Antibiotic Resistance Profiling and Genotyping of Vancomycin-Resistant Enterococci Collected from an Urban River Basin in the Provincial City of Miyazaki, Japan
Abstract
:1. Introduction
2. Materials and Methods
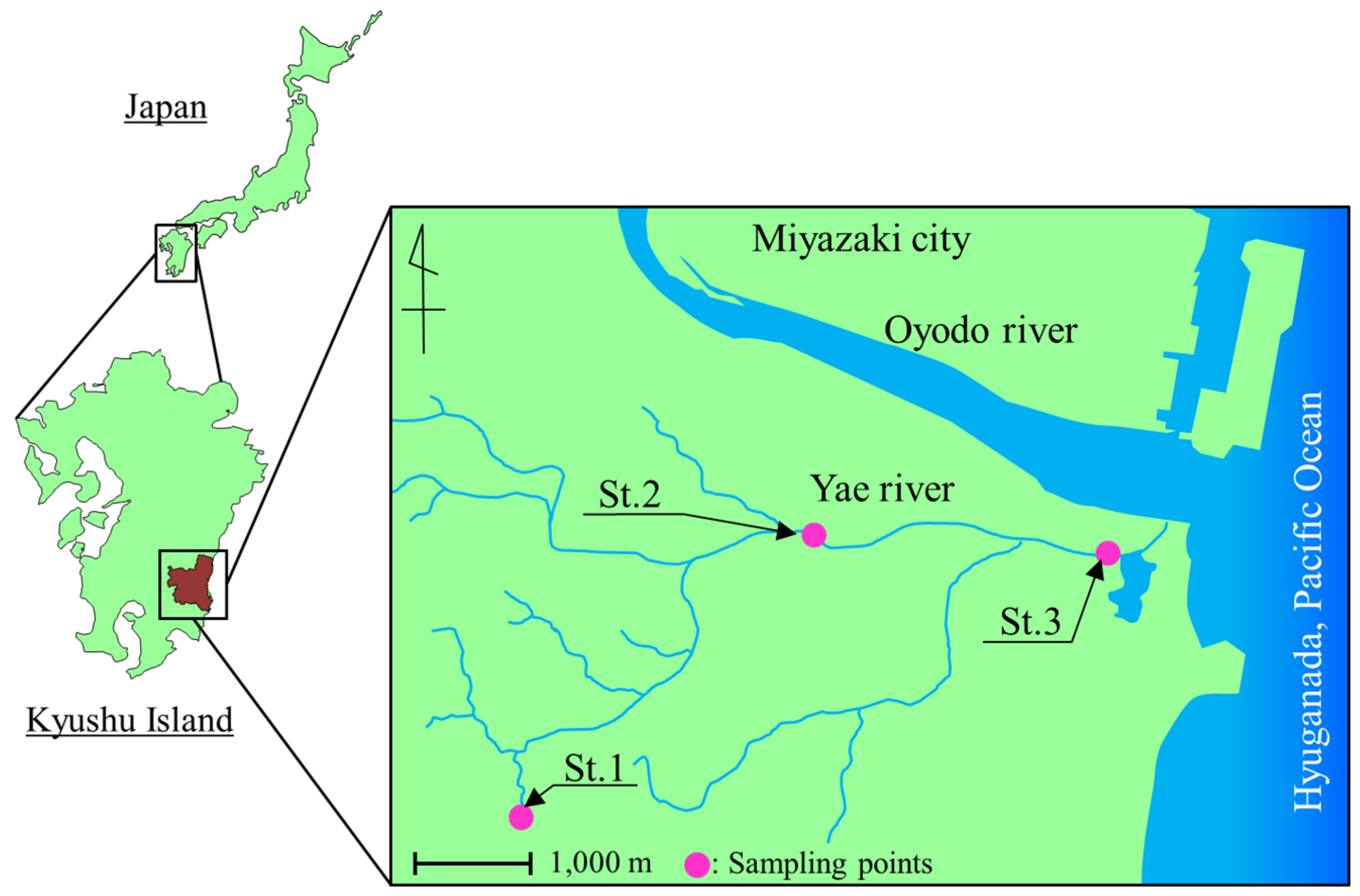
2.1. Sampling
2.2. Enumeration and Isolation of Bacteria
2.3. Identification of Enterococci and Detection of Vancomycin-Resistant Genes by Polymerase Chain Reaction (PCR) Analysis
2.4. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Pulse-Field Gel Electrophoresis (PFGE) Typing
2.6. Dendrogram Analysis of PFGE Patterns
3. Results
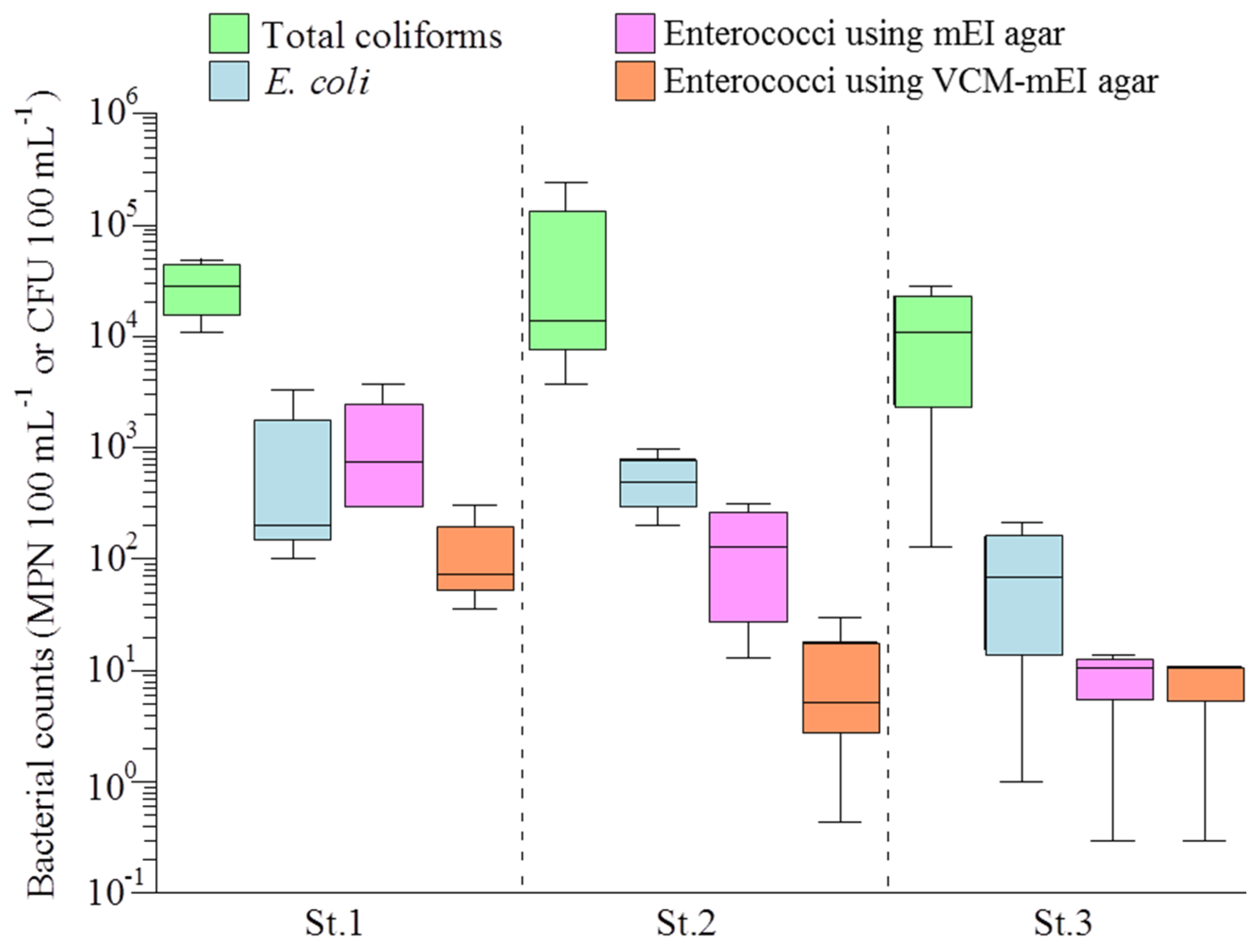
3.1. Bacterial Counts and Water Quality of the Yae River Basin
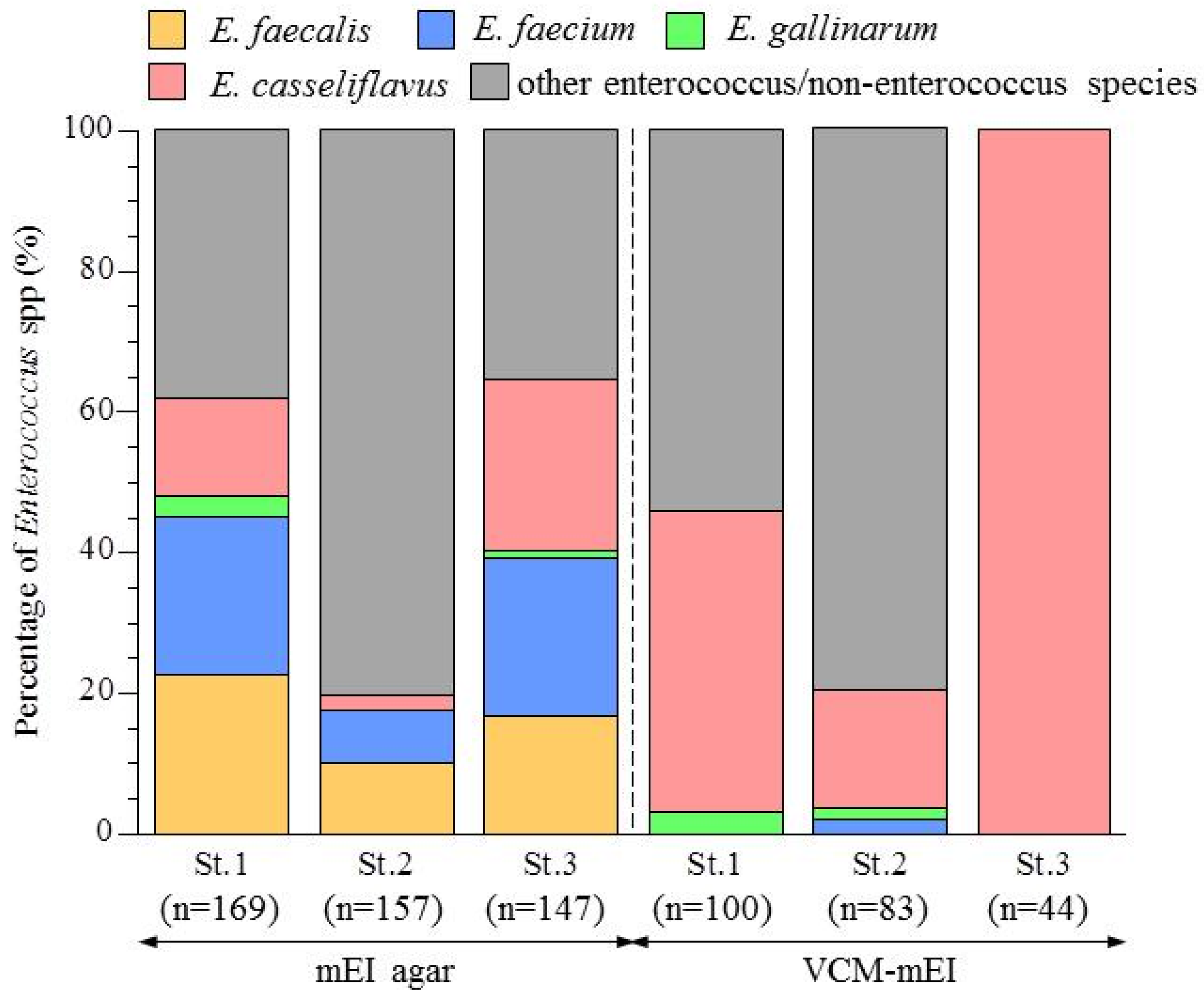
3.2. Abundance of Enterococcus Species in the Yae River Basin
3.3. Antimicrobial Susceptibility of Enterococcal Isolates from the Yae River Basin
3.4. Determination of Vancomycin-Resistant Genes
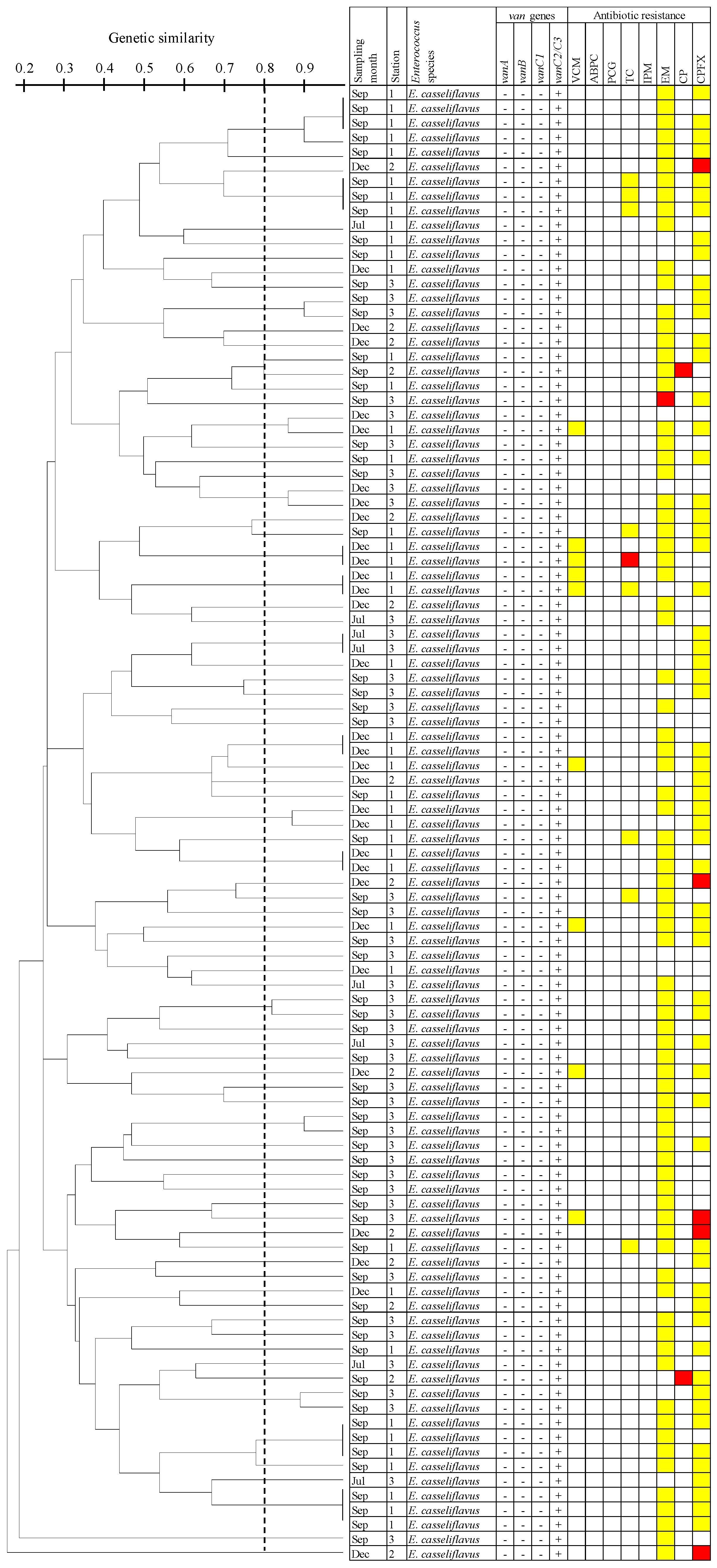
3.5. Dendrogram Analysis of E. Casseliflavus Isolates Carrying the vanC2/C3 Gene Using PFGE
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: http://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 4 May 2014).
- Center for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2013; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- European Center for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2013. European Antimicrobial Resistance Surveillance Network (EARS-Net). ECDC: Stockholm, 2014. Available online: http://ecdc.europa.eu/en/publications/Documents/antibiotic-resistance-in-EU-summary.pdf (accessed on 4 November 2015).
- Alm, E.W.; Zimbler, D.; Callahan, E.; Plomaritis, E. Patterns and persistence of antibiotic resistance in faecal indicator bacteria from freshwater recreational beaches. J. Appl. Microbiol. 2014, 117, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Mesa, R.J.; Blanc, V.; Blanch, A.R.; Cortés, P.; González, J.J.; Lavilla, S.; Miró, E.; Muniesa, M.; Saco, M.; Tórtola, M.T.; et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 2006, 58, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kajii, S.; Nishiyama, M.; Iguchi, A. Susceptibility of Pseudomonas aeruginosa isolates collected from river water in Japan to antipseudomonal agents. Sci. Total. Environ. 2013, 450–451, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bating waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Facklam, R.R.; Carvalho, M.S.; Teixeira, L.M. History, taxonomy, biochemical characteristics, and antibiotic susceptibility testing of enterococci. In The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; Gilmore, M.S., Ed.; ASM Press: Washington, DC, USA, 2002; pp. 1–54. [Google Scholar]
- U.S. Environmental Protection Agency. Ambient Water-Quality Criteria for Bacteria-1986; Office of Water Regulations and Standards, U.S. Environmental Protection Agency: Washington, DC, USA, 1986; Volume 17.
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Eur. Surveill. 2008, 13, 19046. [Google Scholar]
- Huycke, M.M.; Sahm, D.F.; Gilmore, M.S. Multiple-drug resistant enterococci: The nature of the problem and an agenda for future. Emerg. Infect. Dis. 1998, 4, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, M.; Domenech, M.; García, E. Vancomycin tolerance in Gram-positive cocci. Environ. Microbiol. Rep. 2011, 3, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.M.; Fritsche, T.R.; Moet, G.J.; Biedenbach, D.J.; Jones, R.N. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North American and Europe: A report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 2007, 15, 838–839. [Google Scholar]
- Hsueh, P.R.; Chen, M.L.; Sun, C.C.; Chen, W.H.; Pan, H.J.; Yang, L.S.; Chang, S.C.; Ho, S.W.; Lee, C.Y.; Hsieh, W.C.; et al. Antimicrobial drug resistance in pathogens causing nosocomial infections at a university hospital in Taiwan, 1981–1999. Emerg. Infect. Dis. 2002, 8, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Takakura, S.; Yamamoto, M.; Matsumura, Y.; Shirano, M.; Nagano, M.; Ito, Y.; Iinuma, Y.; Shimizu, T.; Fujita, N.; et al. Regional spread and control of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Kyoto, Japan. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.G.; Vemer-Jeffreys, D.W.; Baker-Austin, C. Aquatic systems: Maintaining, mixing and mobilizing antimicrobial resistance? Trends Ecol. Evol. 2011, 26, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg Goldstein, R.E.; Micallef, S.A.; Gibbs, S.G.; George, A.; Claye, E.; Sapkota, A.; Joseph, S.W.; Sapkota, A.R. Detection of vancomycin-resistant enterococci (VRE) at four U. S. wastewater treatment plants that provide effluent for reuse. Sci. Total Environ. 2014, 466–467, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.; Kuhn, I.; Franklin, A.; Mollby, R. High prevalence of vancomycin-resistant enterococci in Swedish sewage. Appl. Environ. Microbiol. 2002, 68, 2838–2842. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, M.; Iguchi, A.; Suzuki, Y. Identification of Enterococcus faecium and Enterococcus faecalis as vanC-type vancomycin-resistant enterococci (VRE) from sewage and river water in the provincial city of Miyazaki, Japan. J. Environ. Sci. Health A 2015, 50, 16–25. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Method 1600: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-02-022; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Kariyama, R.; Mitsuhata, R.; Chow, J.W.; Clewell, D.B.; Kumon, H. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J. Clin. Microbiol. 2000, 38, 3092–3095. [Google Scholar] [PubMed]
- Nagayama, A.; Yamaguchi, K.; Watanabe, K.; Tanaka, M.; Kobayashi, I.; Nagasawa, Z. Final report from the committee on antimicrobial susceptibility testing, Japanese Society of Chemotherapy, on the agar dilution method (2007). J. Infect. Chemother. 2008, 14, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-second Informational Supplement, M100-S22. 2012, 27, 90–92. Available online: http://mazums.ac.ir/dorsapax/userfiles/file/moavenat%20darman/M100-S22.pdf (accessed on 26 January 2017). [Google Scholar]
- Furukawa, T.; Suzuki, Y. A proposal for source tracking of fecal pollution in recreational water by pulsed-field gel electrophoresis. Microbes Environ. 2013, 28, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Courvalin, P. Acquired and intrinsic glycopeptide resistance in enterococci. Int. J. Antimicrob. Agents 2000, 16, S11–S17. [Google Scholar] [CrossRef]
- Murray, B.E. The life and times of the Enterococcus. Clin. Microbiol. Rev. 1990, 3, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Lleó, M.M.; Bonato, B.; Benedetti, D.; Canepari, P. Survival of enterococcal species in aquatic environments. FEMS Microbiol. Ecol. 2005, 54, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Yoshida, T.; Suzuki, Y. Application of PFGE to source tracking of fecal pollution in coastal recreation area: A case study in Aoshima Beach, Japan. J. Appl. Microbiol. 2011, 110, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Taučer-Kapteijn, M.; Hoogenboezem, W.; Heiliegers, L.; de Bolster, D.; Medema, G. Screening municipal wastewater effluent and surface water used for drinking water production for the presence of ampicillin and vancomycin resistant enterococci. Int. J. Hyg. Environ. Health 2016, 219, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Galvin, S.; Boyle, F.; Hickey, P.; Mulligan, M.; Cormican, M. Enterococcus faecium of the vanA genotype in rural drinking water, effluent, and the aqueous environment. Appl. Environ. Microbiol. 2012, 78, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Soge, O.O.; Giardino, M.A.; Mazengia, E.; Ma, G.; Meschke, J.S. Vancomycin-resistant Enterococcus spp. in marine environments from the West Coast of the USA. J. Appl. Microbiol. 2009, 107, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Ishimaru, M.; Endoh, Y.S.; Suginaka, M.; Yamatani, S. Isolation of glycopeptide-resistant enterococci from chickens in Japan. Antimicrob. Agents Chemother. 1998, 42, 3333. [Google Scholar] [PubMed]
- Kirst, H.A.; Thompson, D.G.; Nicas, T.I. Historical yearly usage of vancomycin. Antimicrob. Agents Chemother 1998, 42, 1303–1304. [Google Scholar] [PubMed]
- Garcia-Migura, L.; Liebana, E.; Jensen, L.B.; Barnes, S.; Pleydell, E. A longitudinal study to assess the persistence of vancomycin-resistant Enterococcus faecium (VREF) on an intensive broiler farm in the United Kingdom. FEMS Microbiol. Lett. 2007, 275, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kotzamanidis, C.; Zdraags, A.; Kourelis, A.; Moraitou, E.; Papa, A.; Yiantzi, V.; Pantelidou, C.; Yiangou, M. Characterization of vanA-type Enterococcus faecium isolates from urban and hospital wastewater and pig. J. Appl. Microbiol. 2009, 107, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, W.; Alpízar, A.; Hernández, S.; Pacheco, A.; Vargas, N.; Herrera, M.L.; Vargas, Á.; Caballero, M.; García, F. Predominance of vanA genotype among vancomycin-resistant Enterococcus isolates frim poultry and swine in Costa Rica. Appl. Environ. Microbiol. 2003, 69, 7414–7419. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.M.; Smith, J.M.B.; Cook, G.M. Persistence of vancomycin resistant enterococci in New Zealand broilers after discontinuation of avoparcin use. Appl. Environ. Microbiol. 2004, 70, 5764–5768. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Mander, M.; Jensen, L.B.; Olsen, J.E.; Guardabassi, L. Persistence of vancomycin resistance in multiple clones of Enterococcus faecium isolated from Danish broilers 15 years after the bans of avoparcin. Antimicrob. Agents Chemother. 2015, 59, 2926–2929. [Google Scholar] [CrossRef] [PubMed]
- Tzavaras, I.; Siarkou, V.I.; Zdragas, A.; Kotzamanidis, C.; Vafeas, G.; Bourtzi-Hatzopoulou, E.; Poumaras, S.; Sofianou, D. Diversity of vanA-type vancomycin-resistant Enterococcus faecium isolated from broilers, poultry slaughterers and hospitalized humans in Greece. J. Antimicrob. Chemother. 2012, 67, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Nagulapally, S.R.; Ahmad, A.; Henry, A.; Marchin, G.L.; Zurek, L.; Bhandari, A. Occurrence of ciprofloxacin-, trimethoprim-sulfamethoxazole-, and vancomycin-resistant bacteria in a municipal wastewater treatment plant. Water Environ. Res. 2009, 81, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.R.; Ferro, G.; Vredenburg, J.; Yanik, M.; Vieira, L.; Rizzo, L.; Lameiras, C.; Manaia, C.M. Vancomycin resistant enterococci: From the hospital effluent to the urban wastewater treatment plant. Sci. Total Environ. 2013, 450–451, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Kim, M.J.; Park, C.; Park, J.G.; Maeng, P.J.; Lee, G.C. Detection and genotyping of vancomycin-resistant Enterococcus spp. by multiplex polymerase chain reaction in Korean aquatic environmental samples. Int. J. Hyg. Environ. Health 2013, 216, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Zdragas, A.; Partheniou, P.; Kotzamanidis, C.; Psoni, L.; Koutita, O.; Moraitou, E.; Tzanetakis, N.; Yiangou, M. Molecular characterization of low-level vancomycin-resistant enterococci found in coastal water of Thermaikos Gulf, Northern Greece. Water Res. 2008, 42, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Kak, V.; Chow, J.W. Acquired antibiotic resistances in enterococci. In The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; Gilmore, M.S., Ed.; ASM Press: Washington, DC, USA, 2002; pp. 355–383. [Google Scholar]
- Łuczkiewicz, A.; Jankowska, K.; Kurlenda, J.; Olańczuk-Neyman, K. Identification and antimicrobial resistance of Enterococcus spp. isolated from surface water. Water Sci. Technol. 2010, 62, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Farrow, J.A.E.; Jones, D. Enterococcus mundtii sp. nov. Int. J. Syst. Bacteriol. 1986, 36, 8–12. [Google Scholar] [CrossRef]
- Ulrich, A.; Müller, T. Heterogeneity of plant associated streptococci as characterized by phenotypic features and restriction analysis of PCR amplified 16S rDNA. J. Appl. Microbiol. 1998, 84, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Murcia, A.J.; Collins, M.D. Enterococcus sulfureus, a new yellow pigmented Enterococcus species. FEMS Microbiol. Lett. 1991, 80, 69–74. [Google Scholar] [CrossRef]
- Courvalin, P. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 2006, 42, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Beuckers, A.G.; Zaheer, R.; Cook, S.R.; Stanford, K.; Chaves, A.V.; Ward, M.P.; McAllister, T.A. Effect of in-feed administration and withdrawal of tylosin phosphate on antibiotic resistance in enterococci isolated from feedlot steers. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Harnisz, M.; Korzeniewska, E.; Gołaś, I. The impact of a freshwater fish warm on the community of tetracycline-resistant bacteria and the structure of tetracycline resistance genes in river water. Chemosphere 2015, 128, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulphonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobial agent | MIC test range (μg·mL−1) | St.1 (100 isolates) | MIC50 a (μg·mL−1) | MIC90 b (μg·mL−1) | St.2 (31 isolates) | MIC50 (μg·mL−1) | MIC90 (μg·mL−1) | St.3 (95 isolates) | MIC50 (μg·mL−1) | MIC90 (μg·mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | ||||||||
| No. isolates (% isolates) | No. isolates (% isolates) | No. isolates (% isolates) | ||||||||||||||
| VCM | 0.25-256 | 100 (100%) | 0 (0%) | 0 (0%) | 2 | 4 | 29 (94%) | 2 (6%) | 0 (0%) | 1 | 4 | 95 (100%) | 0 (0%) | 0 (0%) | 0.5 | 2 |
| PCG | 0.25-128 | 100 (100%) | 0 (0%) | 0 (0%) | 1 | 2 | 30 (97%) | 0 (0%) | 1 (3%) | 2 | 2 | 95 (100%) | 0 (0%) | 0 (0%) | 2 | 2 |
| ABPC | 0.25-128 | 100 (100%) | 0 (0%) | 0 (0%) | 0.5 | 1 | 31 (100%) | 0 (0%) | 0 (0%) | 0.5 | 1 | 95 (100%) | 0 (0%) | 0 (0%) | 0.5 | 1 |
| IPM | 0.25-128 | 100 (100%) | 0 (0%) | 0 (0%) | 1 | 2 | 31 (100%) | 0 (0%) | 0 (0%) | 1 | 2 | 95 (100%) | 0 (0%) | 0 (0%) | 2 | 4 |
| EM | 0.25-128 | 25 (25%) | 72 (72%) | 3 (3%) | 1 | 2 | 14 (45%) | 16 (52%) | 1 (3%) | 1 | 2 | 65 (68%) | 29 (31%) | 1 (1%) | 0.5 | 1 |
| TC | 0.25-128 | 88 (88%) | 3 (3%) | 9 (9%) | 2 | 8 | 27 (87%) | 0 (0%) | 4 (13%) | 2 | 16 | 80 (84%) | 1 (1%) | 14 (15%) | 2 | 32 |
| CPFX | 0.0625-32 | 22 (22%) | 64 (64%) | 14 (14%) | 2 | 4 | 13 (42%) | 13 (42%) | 5 (16%) | 4 | 8 | 71 (75%) | 20 (21%) | 4 (4%) | 0.5 | 2 |
| CP | 0.25-128 | 100 (100%) | 0 (0%) | 0 (0%) | 8 | 8 | 31 (100%) | 0 (0%) | 0 (0%) | 8 | 8 | 95 (100%) | 0 (0%) | 0 (0%) | 8 | 8 |
| GEN | 500 | 100 (100%) | 0 (0%) | 0 (0%) | c | 28 (100%) | 0 (0%) | 0 (0%) | 95 (100%) | 0 (0%) | 0 (0%) | |||||
| STM | 2,000 | 100 (100%) | 0 (0%) | 0 (0%) | 28 (100%) | 0 (0%) | 0 (0%) | 95 (100%) | 0 (0%) | 0 (0%) | ||||||
| Antimicrobial agent | MIC test range (μg·mL−1) | St.1 (46 isolates) | MIC50 a (μg·mL−1) | MIC90 b (μg·mL−1) | St.2 (17 isolates) | MIC50 (μg·mL−1) | MIC90 (μg·mL−1) | St.3 (44 isolates) | MIC50 (μg·mL−1) | MIC90 (μg·mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | ||||||||
| No. isolates (% isolates) | No. isolates (% isolates) | No. isolates (% isolates) | ||||||||||||||
| VCM | 0.25-256 | 37 (80%) | 9 (20%) | 0 (0%) | 4 | 4 | 16 (94%) | 1 (6%) | 0 (0%) | 4 | 4 | 43 (98%) | 1 (2%) | 0 (0%) | 4 | 4 |
| PCG | 0.25-128 | 46 (100%) | 0 (0%) | 0 (0%) | 0.25 | 0.5 | 17 (100%) | 0 (0%) | 0 (0%) | 0.5 | 2 | 44 (100%) | 0 (0%) | 0 (0%) | 0.5 | 0.5 |
| ABPC | 0.25-128 | 46 (100%) | 0 (0%) | 0 (0%) | 0.25 | 0.5 | 17 (100%) | 0 (0%) | 0 (0%) | 0.25 | 0.5 | 44 (100%) | 0 (0%) | 0 (0%) | 0.5 | 0.5 |
| IPM | 0.25-128 | 46 (100%) | 0 (0%) | 0 (0%) | 0.5 | 1 | 17 (100%) | 0 (0%) | 0 (0%) | 0.25 | 2 | 44 (100%) | 0 (0%) | 0 (0%) | 0.25 | 0.5 |
| EM | 0.25-128 | 8 (17%) | 38 (83%) | 0 (0%) | 2 | 2 | 4 (24%) | 12 (71%) | 1 (6%) | 2 | 2 | 10 (23%) | 33 (75%) | 1 (2%) | 1 | 2 |
| TC | 0.25-128 | 37 (80%) | 7 (15%) | 2 (4%) | 4 | 8 | 17 (100%) | 0 (0%) | 0 (0%) | 1 | 1 | 43 (98%) | 1 (2%) | 0 (0%) | 4 | 4 |
| CPFX | 0.0625-32 | 12 (46%) | 34 (74%) | 0 (0%) | 2 | 2 | 5 (29%) | 7 (41%) | 5 (29%) | 2 | 4 | 22 (50%) | 20 (45%) | 2 (5%) | 1 | 2 |
| CP | 0.25-128 | 46 (100%) | 0 (0%) | 0 (0%) | 8 | 8 | 17 (100%) | 0 (0%) | 0 (0%) | 4 | 8 | 44 (100%) | 0 (0%) | 0 (0%) | 2 | 2 |
| GEN | 500 | 46 (100%) | 0 (0%) | 0 (0%) | c | 17 (100%) | 0 (0%) | 0 (0%) | 44 (100%) | 0 (0%) | 0 (0%) | |||||
| STM | 2,000 | 46 (100%) | 0 (0%) | 0 (0%) | 17 (100%) | 0 (0%) | 0 (0%) | 44 (100%) | 0 (0%) | 0 (0%) | ||||||
| Antimicrobial Agent | No. of Resistant or Intermediately Resistant Isolates (% Isolates) | |||
|---|---|---|---|---|
| E. faecalis | E. faecium | E. gallinarum | E. casseliflavus | |
| (n = 80) | (n = 79) | (n = 10) | (n = 164) | |
| VCM | 0 (0%) | 1 (1.3%) | 3 (30%) | 9 (5.5%) |
| PCG | 0 (0%) | 1 (1.3%) | 0 (0%) | 0 (0%) |
| ABPC | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IPM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| EM | 36 (45%) | 65 (82%) | 0 (0%) | 106 (65%) |
| TC | 7 (8.8%) | 18 (23%) | 6 (60%) | 10 (6.1%) |
| CPFX | 33 (41%) | 64 (81%) | 2 (20%) | 89 (54%) |
| CP | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| GEN | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| STM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MDR a | 5 (6.3%) | 7 (8.9%) | 1 (10%) | 15 (9.1%) |
| Date | Station | Total Isolates | vanA | vanB | vanC1 | vanC2/C3 | Negative |
|---|---|---|---|---|---|---|---|
| No. Isolates | No. Isolates (% Isolates) | ||||||
| 13-December | St.1 | 49 | 0 (0%) | 0 (0%) | 3 (6%) | 17 (35%) | 29 (59%) |
| St.2 | 25 | 0 (0%) | 0 (0%) | 0 (0%) | 12 (48%) | 13 (52%) | |
| St.3 | 3 | 0 (0%) | 0 (0%) | 0 (0%) | 3 (100%) | 0 (0%) | |
| 14-May | St.1 | 57 | 0 (0%) | 0 (0%) | 5 (9%) | 24 (42%) | 28 (49%) |
| St.2 | 6 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) | 4 (67%) | |
| St.3 | 32 | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 31 (97%) | |
| 14-July | St.1 | 13 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (8%) | 12 (92%) |
| St.2 | 5 | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 4 (80%) | |
| St.3 | 34 | 0 (0%) | 0 (0%) | 0 (0%) | 7 (21%) | 27 (79%) | |
| 14-September | St.1 | 27 | 0 (0%) | 0 (0%) | 0 (0%) | 25 (93%) | 2 (7%) |
| St.2 | 12 | 0 (0%) | 0 (0%) | 0 (0%) | 3 (25%) | 9 (75%) | |
| St.3 | 70 | 0 (0%) | 0 (0%) | 0 (0%) | 70 (100%) | 0 (0%) | |
| All isolates | 333 | 0 (0%) | 0 (0%) | 10 (3%) | 164 (49%) | 159 (48%) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, M.; Ogura, Y.; Hayashi, T.; Suzuki, Y. Antibiotic Resistance Profiling and Genotyping of Vancomycin-Resistant Enterococci Collected from an Urban River Basin in the Provincial City of Miyazaki, Japan. Water 2017, 9, 79. https://doi.org/10.3390/w9020079
Nishiyama M, Ogura Y, Hayashi T, Suzuki Y. Antibiotic Resistance Profiling and Genotyping of Vancomycin-Resistant Enterococci Collected from an Urban River Basin in the Provincial City of Miyazaki, Japan. Water. 2017; 9(2):79. https://doi.org/10.3390/w9020079
Chicago/Turabian StyleNishiyama, Masateru, Yoshitoshi Ogura, Tetsuya Hayashi, and Yoshihiro Suzuki. 2017. "Antibiotic Resistance Profiling and Genotyping of Vancomycin-Resistant Enterococci Collected from an Urban River Basin in the Provincial City of Miyazaki, Japan" Water 9, no. 2: 79. https://doi.org/10.3390/w9020079




