200 kHz Sonication of Mixed-Algae Suspension from a Eutrophic Lake: The Effect on the Caution vs. Outbreak Bloom Alert Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Experimental Setup
2.2. Analytical Methods
3. Results and Discussions
3.1. Effect of the Sonication on Chlorophyll-a Content and Sedimentation
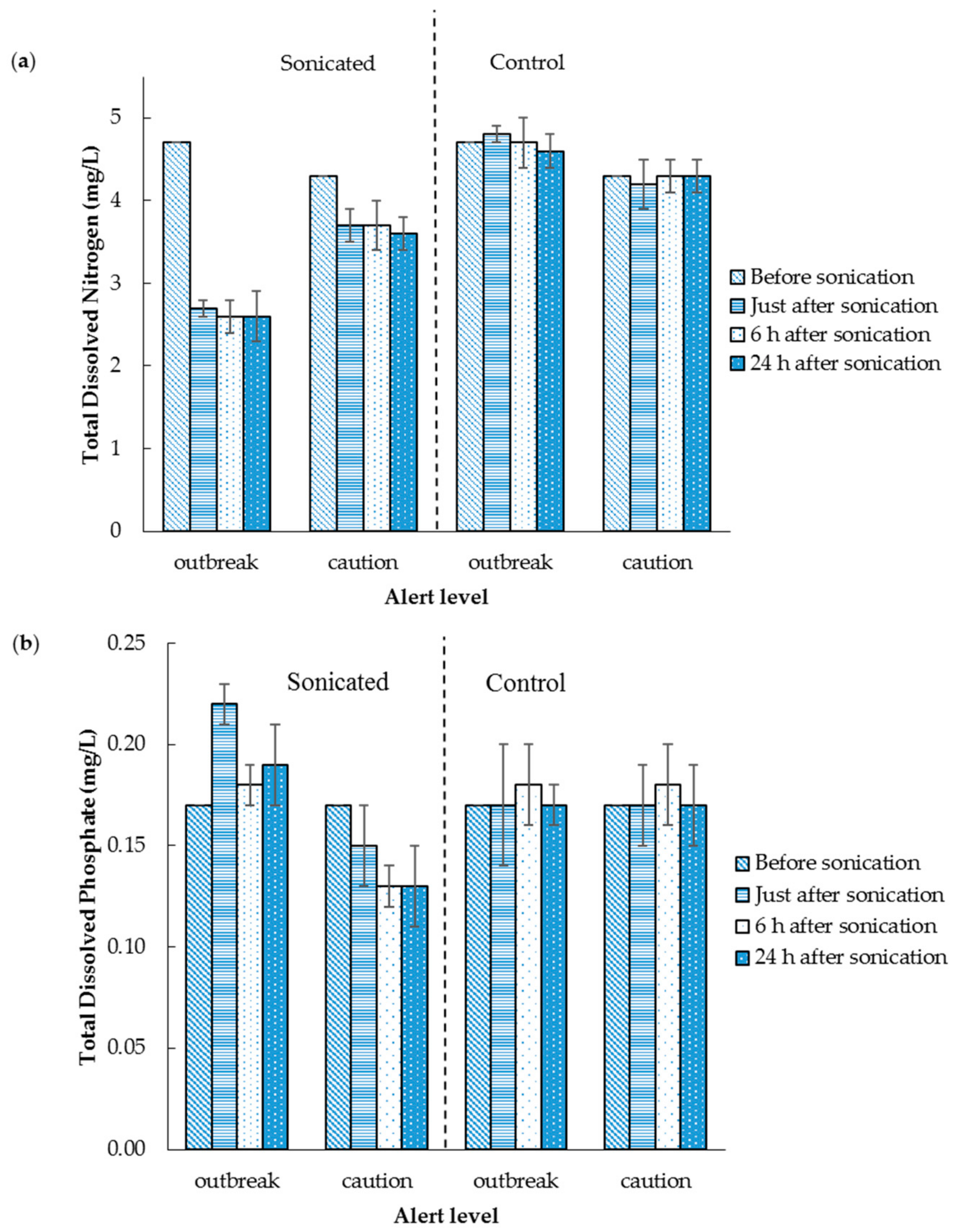
3.2. The Effect of Sonication on the Mixed Algae Suspension TDN and TDP
3.3. The Effect of Sonication on the Mixed Algae Suspension COD
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palaniappan, M.; Gleick, P.H.; Allen, L.; Cohen, M.J.; Christian-Smith, J.; Smith, C. Clearing the Waters: A Focus on Water Quality Solutions; United Nations Environment Programme, Pacific Institute: Oakland, CA, USA; UNON: Nairobi, Kenya, 2010; ISBN 978-92-807-3074-6. [Google Scholar]
- Dokulil, M.T. Old wine in new skins—Eutrophication reloaded: Global perspectives of potential amplification by climate warming, altered hydrological cycle and human interference. In Eutrophication: Causes, Economic Implications and Future Challenges; Lambert, A., Roux, C., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2013; pp. 95–125. ISBN 978-1-62808-498-6. [Google Scholar]
- Beklioglu, M.; Meerfhoff, M.; Sondergaard, M.; Jeppesen, E. Eutrophication and Restoration of Shallow Lakes from a Cold Temperate to a Warm Mediterranean and a (Sub)Tropical Climate. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Lanza, G.R., Rast, W., Eds.; Springer: Dordrecht/Heidelberg, Germany; London, UK; New York, NY, USA, 2011; pp. 91–108. ISBN 978-90-481-9624-1. [Google Scholar]
- Hilton, J.; Ohare, M.; Bowes, M.J.; Jones, J.I. How green is my river? A new paradigm of eutrophication in rivers. Sci. Total Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Webster, I.T.; Sherman, B.S.; Bormans, M.; Jones, G. Management strategies for cyanobacterial blooms in an impounded lowland river. Regul. Rivers 2000, 16, 513–525. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, P.; Shen, H.; Xu, J.; Wang, P.; Zhang, B. A novel flushing strategy for diatom bloom prevention in the lower-middle Hanjiang River. Water Res. 2012, 46, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A. Managing agricultural phosphorus to minimize water quality impacts. Sci. Agric. 2016, 73, 1–8. [Google Scholar] [CrossRef]
- Jancula, D.; Marsalek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Sierp, M.T.; Qin, J.G.; Recknagel, F. Biomanipulation: A review of biological control measures in eutrophic waters and the potential for Murray cod Maccullochella peelii peelii to promote water quality in temperate Australia. Rev. Fish Biol. Fish. 2009, 19, 143–165. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef]
- Beutel, M.W.; Horne, A.J. A Review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv. Manag. 1999, 15, 285–297. [Google Scholar] [CrossRef]
- Hickey, C.W.; Gibbs, M.M. Lake sediment phosphorus release management-Decision support and risk assessment framework. N. Z. J. Mar. Freshw. Res. 2009, 43, 819–856. [Google Scholar] [CrossRef]
- Joyce, E.M.; Wu, X.; Mason, T.J. Effect of ultrasonic frequency and power on algae suspensions. J. Environ. Sci. Health A 2010, 45, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Tekile, A.; Kim, I.; Kim, J. Mini-review on river eutrophication and bottom improvement techniques, with special emphasis on the Nakdong River. J. Environ. Sci. 2015, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.; Parsons, S.A.; Jefferson, B. The influence of ultrasound frequency and power, on the algal species Microcystis aeruginosa, Aphanizomenon flos-aquae, Scenedesmus subspicatus and Melosira sp. Environ. Technol. 2013, 34, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.D.; Weinrich, L.A.; Brezinski, S. Ultrasonic treatment of algae in a New Jersey Reservoir. J. Am. Water Works Assoc. 2015, 107, E533–E542. [Google Scholar] [CrossRef]
- Hao, H.; Wu, M.; Chen, Y.; Tang, J.; Wu, Q. Cavitation mechanism in cyanobacterial growth inhibition by ultrasonic irradiation. Colloids Surf. B 2004, 33, 151–156. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Guo, N.; Teh, C.Y.; Hay, J.W. Advances in Ultrasound Technology for Environmental Remediation; Springer Science and Business Media: New York, NY, USA, 2013; pp. 95–98. ISBN 978-94-007-5532-1. [Google Scholar]
- Lee, T.J.; Nakano, K.; Matsumara, M. Ultrasonic irradiation for blue-green algae bloom control. Environ. Technol. 2001, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.; Nan, J.; He, W.-J.; Li, G. Algae removal by ultrasonic irradiation–coagulation. Desalination 2009, 239, 191–197. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Wang, B.; Liu, H. Ultrasonic frequency effects on the removal of Microcystis aeruginosa. Ultrason. Sonochem. 2006, 13, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. Impact of sonication at 20 kHz on Microcystis aeruginosa, Anabaena circinalis and Chlorella sp. Water Res. 2012, 46, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Lee, T.J.; Matsumura, M. In situ algal bloom control by the integration of ultrasonic radiation and jet circulation to flushing. Environ. Sci. Technol. 2001, 35, 4941–4946. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Nakano, K.; Matsumura, M. A novel strategy for cyanobacterial bloom control by ultrasonic irradiation. Water Sci. Technol. 2002, 46, 207–215. [Google Scholar] [PubMed]
- Srisuksomwong, P.; Whangchai, N.; Yagita, Y.; Okada, K.; Peerapornpisal, Y.; Nomura, N. Effects of ultrasonic irradiation on degradation of microcystin in fish ponds. Int. J. Agric. Biol. 2011, 13, 67–70. [Google Scholar]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Park, J.; Church, J.; Son, Y.; Kim, K.T.; Lee, W.H. Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason. Sonochem. 2017, 38, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.; Dickson, S.; Burch, M.; Thorne, O.; Tsymbal, L.; House, J.; Brookes, J.; Chang, D.; Kao, S.; Lin, T.; et al. Alternative and Innovative Methods for Source Water Management of Algae and Cyanobacteria; Water Research Foundation: Denver, CO, USA, 2012. Available online: http://trove.nla.gov.au/version/190037736 (accessed on 15 June 2017).
- Li, J.; Long, H.; Song, C.; Wu, W.; Yeabah, T.O.; Qiu, Y. Study on the removal of algae from lake water and its attendant water quality changes using ultrasound. Desalin. Water Treat. 2014, 52, 4762–4771. [Google Scholar] [CrossRef]
- Srivastava, A.; Ahn, C.Y.; Asthana, R.K.; Lee, H.G.; Oh, H.M. Status, alert system, and prediction of cyanobacterial bloom in South Korea. BioMed Res. Int. 2015, 2015, 584696. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Kim, B.; Kim, E.; Okino, T. Hepatotoxic microcystins and neurotoxic anatoxin-a in cyanobacterial blooms from Korean lakes. Environ. Toxicol. Water Qual. 1998, 13, 225–234. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Dickson, S.; Hobson, P.; Howard, C.; Zander, A.; Burch, M. Quantification of the ultrasound induced sedimentation of Microcystis aeruginosa. Ultrason. Sonochem. 2014, 21, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Jachlewski, S.; Botes, M.; Cloete, T.E. The effect of ultrasound at 256 KHz on Microcystis aeruginosa, with and without gas vacuoles. Water SA 2013, 39, 171–174. [Google Scholar] [CrossRef]
- Kurokawa, M.; King, P.M.; Wu, X.; Joyce, E.M.; Mason, T.J.; Yamamoto, K. Effect of sonication frequency on the disruption of algae. Ultrason. Sonochem. 2016, 31, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.Y.; Park, M.H.; Joung, S.H.; Kim, H.S.; Jang, K.Y.; Oh, A.M. Growth inhibition of cyanobacteria by ultrasonic radiation: Laboratory and enclosure studies. Environ. Sci. Technol. 2003, 37, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.Y.; Joung, S.H.; Choi, A.; Kim, H.S.; Jang, K.Y.; Oh, H.M. Selective control of cyanobacteria in eutrophic pond by a combined device of ultrasonication and water pumps. Environ. Technol. 2007, 28, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Lurling, M.; Tolman, Y. Beating the blues: Is there any music in fighting cyanobacteria with ultrasound? Water Res. 2014, 66, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Wu, M.; Chen, Y.; Tang, J.; Wu, Q. Cyanobacterial bloom control by ultrasonic irradiation at 20 kHz and 1.7 MHz. J. Environ. Sci. Health A 2004, 39, 1435–1446. [Google Scholar] [CrossRef]
- Yamamoto, K.; King, P.M.; Wu, X.; Mason, T.J.; Joyce, E.M. Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason. Sonochem. 2015, 24, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lurling, M.; Tolman, Y. Effects of commercially available ultrasound on the zooplankton grazer daphnia and consequent water greening in laboratory experiments. Water 2014, 6, 3247–3263. [Google Scholar] [CrossRef]
- Purcell, D.; Parsons, S.A.; Jefferson, B.; Holden, S.; Campbell, A.; Wallen, A.; Chipps, M.; Holden, B.; Ellingham, A. Experiences of algal bloom control using green solutions barley straw and ultrasound, an industry perspective. Water Environ. J. 2013, 27, 148–156. [Google Scholar] [CrossRef]
- Tang, J.W.; Wu, Q.Y.; Hao, H.W.; Chen, Y.; Wu, M. Effect of 1.7 MHz ultrasound on a gas-vacuolate cyanobacterium and a gas-vacuole negative cyanobacterium. Colloids Surface B 2004, 36, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Klemencic, A.K.; Griessler-Bulc, T. The efficiency of ultrasound on algal control in a closed loop water treatment system for cyprinid fish farms. Fresenius Environ. Bull. 2010, 19, 919–931. [Google Scholar]
- Zhang, G.; Zhang, P.; Liu, H.; Wang, B. Ultrasonic damages on cyanobacterial photosynthesis. Ultrason. Sonochem. 2006, 13, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, P.; Fan, M. Ultrasound-enhanced coagulation for Microcystis aeruginosa removal. Ultrason. Sonochem. 2009, 16, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Chen, Y.; Hao, H.; Wu, M.; Wang, B.; Lv, H.; Zhang, G. Influence of ultrasonic field on microcystins produced by bloom-forming algae. Colloids Surface B 2005, 41, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 1998; ISBN-13 978-0875532356. [Google Scholar]
- Jirka, A.M.; Carter, M.J. Micro semi-automated analysis of surface and wastewaters for chemical oxygen demand. Anal. Chem. 1975, 47, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.; Foreman, J.A.; Searcy, R.L. New reactants for the colorimetric determination of ammonia. Clin. Chim. Acta 1966, 14, 403–405. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications, 2nd ed.; Woodhead Publishing: Philadelphia, PA, USA, 2002; pp. 5–11. ISBN 978-1-898563-83-9. [Google Scholar]
- Liu, C.; Wang, J.; Cao, Z.; Chen, W.; Bi, H. Variation of dissolved organic nitrogen concentration during the ultrasonic pretreatment to Microcystis aeruginosa. Ultrason. Sonochem. 2016, 29, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, L.; Liu, H. Using low intensity ultrasound to improve the efficiency of biological phosphorus removal. Ultrason. Sonochem. 2008, 15, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, V.; Belgiorno, V.; Napoli, R.M.A. Behavior of natural organic matter during ultrasonic irradiation. Desalination 2007, 210, 175–182. [Google Scholar] [CrossRef]
- Lurling, M.; Waajen, G.; de Senerpont Domis, L.N. Evaluation of several end-of-pipe measures proposed to control cyanobacteria. Aquat. Ecol. 2016, 50, 499–519. [Google Scholar] [CrossRef]
- Tekile, A.; Kim, I.; Lee, J.-Y. Field trial of water flow and ultrasonic irradiation to improve the water quality of a stagnant river reach. Desalin. Water Treat. 2017, 74, 115–124. [Google Scholar] [CrossRef]

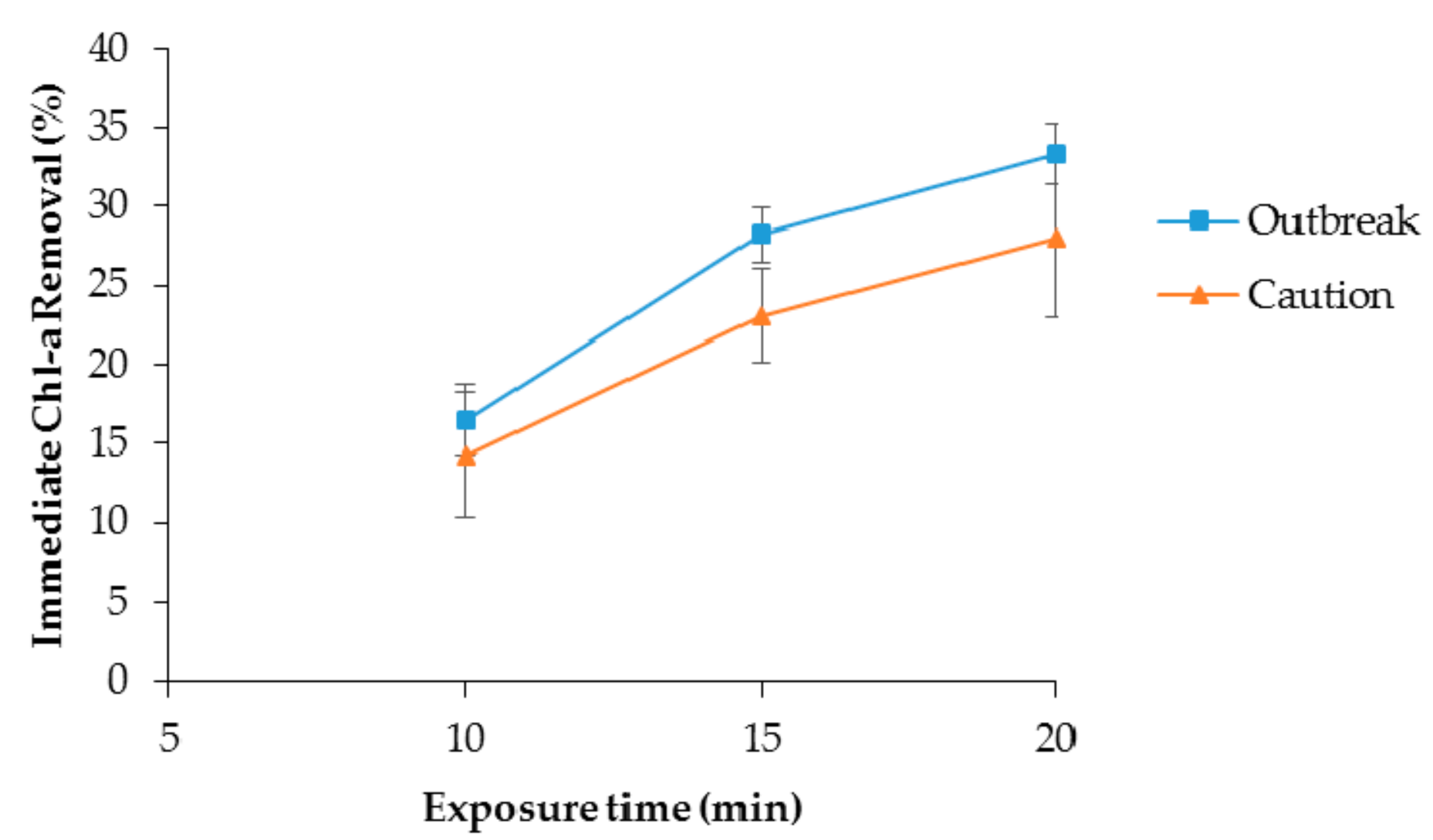

 ,
,  and
and  represent 10, 15 and 20 min treated suspensions, respectively and the broken lines,
represent 10, 15 and 20 min treated suspensions, respectively and the broken lines,  ,
,  and
and  represent control groups corresponding to 10, 15, and 25 min treatment, respectively.
represent control groups corresponding to 10, 15, and 25 min treatment, respectively.
 ,
,  and
and  represent 10, 15 and 20 min treated suspensions, respectively and the broken lines,
represent 10, 15 and 20 min treated suspensions, respectively and the broken lines,  ,
,  and
and  represent control groups corresponding to 10, 15, and 25 min treatment, respectively.
represent control groups corresponding to 10, 15, and 25 min treatment, respectively.

| Target Species | Sonication Condition (Frequency, Power) | Study Scale (Volume) | Treatment Duration | Effect | Ref. |
|---|---|---|---|---|---|
| Cyanobacteria and other algae types | 4 buoys (Not given) | Reservoir (2.9 Mm3) | 6 months | Reduced taste and odor and algae levels; 22% reduction of alum dose and 20 h longer filter run ($87,800 related operation costs saved). | [16] |
| M. aeruginosa | Jet flow, flushing, and 10 transducers (100 W, 200 kHz each) | Lake (365,000 m3) | 5 s contact time, for 2 years | Chl-a and SS decreased, from 200 to 50 µg/L and 100 to 20 g/m3, res.; however, following decrease in flushing rate, bloom reappeared (130 µg/L Chl-a). | [25,26] |
| C. gracilis, C. calcitrans, and Nannochloropsis sp. | 0.02, 0.4, 1.0, 2.2, 3.3, and 4.3 MHz, 10 W | Lab Scale (100 mL) | 0–10 min | Highest algae reduction efficiency at 2.2, 3.3, and 4.3 MHz for C. gracilis (100% at 2 min), C. calcitrans (100% at 2 min), and Nannochloropsis sp. (90% at 10 min), res. | [36] |
| Cyanobacteria and other algae types | 630 W, 22 kHz | Pond enclosures (200 L) | 40 s with 210 s gap, for 7 days | Chl-a decreased from 111.3 to 32.5 µg/L after 3 days of sonication during which that of control doubled. Sonication selectively inhibited cyanobacteria compared to other algae cells. | [37] |
| Cyanobacteria and diatoms | 2 pumps with ultrasonic apparatus (630 W, and 22 kHz) | Pond (9000 m3), control (7000 m3) | 7 weeks | Chl-a in treated pond remained low at 10 μg/L, while it increased in control (20 to 87 μg/L). It increased in the treated pond, only when the device stopped. | [38] |
| Anabaena sp., C. raciborskii, M. aeruginosa, S. obliquus, and D. magna | Mix of 20, 28, and 44 kHz, 26.4 W | Lab scale (800 mL) | 19, 10, 7, and 5 days | Chl-a of Anabaena decreased. However, no effect on C. raciborskii, M. aeruginosa, and S. obliquus growth rates. Ultrasound killed all Daphnia within 15 min. | [39] |
| Spirulina platensis | Beaker system (1.7 MHz, 14 W) and probe (20 kHz, 70 W) | Lab Scale (800 mL) | 0–9 min | The inhibition at 1.7 MHz was 50% better than that at 20 kHz. 5 min sonication at 1.7 MHz inhibited growth for 3 days. | [40] |
| Filamentous cyanobacteria (Spirulina platensis) | 200 kHz and 1.7 MHz, 40 W) and (20 kHz, 0, 20, 40, 60, and 80 W) | Lab Scale (800 mL) | 0–10 min | Inhibition most effective at 200 kHz and became saturated with the increased power. | [17] |
| Spherical shaped (C. concordia) and ovoid shaped (D. salina) | (20 kHz, 32.3 W), and (580, 864, and 1146 kHz, 3, 20, and 60 W) | Lab Scale (400 mL) | 1–30 min | Disruption efficiency of C. concordia was in the order of 20 < 580 < 864 < 1146 kHz frequency, and for D. salina was 20 < 580 ≅ 864 ≤ 1146 kHz. | [41] |
| M. aeruginosa | 20, 40, 580, 864, and 1146 kHz at 0.0178, 0.0213, 0.0018, 0.0042, and 0.0026 W/cm3, res. | Lab Scale (200 mL) | 0, 5, 10, 20, 30 min | The order of efficiency for algae reduction: 20 < 1146 < 864 < 580 kHz. Ultrasound is suitable method for algae inactivation or control under proper sonication conditions. | [13] |
| D. magna | 20, 28, 36, or 44 kHz, 0.63 W | Lab scale (100–3200 mL) | 5–30 min | Differently sized Daphnia (0.7–3.2 mm) were all killed between 5 and 30 min when exposed to 44 kHz. | [42] |
| Green algae, cyanobacteria (with D. magna) | 20, 28, 36, or 44 kHz, 0.63 W | Lab scale (85 L) | 25 days | In controls Daphnia flourished and algal biomass dropped. In the treatment, Daphnia number extremely low and phytoplankton biomass high. | [42] |
| M. aeruginosa, Peridinium sp., and B. braunii | 29, 43, 108, 200 and 1000 kHz, 3 W | Lab scale (30 mm scum) | 0–10 min | 200 kHz settled Microcystis scum successfully without cell disintegration, and recommended for reducing microcystin and musty odor substances of aquaculture ponds. | [27] |
| Microcystis aeruginosa | 20, 580, and 1146 kHz, 0.0403 W/cm3 | Lab scale (200 mL) | 0–30 min | 20 kHz ultrasound at high intensity (0.0403 W/cm3) was effective for inactivation of cyanobacterial cells. | [23] |
| Cyanobacteria, Green algae, Diatoms | 28 and 40–50 kHz, 40 W | Reservoir (0.8–1 Mm3) | 5 months | Inconsistent results with reductions across all algal groups in some cases, but no overall effect in others. | [43] |
| M. aeruginosa, A. flos-aquae, S. subspicatus, and Melosira sp. | Probe (20 kHz, 600 W) and Multi-frequency type (582, 862, and 1144 kHz, 200 W) | Lab scale (1500 mL) | 5–500 s | High removal rate of A. flos-aquae (99%) and Melosira sp. (83%). M. aeruginosa and S. subspicatus non-susceptible to ultrasound. 65% photosynthetic activity reduction of M. aeruginosa shows possibility of controlling bloom growth. | [15] |
| M. aeruginosa | Bath-type (21.5 kHz, 8.24 W) | Lab scale (600 mL) | 10 min | Slight recovery of cells after 14 days of incubation proved that ultrasound induced sedimentation is a long-term effect. | [34] |
| Vacuolated (M. aeruginosa) and vacuole negative (Synechococcus) | 1.7 MHz, 0.6 W/cm2 | Lab scale | 5 min every day, for 4 days | Cavitation effect depended on intracellular gas-vacuoles. Compared to control, 65% decrease in M. aeruginosa biomass increment, while no effect on Synechococcus culture. | [44] |
| M. aeruginosa, A. circinalis, and Chlorella sp. | Probe system (20 kHz, 0.085 W/mL) | Lab scale (200 mL) | 5, 10, 15, and 20 min | The order of growth inhibition was: A. circinalis > M. aeruginosa > Chlorella sp., demonstrating selectively removal of cyanobacteria. | [24] |
| Blue-green algae and M. aeruginosa | 20–1100 kHz, 10–30 W | Lab scale (2000 mL) | 0–6 min | At 20 kHz and 30 W, effective algae removal (96%) and significant improvement of water quality achieved in 6 min. | [31] |
| M. aeruginosa (with and without gas vacuoles) | Ultrasonic flow device (256 KHz) | Lab scale (35 L) | 9 days | Disruption of gas vacuoles (in gas-vacuole type) and destruction of cell membranes (for those without). Chl-a was lower in both cases, compared to controls. | [35] |
| More than 30 different algae species | 20–200 kHz, 12 W | Fish pond (36 m3) | June–September and October–November 2007 | Efficient removal of planktonic algae, but flushing system required to remove the sedimented algae. | [45] |
| M. aeruginosa | Probe system (25 kHz, 0.32 W/mL) | Lab scale (250 mL) | 5 min | Cell growth and extracellular microcystins release were inhibited effectively. | [46] |
| M. aeruginosa | 20, 80 150, 410, 690, 1320 kHz, 32–80 W | Lab scale (200 mL) | 0–10 min | High power and long duration increased microcystins, but frequency had little impact. | [22] |
| Blue green algae (BGA) | 28 kHz, 120 and 1200 W | Lab scale (700 mL) | 0–5 min | Sonication collapsed gas vacuoles, precipitated BGA. Microcystin concentration did not increase even at 1200 W, 28 kHz and 5 min sonication. | [20] |
| M. aeruginosa | 150 kHz, 30 W | Lab scale (1000 mL) | 0–60 s | Short sonication enhanced reduction of algae cells and Chl-a without increasing concentration of microcystins. | [47] |
| Microcystis | Beaker (150, 410, and 1.7 MHz, 30 W) and horn (20 kHz, 0–90 W) | Lab scale (400 mL) | 0–12 min | Growth of Microcystis efficiently inhibited and after 20 min of sonication at 150 kHz and 30 W, 70% microcystins removed. | [48] |
| Sonication Time (min) | Ultrasonic Dose (kWh/m3) | Chl-a Reduction Efficiency (m3/kWh) | |||
|---|---|---|---|---|---|
| Outbreak | Outbreak | ||||
| Average | min., max. | Average | min., max. | ||
| 10 | 20 | 0.82 | 0.71, 0.94 | 0.71 | 0.43, 1.00 |
| 15 | 30 | 0.94 | 0.88, 1.00 | 0.77 | 0.60, 0.94 |
| 20 | 40 | 0.83 | 0.78, 0.88 | 0.70 | 0.53, 0.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekile, A.; Kim, I.; Lee, J.-Y. 200 kHz Sonication of Mixed-Algae Suspension from a Eutrophic Lake: The Effect on the Caution vs. Outbreak Bloom Alert Levels. Water 2017, 9, 915. https://doi.org/10.3390/w9120915
Tekile A, Kim I, Lee J-Y. 200 kHz Sonication of Mixed-Algae Suspension from a Eutrophic Lake: The Effect on the Caution vs. Outbreak Bloom Alert Levels. Water. 2017; 9(12):915. https://doi.org/10.3390/w9120915
Chicago/Turabian StyleTekile, Andinet, Ilho Kim, and Jai-Yeop Lee. 2017. "200 kHz Sonication of Mixed-Algae Suspension from a Eutrophic Lake: The Effect on the Caution vs. Outbreak Bloom Alert Levels" Water 9, no. 12: 915. https://doi.org/10.3390/w9120915





