 141.37 | 90.14 | 2.01 | −1.2 | |
| 23 September 2014 | 7.4 | 15.7 | 994 | 506 | 21.85 | 2.22 | 31.56 | 28.23 | 28.43 | 129.66 | 92.67 | 2.37 | 1.5 | |
| 5 November 2014 | 7.5 | 10.6 | 1084 | 560 | 52.59 | 4.88 | 38.27 | 32.36 | 35.96 | 191.22 | 106.43 | 2.79 | 5.9 | |
| 5 | 18 July 2013 | 7.4 | 18.5 | 970 | 484 | 33.28 | 3.28 | 35.04 | 51.2 | 98.39 | 53.49 | 222.07 | 1.57 | −8.4 |
| 16 October 2013 | 7.7 | 18.4 | 952 | 474 | 15 | 1.45 | 20.64 | 39.2 | 24.79 | 153.09 | 60.65 | 1.79 | −1.4 | |
| 22 March 2014 | 7.7 | 14.5 | 882 | 442 | 31.54 | 2.87 | 26.51 | 24.46 | 31.66 | 158.55 | 77.54 | 2.54 | −2.7 | |
| 28 June 2014 | 6.9 | 15 | 1110 | 716 | 31.22 | 2.72 | 21.4 | 25.19 | 34.74 | 138.25 | 80.45 | 2.44 | −5.0 | |
| 23 September 2014 | 7.4 | 17.7 | 1058 | 528 | 30.13 | 2.6 | 27.28 | 26.01 | 36.48 | 135.12 | 83.97 | 2.63 | −0.8 | |
| 5 November 2014 | 7.5 | 11.2 | 1012 | 505 | 69.86 | 5.54 | 33.85 | 28.54 | 44.39 | 210.28 | 94.51 | 4.26 | 4.9 | |
| 6 | 18 July 2013 | 7.6 | 13.8 | 990 | 484 | 29.57 | 3.28 | 33.6 | 77.6 | 19.15 | 343.05 | 63.62 | 0.67 | 3.5 |
| 16 October 2013 | 7.6 | 11.4 | 978 | 490 | 15.24 | 1.68 | 37.2 | 60.8 | 32.91 | 210.18 | 83.65 | 4.29 | 5.0 | |
| 22 March 2014 | 8.0 | 13.8 | 1066 | 532 | 28.2 | 2.91 | 35.34 | 33.79 | 34.31 | 173.39 | 89.86 | 5.38 | 1.4 | |
| 28 June 2014 | 6.7 | 11 | 1330 | 848 | 26.72 | 2.49 | 34.94 | 33.7 | 38.99 | 174.18 | 95.39 | 4.8 | −1.6 | |
| 23 September 2014 | 7.7 | 15.2 | 916 | 894 | 20.81 | 2.27 | 26.5 | 26.66 | 32.62 | 118.72 | 75.34 | 2.62 | 0.3 | |
| 5 November 2014 | 7.2 | 10.5 | 1120 | 562 | 63.13 | 5.51 | 40.63 | 36.76 | 49.25 | 210.09 | 112.22 | 6.92 | 5.0 | |
| 7 | 18 July 2013 | 7.5 | 14.2 | 1128 | 556 | 31.07 | 3.52 | 45.6 | 80 | 111.57 | 31.06 | 318.56 | 4.66 | −5.7 |
| 16 October 2013 | 7.5 | 13.9 | 1296 | 648 | 22.82 | 2.38 | 58.08 | 71.6 | 40.41 | 300.8 | 125.99 | 7.38 | 3.6 | |
| 22 March 2014 | 7.2 | 13.9 | 1006 | 504 | 26.57 | 2.84 | 35.05 | 29.65 | 31.12 | 162.46 | 99.91 | 5.19 | −0.6 | |
| 28 June 2014 | 6.7 | 11 | 1424 | 908 | 28.32 | 2.75 | 30.61 | 34.68 | 39.04 | 158.86 | 112 | 5.62 | −4.6 | |
| 23 September 2014 | 7.5 | 14.2 | 1588 | 776 | 35.22 | 3.84 | 46.34 | 40.29 | 50.57 | 206.2 | 148.61 | 7.76 | −3.3 | |
| 5 November 2014 | 6. | 10.7 | 1114 | 562 | 63.49 | 5.32 | 40.12 | 33.84 | 48.86 | 176.99 | 128.98 | 6.88 | 5.7 | |
| 8 | 18 July 2013 | 7.7 | 15.2 | 790 | 394 | 19.34 | 2.74 | 24.96 | 58.4 | 78.38 | 36.71 | 185.01 | 1.82 | −6.2 |
| 16 October 2013 | 7.7 | 13.7 | 746 | 374 | 11.31 | 1.54 | 28.08 | 37.2 | 24.34 | 137.79 | 59.19 | 2.32 | 5.7 | |
| 22 March 2014 | 8.4 | 16.7 | 892 | 486 | 20.19 | 2.57 | 25.91 | 28.55 | 27.85 | 124.19 | 64.15 | 2.58 | 3.8 | |
| 28 June 2014 | 6.7 | 11.5 | 964 | 622 | 18.74 | 2.19 | 24.97 | 23.14 | 32.45 | 114.82 | 71.97 | 2.1 | −2.6 | |
| 23 September 2014 | 7.5 | 12.1 | 820 | 398 | 18.12 | 2.22 | 26.48 | 24.3 | 33.1 | 107 | 73.49 | 2.38 | 0.1 | |
| 5 November 2014 | 7.4 | 10 | 786 | 422 | 40.81 | 4.5 | 33.98 | 29.23 | 41.21 | 162.47 | 84.61 | 3.24 | 4.6 | |
| 9 | 18 July 2013 | 7.0 | 19 | 2176 | 1080 | 24.66 | 3.04 | 77.76 | 108 | 143.89 | 175.44 | 362.77 | 3.15 | −5.4 |
| 16 October 2013 | 7 | 14.4 | 1940 | 970 | 35.73 | 3.07 | 81.12 | 90.4 | 66.4 | 342.13 | 215.53 | 17.27 | 2.6 | |
| 22 March 2014 | 7.8 | 9.2 | 1756 | 874 | 80.45 | 5.67 | 88.9 | 176.5 | 61.73 | 684.32 | 215.58 | 11.95 | 5.9 | |
| 28 June 2014 | 6.8 | 14.5 | 2368 | 1506 | 58.43 | 4.73 | 67.85 | 37.68 | 167.9 | 102.76 | 228.24 | 15.69 | −5.0 | |
| 23 September 2014 | 7.2 | 16.3 | 2174 | 1084 | 56.79 | 4.91 | 70.04 | 55.28 | 82.33 | 206.2 | 259.34 | 16.54 | −0.7 | |
| 5 November 2014 | 7.0 | 10.8 | 2040 | 1100 | 129.2 | 8.83 | 68.65 | 55.05 | 97.93 | 231.77 | 291.04 | 18.12 | 5.0 | |
| 10 | 18 July 2013 | 6.8 | 18.8 | 2338 | 1176 | 65.41 | 5.28 | 87.72 | 78.5 | 226.17 | 202.08 | 293.82 | 1.61 | −5.3 |
| 16 October 2013 | 6.9 | 17.1 | 2142 | 1070 | 34.11 | 2.82 | 78.24 | 50.8 | 64.48 | 392.87 | 168.12 | 2.25 | −5.2 | |
| 22 March 2014 | 7.4 | 6.1 | 1934 | 960 | 85.14 | 4.8 | 107.3 | 194.1 | 80.91 | 793.67 | 243.39 | 3.95 | 4.8 | |
| 28 June 2014 | 6.4 | 12.5 | 3382 | 2266 | 85.01 | 5.09 | 95.85 | 29.66 | 265.84 | 105.37 | 258.72 | 3.92 | −4.9 | |
| 23 September 2014 | 7.0 | 15.8 | 2794 | 1380 | 70.45 | 4.99 | 82.21 | 48.36 | 99.49 | 283.5 | 264.5 | 7.73 | −2.4 | |
| 5 November 2014 | 6.9 | 11.2 | 2308 | 1152 | 139.5 | 8.82 | 95.54 | 70.24 | 107.99 | 456.94 | 261.47 | 8.07 | 4.9 | |
| 11 | 18 July 2013 | 8 | 19.1 | 1778 | 906 | 25.8 | 10.7 | 48.5 | 59.6 | 148.23 | 114.8 | 159.2 | 0.52 | −5.4 |
| 16 October 2013 | 7.6 | 14.2 | 1758 | 878 | 22.45 | 1.76 | 37.92 | 55.2 | 36.36 | 124.97 | 160.77 | 1.26 | 3.7 | |
| 22 March 2014 | 7.2 | 9.4 | 4870 | 2410 | 237.1 | 40.69 | 313.2 | 229.3 | 191.77 | 938.2 | 1097.89 | 5.31 | 5.6 | |
| 28 June 2014 | 6.6 | 11 | 8840 | 6220 | 341.9 | 59.96 | 320.1 | 65.5 | 398.17 | 346.01 | 1617.76 | 0.89 | −4.3 | |
| 23 September 2014 | 7.9 | 15.8 | 1478 | 728 | 99.56 | 6.6 | 75.49 | 55.58 | 131.37 | 149.18 | 431.49 | 2.19 | −5.5 | |
| 5 November 2014 | 7.4 | 11.2 | 1054 | 528 | 62.52 | 5.13 | 44.34 | 33.17 | 124.98 | 160.91 | 68.28 | 1.84 | 3.7 | |
| 12 | 18 July 2013 | 7.3 | 11.9 | 2598 | 1296 | 63.8 | 5.82 | 123.6 | 98.9 | 410.03 | 175.06 | 280.28 | 2.57 | −5.5 |
| 16 October 2013 | 7.3 | 12.7 | 2660 | 1320 | 69.8 | 5.49 | 128.88 | 104.4 | 131.75 | 310.1 | 417.73 | 3.17 | 4.3 | |
| 22 March 2014 | 7.3 | 12.7 | 2660 | 1320 | 140.1 | 12.29 | 126.52 | 220.8 | 124.05 | 786.2 | 411.09 | 1.77 | 5.6 | |
| 28 June 2014 | 6.9 | 12.3 | 3478 | 2244 | 104.9 | 8.49 | 89.45 | 53.6 | 451.56 | 26.06 | 262.88 | 2.55 | −11.1 | |
| 23 September 2014 | 7.6 | 11.3 | 2012 | 1004 | 87.52 | 5.9 | 64.42 | 57.86 | 114.9 | 168.71 | 343.66 | 12.63 | −4.5 | |
| 5 November 2014 | 7.2 | 10.5 | 3064 | 1532 | 121.7 | 11.23 | 79.33 | 59.31 | 161.3 | 179.64 | 450.85 | 8.97 | −5.8 | |
| Mean | 7.3 | 13.4 | 1575 | 855.5 | 51.6 | 5.2 | 55.8 | 56.8 | 82.4 | 205.6 | 197.8 | 4.3 | - | |
| Min. | 6.4 | 6.1 | 746 | 374 | 11.3 | 1.16 | 14.4 | 23.1 | 19.0 | 26.1 | 59.08 | 0.4 | - | |
| Max. | 8.4 | 19.1 | 8840 | 6220 | 341.9 | 59.9 | 320.1 | 229.3 | 451.56 | 938.2 | 1617.76 | 18.12 | - |
| No. | Sampling Date | δ18O (‰) | δD (‰) | d (‰) | 3H (TU) | CFC Concentration pmol/kg CFC-11 CFC-12 CFC-113 | Apparent Age Years CFC-11 CFC-12 CFC-113 Average | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 July 2013 | −8.51 | −55.57 | 12.51 | 10.91 | - | - | - | - | - | - | - |
| 16 October 2013 | -9.09 | −55.74 | 16.98 | 12.45 | - | - | - | - | - | - | - | |
| 2 | 18 July 2013 | −8.78 | −55.67 | 14.57 | 14.85 | 4.7 | 2.41 | 0.35 | 27 | 25 | 25 | 26 |
| 16 October 2013 | −8.95 | −58.28 | 13.32 | 14.48 | 5.32 | 2.51 | 0.54 | 25 | 25 | 19 | 23 | |
| 3 | 18 July 2013 | −8.96 | −55.92 | 15.76 | 16.16 | 5.17 | 2.77 | 0.4 | 25 | 20 | 25 | 23 |
| 16 October 2013 | −9.14 | −59.13 | 13.99 | 13.50 | 5.5 | 2.57 | 0.36 | 24 | 24 | 26 | 25 | |
| 4 | 18 July 2013 | −8.52 | −56.34 | 11.82 | 19.76 | 5.05 | 3.24 | 0.46 | 26 | Contam. | 24 | 25 |
| 16 October 2013 | −8.52 | −55.41 | 12.75 | 16.77 | 5.13 | 3.09 | 0.49 | 26 | Contam. | 23 | 25 | |
| 5 | 18 July 2013 | −10.13 | −64.38 | 16.66 | 0.80 | 1.78 | 0.95 | 0.06 | 40 | 40 | 39 | 40 |
| 16 October 2013 | −10.06 | −65.4 | 15.08 | 0.90 | 2.12 | 1.05 | 0.18 | 39 | 39 | 32 | 36 | |
| 6 | 18 July 2013 | −9.44 | −60.72 | 14.8 | 18.32 | 3.14 | 2.08 | 0.25 | 35 | 29 | 29 | 31 |
| 16 October 2013 | −9.11 | −61.23 | 11.65 | 15.84 | 3.86 | 2.07 | 0.22 | 31 | 29 | 29 | 30 | |
| 7 | 18 July 2013 | −9.28 | −57.5 | 16.74 | 25.69 | 5.91 | 2.25 | 0.22 | Contam. | 27 | 29 | 28 |
| 16 October 2013 | −8.96 | −58.55 | 13.13 | 25.99 | 7.41 | 2.29 | 0.28 | Contam. | 26 | 28 | 27 | |
| 8 | 18 July 2013 | −8.81 | −57.9 | 12.58 | 8.89 | 2.58 | 0.83 | 0.11 | 38 | 41 | 36 | 38 |
| 16 October 2013 | −8.92 | −59.37 | 11.99 | 10.74 | 1.7 | 0.85 | 0.1 | 40 | 41 | 36 | 39 | |
| 9 | 18 July 2013 | −8.87 | −57.03 | 13.93 | 10.11 | 3.58 | 2.24 | 0.28 | 32 | 27 | 28 | 29 |
| 16 October 2013 | −8.89 | −57.4 | 13.72 | 11.53 | 4.4 | 2.63 | 0.34 | 29 | 23 | 27 | 26 | |
| 10 | 18 July 2013 | −8.1 | −52.69 | 12.11 | 20.69 | 4.18 | 2.52 | 0.34 | 29 | 24 | 27 | 27 |
| 16 October 2013 | −8.26 | −52.63 | 13.45 | 17.61 | 3.46 | 2.68 | 0.32 | 33 | 22 | 27 | 27 | |
| 11 | 18 July 2013 | −8.03 | −52.71 | 11.53 | 18.64 | 3.14 | 3.0 | 0.28 | 35 | Contam. | 28 | 32 |
| 16 October 2013 | −9.09 | −56.72 | 16. | 18.40 | 3.16 | 3.2 | 0.29 | 35 | Contam. | 28 | 32 | |
| 12 | 18 July 2013 | −8.65 | −59.01 | 10.19 | 14.16 | 1.90 | 1.52 | 0.17 | 40 | 35 | 33 | 36 |
| 16 October 2013 | −8.86 | −58.19 | 12.69 | 14.18 | 1.92 | 1.53 | 0.18 | 40 | 35 | 32 | 36 | |
| 13 | 18 July 2013 | 1.68 | 16.32 | 2.88 | - | - | - | - | - | - | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Zhou, Z.; Wang, S. The Source, Flow Rates, and Hydrochemical Evolution of Groundwater in an Alluvial Fan of Qilian Mountain, Northwest China. Water 2017, 9, 912. https://doi.org/10.3390/w9120912
Guo Q, Zhou Z, Wang S. The Source, Flow Rates, and Hydrochemical Evolution of Groundwater in an Alluvial Fan of Qilian Mountain, Northwest China. Water. 2017; 9(12):912. https://doi.org/10.3390/w9120912
Chicago/Turabian StyleGuo, Qiaona, Zhifang Zhou, and Shan Wang. 2017. "The Source, Flow Rates, and Hydrochemical Evolution of Groundwater in an Alluvial Fan of Qilian Mountain, Northwest China" Water 9, no. 12: 912. https://doi.org/10.3390/w9120912




