Raw Water Quality and Pretreatment in Managed Aquifer Recharge for Drinking Water Production in Finland
Abstract
:1. Introduction
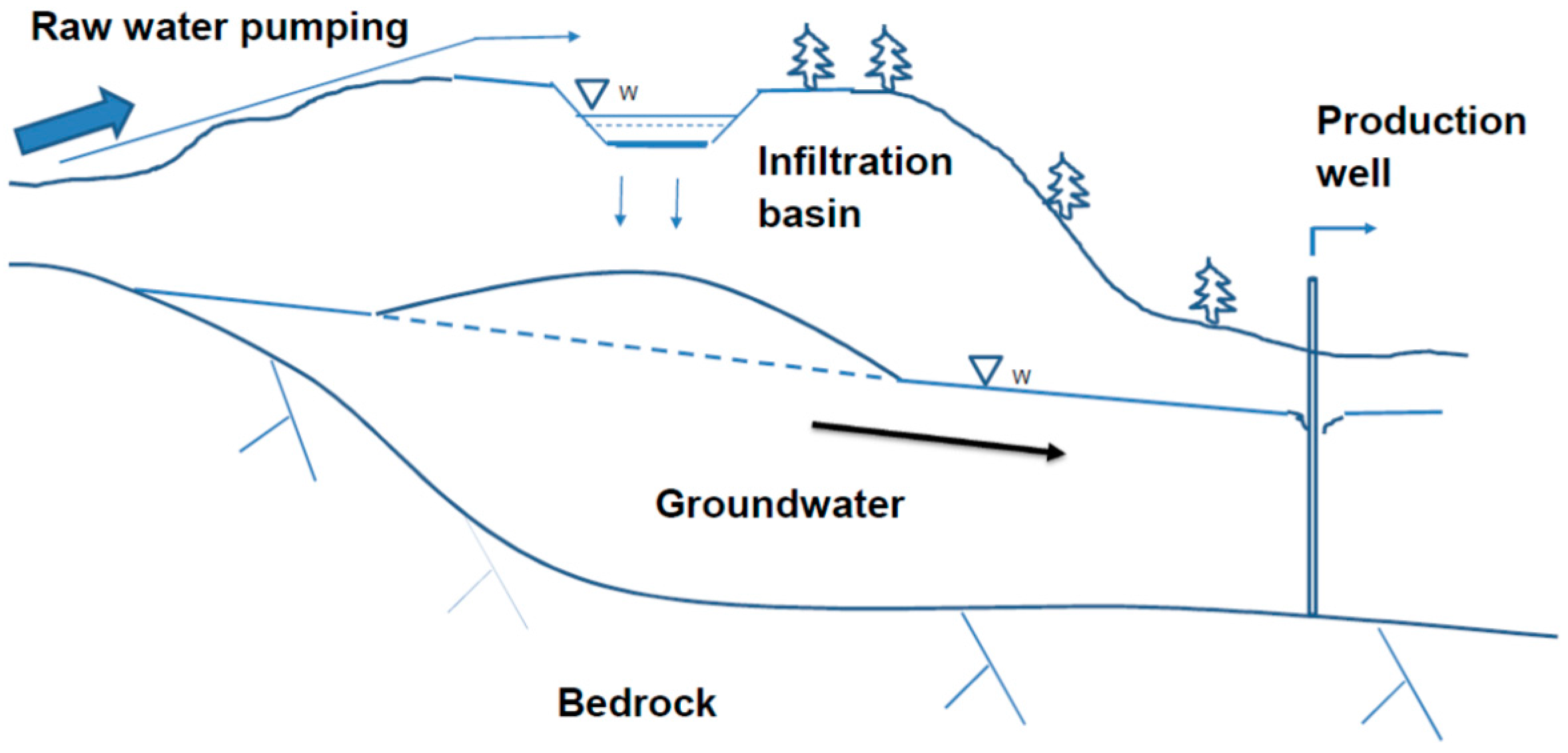
2. Description of the MAR Plants
2.1. Locations and Key Figures of the MAR Plants
2.2. Ahvenisto MAR Plant
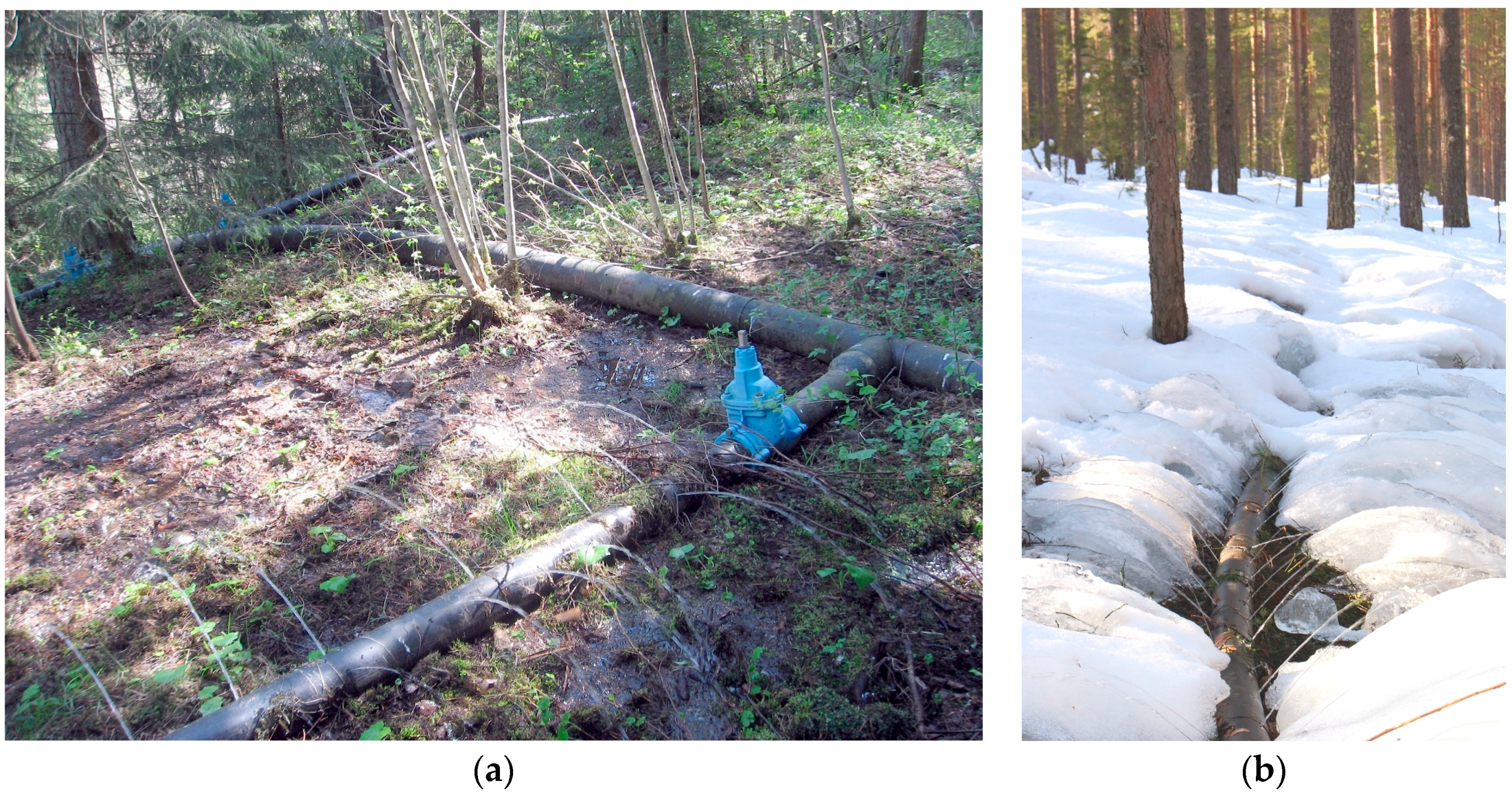
2.3. Vuontee MAR Plant
2.4. Kuivala MAR Plant
2.5. Vehoniemi MAR Plant
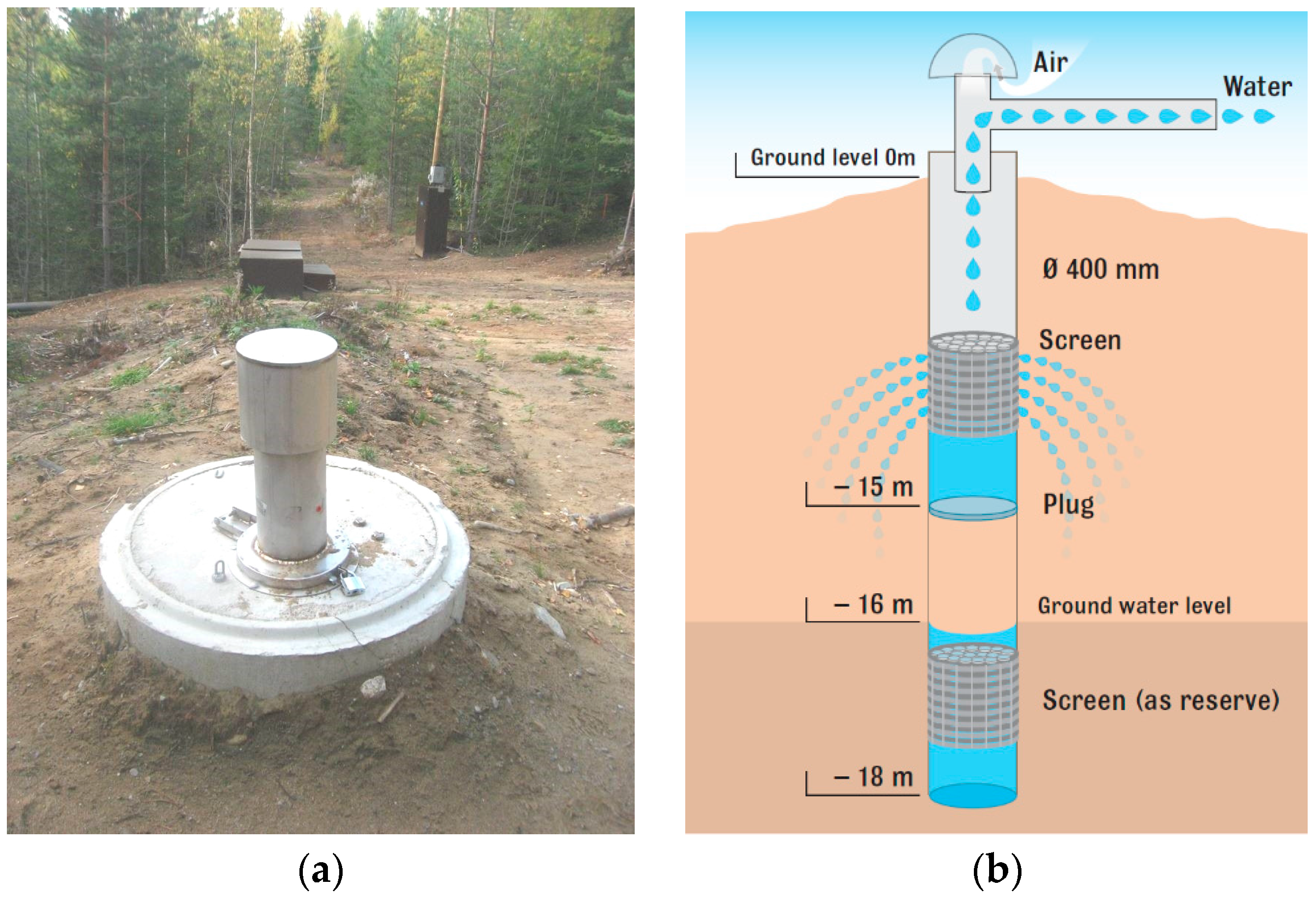
2.6. Rusutjärvi MAR Plant
2.7. Virttaankangas MAR Plant
3. Comparison of the Raw Water Sources and MAR Processes
4. Aspects on Chemical Pretreatment
4.1. Coping with Raw Water Quality Fluctuations at Virttaankangas MAR Plant
4.2. Maintaining Dissolved Oxygen Levels at Kuivala MAR Plant
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CODMn | Chemical oxygen demand, determined by KMnO4 oxidation |
| FNU | Formazin nephelometric units |
| MAR | Managed aquifer recharge |
| NOM | Natural organic matter |
| RO | Reverse osmosis |
| TOC | Total organic carbon |
References
- Niemi, J.; Heinonen, P.; Mitikka, S.; Vuoristo, H.; Pietiläinen, O.-P.; Puupponen, M.; Rönkä, E. The Finnish Eurowaternet with Information about Finnish Water Resources and Monitoring Strategies; Finnish Environment Institute: Helsinki, Finland, 2001. [Google Scholar]
- Rantakari, M. The Role of Lakes in Carbon Cycling in Boreal Catchments. Monographs of the Boreal Environment Research, No. 35. 2010. Available online: https://helda.helsinki.fi/handle/10138/29867?locale-attribute=en (accessed on 8 March 2016).
- Mattsson, T. Export of Organic Matter, Sulphate, and Base Cations from Boreal Headwater Catchments Downstream to the Coast: Impacts of Land Use and Climate. Monographs of the Boreal Environment Research, No. 36. 2010. Available online: https://helda.helsinki.fi/handle/10138/29867?locale-attribute=en (accessed on 8 March 2016).
- Jokela, P.; Vaahtera, M.; Vuori, T.; Meriluoto, J. The effect of Iron and Aluminium Chemicals in Humic Water Treatment by High-rate Dissolved Air Flotation. In Chemical Water and Wastewater Treatment IX, Proceedings of the 12th Gothenburg Symposium, Ljubljana, Slovenia, 20–23 May 2007; Hahn, H.H., Hoffman, E., Ødegaard, H., Eds.; IWA Publishing: London, UK, 2007. [Google Scholar]
- Jensen, K.H. Introduction to the Concept of Artificial Recharge. In Artificial Recharge of Groundwater. EC Project ENV4-CT95-0071. Energy, Environment and Sustainable Development; Final Report; European Commission: Luxembourg, 2001; pp. 9–11. [Google Scholar]
- Dillon, P.; Escalante, E.F.; Tuinhof, A. Management of aquifer recharge and discharge processes and aquifer storage equilibrium. In Thematic Papers on Groundwater; Food and Agriculture Organization of the United Nations (FAO): Washington, DC, USA, 2016; pp. 223–277. [Google Scholar]
- Bouwer, H. Artificial recharge of groundwater: Hydrogeology and engineering. Hydrogeol. J. 2002, 10, 121–142. [Google Scholar] [CrossRef]
- Kortelainen, N.M.; Karhu, J.A. Tracing the decomposition of dissolved organic carbon in artificial groundwater recharge using carbon isotope ratios. Appl. Geochem. 2006, 21, 547–562. [Google Scholar] [CrossRef]
- Kolehmainen, R.E.; Langwaldt, J.H.; Puhakka, J.A. Natural organic matter (NOM) removal and structural changes in the bacterial community during artificial groundwater recharge with humic lake water. Water Res. 2007, 41, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, R. Natural Organic Matter Biodegradation and Microbial Community Dynamics in Artificial Groundwater Recharge. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 2008. [Google Scholar]
- Lahti, K.; Rapala, J.; Kivimäki, A.-L.; Kukkonen, J.; Niemelä, M.; Sivonen, K. Occurrence of microcystins in raw water sources and treated drinking water of Finnish waterworks. Water Sci. Technol. 2001, 43, 225–228. [Google Scholar] [PubMed]
- Kolehmainen, R.E.; Kortelainen, N.M.; Langwaldt, J.H.; Puhakka, J.A. Biodegradation of natural organic matter in long-term, continuous-flow experiments simulating artificial ground water recharge for drinking water production. J. Environ. Qual. 2009, 38, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jokela, P.; Kallio, E. Sprinkling and well infiltration in managed aquifer recharge for drinking water quality improvement in Finland. J. Hydrol. Eng. 2015, 20. [Google Scholar] [CrossRef]
- Jokela, P.; Valtonen, J. Managed aquifer recharge and public participation. In Proceedings of the 7th International Symposium on Managed Aquifer Recharge (ISMAR7), Abu Dhabi, UAE, 9–13 October 2010; pp. 461–468.
- Kurki, V.; Lipponen, A.; Katko, T. Managed aquifer recharge in community water supply; the Finnish experience and some international comparisons. Water Int. 2013, 38, 774–789. [Google Scholar] [CrossRef]
- De Wit, H.A.; Valinia, S.; Weyhenmeyer, G.A.; Futter, M.N.; Kortelainen, P.; Austnes, K.; Hessen, D.O.; Räike, A.; Laudon, H.; Vuorenmaa, J. Current Browning of Surface Waters Will Be Further Promoted by Wetter Climate. Environ. Sci. Technol. Lett. 2016, 3, 430–435. [Google Scholar] [CrossRef]
- Ojala, J.; Wakeman, K.; Puhakka, J. (Eds.) Improving Artificial Groundwater Recharge through On-Line Monitoring and Control; Final Report of the TEVA Research Project 40232/08; Department of Chemistry and Bioengineering and TUT Foundation, Tampere University of Technology: Tampere, Finland, 2012; 122p.
- Ramboll Finland Ltd. Utti, Pohjavesialueen Suojelusuunnitelma. (Utti, Aquifer Protection Plan). Report, Reference Number 1510005048. 25 June 2014. Available online: http://www.ymparisto.fi/download/noname/%7B9272E465-273B-40F8-AD52-0276140DC221%7D/103226 (accessed on 8 March 2016). (In Finnish)
- Löfgren, S.; Forsius, M.; Andersen, T. The Color of Water. Brochure of the Project “Climate Induced Variation of Dissolved Organic Carbon in Nordic Surface Waters”, Produced under the Umbrella of the Nordic Council of Ministers. Available online: http://info1.ma.slu.se/ima/publikationer/brochure/The_color_of_water.pdf (accessed on 8 March 2016).
- Haaland, S.; Hongve, D.; Laudon, H.; Riise, G.; Vogt, R.D. Quantifying the drivers of increasing coloured organic matter in boreal surface waters. Environ. Sci. Technol. 2010, 44, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Hanson, G. Konstgjord Grundvattenbildning—100-Årig Teknik Inom Svensk Dricksvattenförsörjning (Artificial Groundwater Recharge—A Method Used in Swedish Drinking Water Supply for 100 Years); VA-FORSK Serien 5/2000; VAV Aktiebolag: Stockholm, Sweden, 2000; 220p. (In Swedish) [Google Scholar]
- Pyne, R.D.G. Aquifer Storage Recovery: A Guide to Groundwater Recharge through Wells, 2nd ed.; ASR Systems LLC: Gainesville, FL, USA, 2005; 608p. [Google Scholar]
- Martin, R. Clogging Issues Associated with Managed Aquifer Recharge Methods; IAH Commission on Managing Aquifer Recharge: Adelaide, Australia, 2013; 212p. [Google Scholar]
- Du, X.; Fang, Y.; Wang, Z.; Hou, J.; Ye, X. The Prediction Methods for Potential Suspended Solids Clogging Types during Managed Aquifer Recharge. Water 2014, 6, 961–975. [Google Scholar] [CrossRef]
- Anders, R.; Chrysikopoulos, C.V. Virus fate and transport during artificial recharge with recycled water. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef]
- Masciopinto, C.; La Mantia, R.; Chrysikopoulos, C.V. Fate and transport of pathogens in a fractured aquifer in the Salento area, Italy. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Reclaim Water. Water Reclamation Technologies for Safe Artificial Recharge of Groundwater; EC Project No. 018309, Thematic Priority ‘Global Change and Ecosystems’; Publishable Final Activity Report; European Commission: Brussels, Belgium, 2009; pp. 1–46. [Google Scholar]
- Toze, S.; Bekele, E.; Page, D.; Sidhu, J.; Shackleton, M. Use of static quantitative microbial risk assessment to determine pathogen risks in an unconfined carbonate aquifer used for managed aquifer recharge. Water Res. 2010, 44, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Schijven, J.F.; Hoogenboezem, W.; Hassanizadeh, S.M.; Peters, J.H. Modeling removal of bacteriophages MS2 and PRD1 by dune recharge at Castricum, Netherlands. Water Resour. Res. 1999, 35, 1101–1111. [Google Scholar] [CrossRef]
- Schijven, J.F.; Medema, G.; Vogelaar, A.J.; Hassanizadeh, S.M. Removal of microorganisms by deep well injection. J. Contam. Hydrol. 2000, 44, 301–327. [Google Scholar] [CrossRef]
- Jørgensen, C.; Peters, J.; Eschweiler, B. Removal of Pathogens and Monitoring of Microbial Changes during Artificial Recharge. In Artificial Recharge of Groundwater. EC Project ENV4-CT95-0071. Energy, Environment and Sustainable Development. Final Report; European Commission: Luxembourg, 2001; pp. 221–225. [Google Scholar]
- Jørgensen, C.; Peters, J. Migration and Survival of Viruses. In Artificial Recharge of Groundwater. EC Project ENV4-CT95-0071. Energy, Environment and Sustainable Development. Final Report; European Commission: Luxembourg, 2001; pp. 226–230. [Google Scholar]
- Jørgensen, C. Migration and Survival of Bacteria during Artificial Recharge. In Artificial Recharge of Groundwater. EC Project ENV4-CT95-0071. Energy, Environment and Sustainable Development. Final Report; European Commission: Luxembourg, 2001; pp. 231–234. [Google Scholar]
- Medema, G.J.; Stuyfzand, P.J. Removal of micro-organisms upon basin recharge, deep well injection and river bank filtration in the Netherlands. In Management of Aquifer Recharge for Sustainability, Proceedings of the 4th International Symposium on Artificial Recharge of Groundwater, Adelaide, Australia, 22–26 September 2002; Dillon, P., Ed.; CRC Press: Lisse, The Netherlands, 2002; pp. 125–131. [Google Scholar]
- Lundh, M.; Långmark, J.; Berggren Kleja, D.; Johansson, P.-O. Reduction of microorganisms and natural organic matter in unsaturated zone—A column experiment. In Management of Aquifer Recharge for Sustainability, Proceedings of the 6th International Symposium on Managed Aquifer Recharge of Groundwater, ISMAR6, Phoenix, AZ, USA, 28 October–2 November 2007; Fox, P., Ed.; Acacia Publishing Incorporated: Phoenix, AZ, USA, 2007; pp. 257–271. [Google Scholar]
- Sprenger, C.; Lorenzen, G.; Grunert, A.; Ronghang, M.; Dizer, H.; Selinka, C.; Girones, R.; Lopez-Pila, J.M.; Mittal, A.; Szewzyk, R. Removal of indigenous coliphases and enteric viruses during riverbank filtration from highly polluted river water in Delhi (India). J. Water Health 2014, 12, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, T. Suolistoperäiset Saastelähteet ja Taudinaiheuttajamikrobien Esiintyminen Pintavedessä (Sources of Enteric Pollution and the Occurence of Pathogens in Surface Water). In Presentation at the Final Seminar of CONPAT Project (Aquatic Contaminants—Pathways, Health Risks and Management), Turku, Finland, 15 September 2016; Available online: https://thl.videosync.fi/conpat/ (accessed on 2 February 2017). (In Finnish)
- Meriläinen, P. Aiheutuuko Vesistöjen/Juomavesien Mikrobeista Tai Kemikaaleista Terveysriskejä? (Do Microbes or Chemicals Found in Watersheds/Drinking Waters Cause Health risks?). In Presentation at the Final Seminar of CONPAT Project (Aquatic Contaminants—Pathways, Health Risks and Management), Turku, Finland, 15 September 2016; Available online: https://thl.videosync.fi/conpat/ (accessed on 2 February 2017). (In Finnish)
- Happonen, M.; Koivusalo, H.; Malve, O.; Perkola, N.; Juntunen, J.; Huttula, T. Contamination risk of raw drinking water caused by PFOA sources along a river reach in south-wetestern Finland. Sci. Total Environ. 2016, 541, 74–82. [Google Scholar] [CrossRef]
- Simola, A.; Juntunen, J.; Meriläinen, P. Contaminants and Pathogens in Waterways—Economic Assessment of Risks. In Proceedings of the International Conference on Economic Modeling (EcoMod2016), Lisbon, Portugal, 6–8 July 2016; pp. 1–17.
- Meriläinen, P.; Hokajärvi, A.-M.; Pitkänen, T.; Miettinen, I.; Juntunen, J.; Huttula, T.; Simola, A.; Honkatukia, J. Talousveden mikrobiologisten ja kemiallisten saasteiden kokonaisriskin arviointi (Overall risk assessment of microbiological and chemical pollution in drinking water). Vesitalous 2016, 57, 10–14. (In Finnish) [Google Scholar]



| MAR Plant | Start-Up (Year) | Average Production (m3/Day) | Production Capacity (m3/Day) | Pre-Treatment | Infiltration Method | Residence Time (Months) | TOC | |
|---|---|---|---|---|---|---|---|---|
| Raw Water (mg/L) | Abstracted Water (mg/L) | |||||||
| Ahvenisto | 1976 | 7800 | - | no | basin, sprinkle | 3 | 11 | 1–2 |
| Vuontee | 2000 | 10,000–15,000 | 22,000 | no | sprinkle | 1.5–2 | 7.2 | 1.8 |
| Kuivala | 1992 | 22,000 | 34,000 | chem | basin | 0.5–3 | 11 * | 1.7 * |
| Vehoniemi | planned | - | 70,000 | no/mech | well, sprinkle, basin | 1–2 | 6.5 | 2 ^ |
| Rusutjärvi | 1997 | 7000 | 10,000 | no | well | 1–2 | 7.7 | 2.5 |
| Virttaankangas | 2011 | 63,000 | 105,000 | chem | basin | 3 | 9 | 1.9 |
| Parameter | Unit | Ahvenisto MAR | Vuontee MAR | Vehoniemi MAR | Vehoniemi MAR | Rusutjärvi MAR | Virttaankangas MAR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lake Alajärvi | Lake Kuusvesi | Lake Roine | Lake Roine | Lake Päijänne | River Kokemäenjoki | ||||||||
| Surface 1 | Depth 16 m 1 | ||||||||||||
| 2000–2010 | 2001–2009 | 2003–2010 | 2003–2010 | 2000–2010 | 2000–2010 | ||||||||
| (n = 23) | (n = 13–127) | (n = 12) | (n = 12) | (n = 22) | (n = 120) | ||||||||
| Turbidity | FNU | 1.5 | (0.3–5.6) | 1.2 | (0.4–2.4) | 2.1 | (0.54–3.4) | 2.5 | (1.0–6.1) | 0.5 | (<0.3–0.7) | 6.6 | (0.9–17) |
| Suspended Solids | mg/L | 2.0 | (<1–4.4) | 2.2 | (0.8–4.3) | <2.0 | 5.1 | (0.5–13) | |||||
| pH | 7.1 | (6.7–7.7) | 6.8 | (6.0–8.4) | 7.2 | (6.9–7.5) | 5.9 | (5.7–6.1) | 7.2 | (6.8–7.7) | 7.1 | (6.8–7.5) | |
| Color | mg Pt/L | 60 | (35–100) | 16 | (0–25) | 20 | 24 | (15–40) | 50 | (25–100) | |||
| Electric conductivity | mS/m | 8.5 | (7.8–9.5) | 4.4 | (3.0–7.9) | 6.6 | (6.3–6.8) | 6.7 | (6.4–7.3) | 6.8 | (6.7–7.1) | 9.5 | (7.8–13.2) |
| Iron | µg/L | 380 | (76–660) | 83 | (1–470) | 80 | (23–140) | 95 | (39–150) | 46 | (25–79) | 380 | (120–990) |
| Manganese | µg/L | 29.0 | (5–54) | 53 | (2–490) | 25 | (6–54) | 75 | (19–330) | 11 | (<10–18) | ||
| Nitrogen | µg/L | 590 | (390–930) | 380 | (300–490) | 360 | (290–450) | 380 | (280–490) | 500 | (390–590) | 910 | (450–2300) |
| Phosphorus | µg/L | 11 | (6–22) | 12 | (8–15) | 12 | (9–17) | 7.7 | (5–11) | 25 | (14–50) | ||
| CODMn | mg O2/L | 12 | (8.2–18) | 5.6 2 | 4.5 | (3.6–5.6) | 4.3 | (3.7–5.0) | 6.4 | (5.4–8.4) | 9.1 | (7.2–15) | |
| TOC | mg/L | 10.3 | 6.7 | 6.5 3 | (6.0–7.6) | 6.0 | (5.7–6.1) | 6.7 4 | (6.2–7.0) | 7.9 5 | (6.8–9.0) | ||
| Parameter | n | Unit | Raw Water Quality (before Pretreatment) | After Pretreatment | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Median | Mean | Min. | Max. | Median | Mean | Reduction | |||
| Turbidity | 50 | FNU | 2.6 | 21 | 6.6 | 6.9 | <0.2 | 1.3 | 0.23 | ||
| reduction | % | 97 | |||||||||
| Suspended Solids | 43 | mg/L | 2.2 | 19 | 7.1 | 8.0 | <0.5 | ||||
| reduction | % | >94 | |||||||||
| TOC | 48 | mg/L | 9.2 | 12 | 9.1 | 3.8 | 5.3 | 4.1 | 4.2 | ||
| reduction | % | 54 | |||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokela, P.; Eskola, T.; Heinonen, T.; Tanttu, U.; Tyrväinen, J.; Artimo, A. Raw Water Quality and Pretreatment in Managed Aquifer Recharge for Drinking Water Production in Finland. Water 2017, 9, 138. https://doi.org/10.3390/w9020138
Jokela P, Eskola T, Heinonen T, Tanttu U, Tyrväinen J, Artimo A. Raw Water Quality and Pretreatment in Managed Aquifer Recharge for Drinking Water Production in Finland. Water. 2017; 9(2):138. https://doi.org/10.3390/w9020138
Chicago/Turabian StyleJokela, Petri, Tapani Eskola, Timo Heinonen, Unto Tanttu, Jukka Tyrväinen, and Aki Artimo. 2017. "Raw Water Quality and Pretreatment in Managed Aquifer Recharge for Drinking Water Production in Finland" Water 9, no. 2: 138. https://doi.org/10.3390/w9020138




