2.3. Quantitative Description of the Break-through Curve
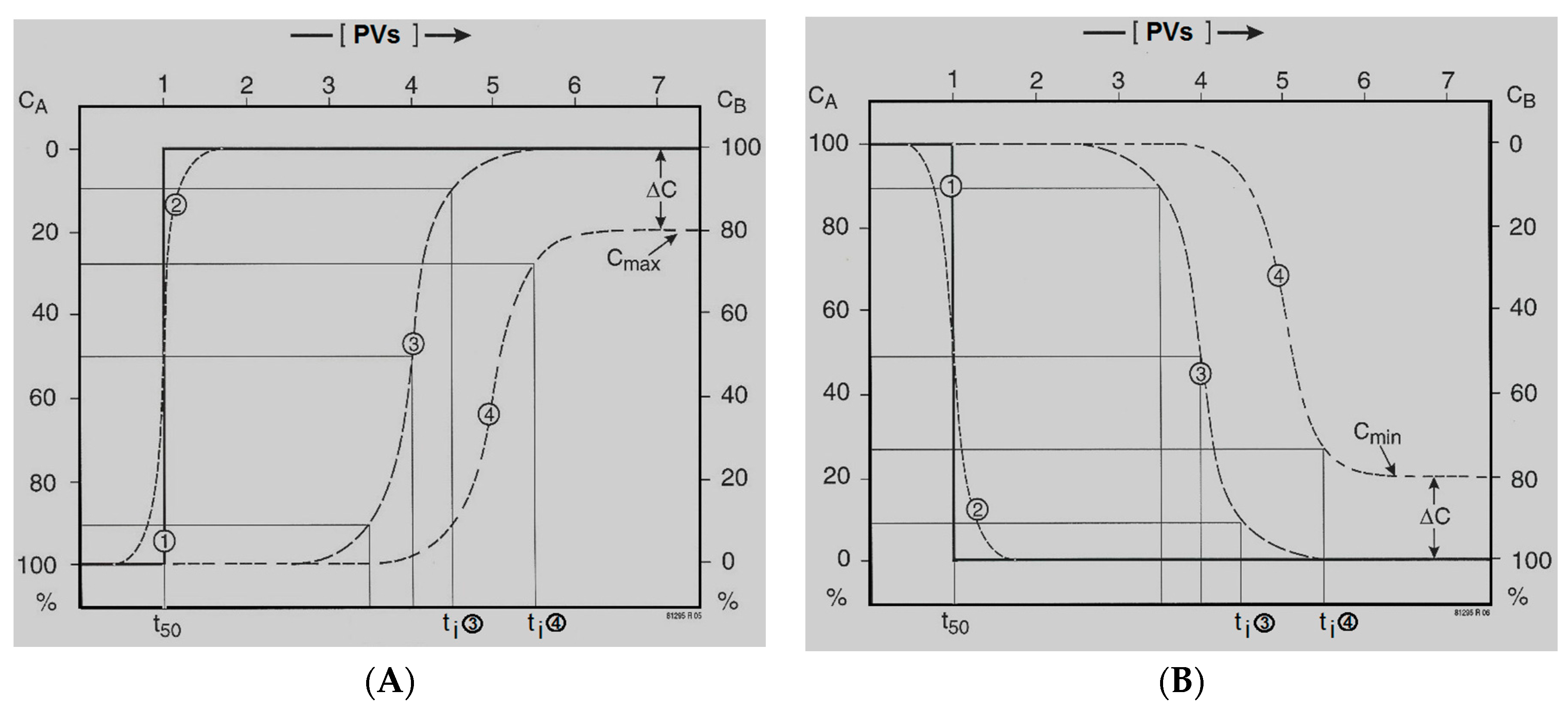
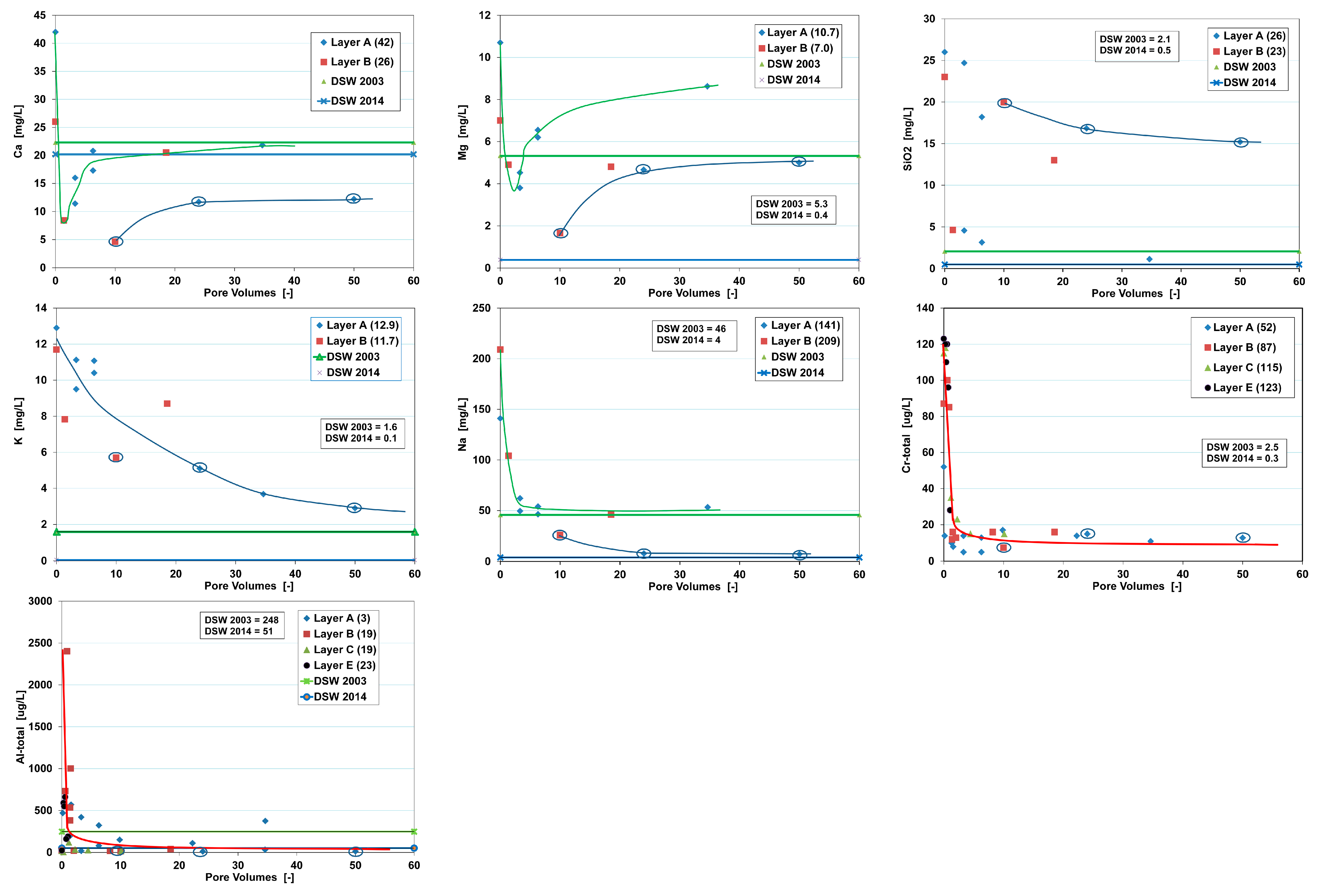
The first infiltration run of the pilot yielded valuable insight into the break-through curve (BTC) of nearly all main constituents and trace elements. These observed BTCs are characterized by 3 parameters: pore volume, retardation or leach factor and (semi)permanent concentration change (
Figure 4).
The dimensionless parameter called “pore volume” (PV) forms the time axis of water quality observations and model predictions:
where
tINF = total infiltration period [d]; and
t50 = the observed 50% breakthrough time of conservative tracer or the calculated travel time via Equation (4).
One PV means that the whole aquifer, from infiltration point to the monitoring or recovery well, has been flushed with the infiltration water exactly one time. Retardation factors R or leach factors L can then be simply deduced from concentration plots against PVs (
Figure 4).
Sorbing and oxidizing solutes as well as desorbing and dissolving solutes are retarded during aquifer passage compared with conservative solutes. In the latter case, raised concentrations drop to the influent level long after passage of the conservative chloride front. These delays are quantified for solute
i by, respectively, the well-known retardation factor
Ri, and the less well known leach factor
Li [
25]:
where
ti = time required for ≥90% break-through (
Ri) or ≥90% leaching (
Li) or till equilibrium is attained with the infiltration water [days];
ρs = density of solids of porous medium [kg/L];
n = porosity [L/L];
Kd = distribution coefficient (slope of the linear portion of the adsorption isotherm) [L/kg];
(solid) = content of reactive phase in aquifer [mmol/kg dry weight]; (
reac) = concentration of reactant in flushing fluid [mmol/L]; (
prod) = concentration of reaction product in fluid during leaching [mmol/L];
rR = reaction coefficient, i.e., the number of mmoles of solid phase which is leached by 1 mmole of reactant [-]; and
rP = reaction coefficient related to
(prod).For practical reasons,
ti is set at ≥90% not at 100%. If for some reason, the BTC shows a partial breakthrough due to a prolonged phase of continued partial immobilization or mobilization, so the additional parameter ΔC is needed to describe the BTC (
Figure 4). Equations (2) and (3) hold for a stationary retardation or leaching process, with a homogeneously distributed reactive phase in the aquifer. Of course, (
reac) or (
prod) should have no other sinks or sources, unless these can be properly accounted for.
2.4. Lithological and Geochemical Stratification
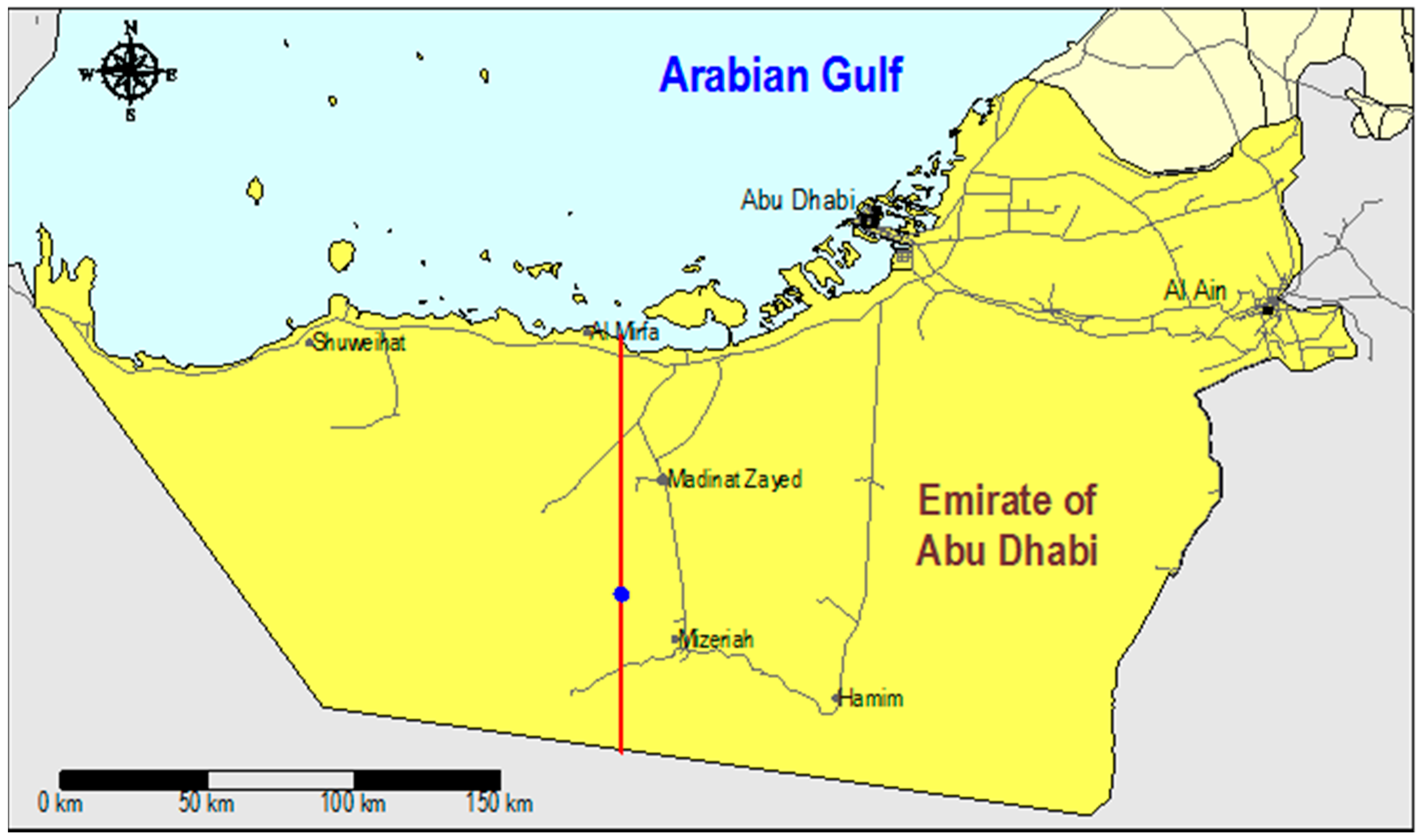
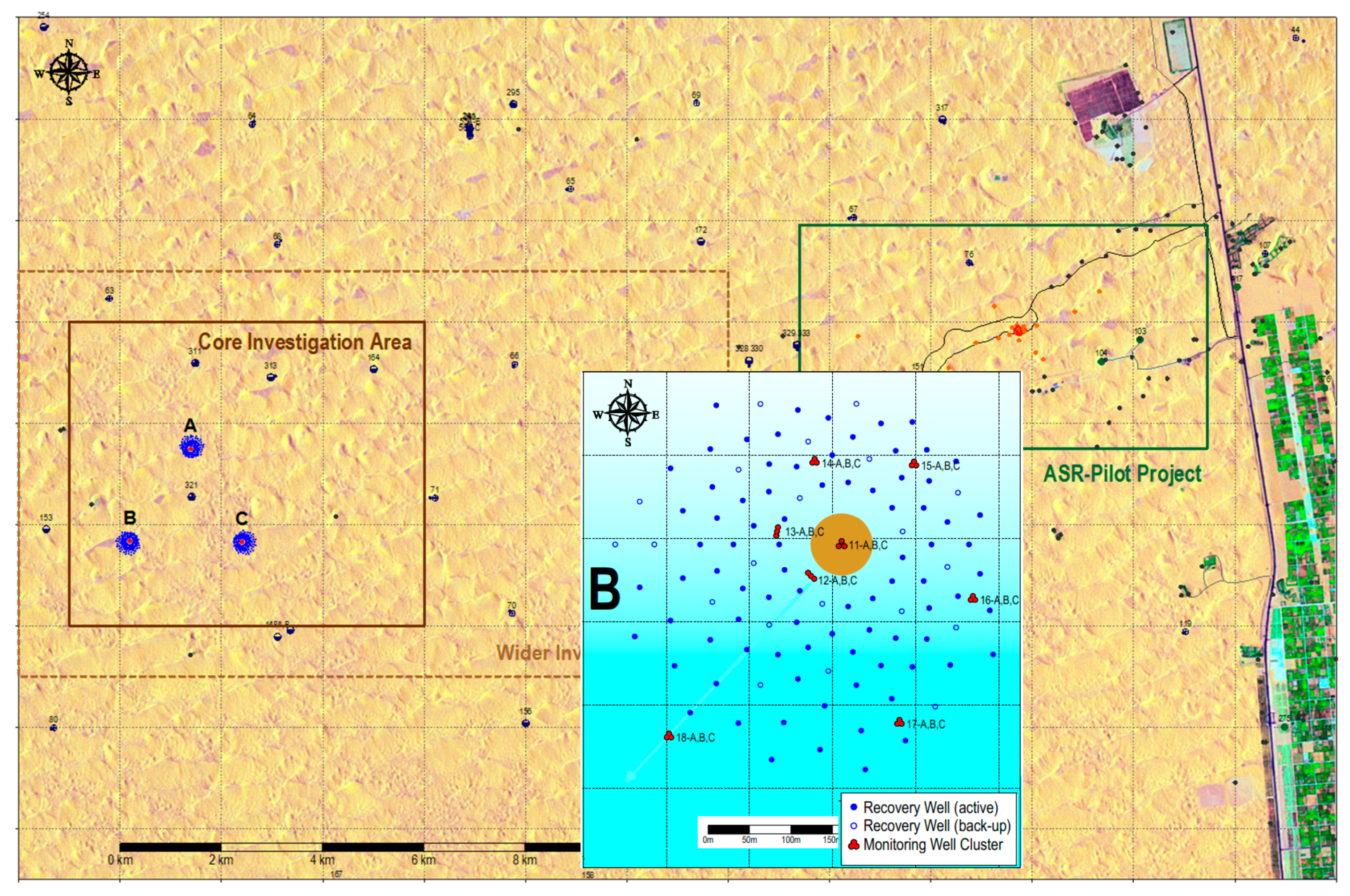
The aquifer system on well fields A, B and C could be schematized into a succession of 14 (sub) horizontal layers in between ground surface and 40 m below sea level [
23], based on all drilling logs (>372), geophysical logs (including the eccentered wireline NMR logging of permeability and porosity), pumping tests, infiltration pilot tests and fluid flow logging, as presented by [
9,
10,
11,
12]. For modeling purposes, the 14 layers were aggregated into 6 main aquifer layers (a–f in
Figure 5), of which the very low permeability aquitard f needs less consideration.
The mean geochemical composition of layers a–f was calculated from the geochemical data of 4 deep core drillings, one in each well field and one in the middle of well fields A, B and C. The data were derived from [
12], containing the following information: Sample description with photographs, petrographic analysis and mineralogical counting by petrological photomicroscopic examination of a thin section impregnated with fluorescent resin, XRD analysis, porosimetric analysis, chemical analysis of main constituents (including LOI and Acid Solubility), chemical analysis of heavy metals (probably in nitric acid; no details given), and particle size distribution (by sieving).
The digital data of all 41 samples were used to calculate mean values for aquifer layers a–f, and to quantify the content of selected minerals by petrochemical calculations [
23]. Petrochemical calculations were needed to correct specific data for water losses (loss on ignition), to calculate the cation exchange capacity (CEC) and to derive the mineral content from data on elements that are present in more than one mineral.
2.5. Hydrochemical Analyses in Period 2003–2013
Samples were taken from practically all recovery and monitoring wells, in both the pilot area and well fields A, B and C. The analyses include data on gases (O2, residual Cl2), turbidity, color, temperature, pH, EC, ORP (Oxidation Reduction Potential, Eh), main anions (Cl, SO4, S, HCO3, CO3, NO3, NO2, PO4, and F), main cations (Na, K, Ca, Mg, NH4, Fe, and Mn), SiO2, TOC (Total Organic Carbon), CN, selected trace elements (Ag, Al, As, B, Ba, Br, Cd, Cr, Cu, Hg, Mo, Ni, Pb, Sb, Se, Sr, V and Zn), and the stable isotopes 2H and 18O. Most samples were filtered in the field, and all concentrations (TOC excluded) refer to total dissolved concentrations, thus without (further) speciation. Microbial parameters and organic micropollutants were analyzed but showed negligible concentration levels.
Samples of the ambient groundwater were taken in the pilot area in 2003, and within and around well fields A, B and C in 2011–2013. Analytical data of DSW samples prior to infiltration, during and after aquifer passage were exclusively available from the pilot. All data (obtained from [
9,
10,
11,
12] and from data files supplied by Dr. G. Koziorowski (GTZ International Services) were controlled, elaborated and stored with Hydrogeochemcal.xlsx [
26].
2.6. Monitoring Campaign in August 2014
In the period of 3–7 August 2014 a sampling campaign was conducted to take 31 samples from divergent observation wells that had been sampled earlier and from DSW, in order to: (i) check potential bias in the existing hydrochemical data set of well fields A, B and C; and (ii) obtain data on chromium speciation: Cr(III) versus Cr(VI).
Important aspects that were tackled with great care, are: sufficient well purging based on purged volume and stable field parameters, sampling without applying vacuum and excluding atmospheric exposition, reducing exposure to sunlight and wind, flow regulation of the pump, field measurements (EC, pH, temp, O2), filtration of water over a 0.45 μm membrane, dedicated sample preservation for specific parameter groups, cooling, nearly daily shipment to the Netherlands, and rapid analysis in the certified Vitens Laboratory (Leeuwarden). The quality of the analysis was validated using HGC 2.0 to exclude potential impact of errors.
2.7. Predictions by EL Modeling
Two models were constructed, an Excel based Easy-Leacher (EL) model [
25] and a PHREEQC-2 [
27] flowtube model.
PHREEQC-2 was applied to model more in detail the behavior of chromate and arsenate along a small number of flowlines during infiltration phase. PHREEQC-2 and EL produced nearly identical results for chromate and arsenate behavior during infiltration, justifying the application of the simpler EL model. Further details about the PHREEQC-2 modeling and its results are given by [
24] and not considered here further.
EL simplifies 3D groundwater flow into a 2D set of maximum 50 flow tubes through a maximum of 10 horizontal aquifer layers. The travel times are either derived from a hydrological model, or calculated analytically. Chemical transport is calculated on the basis of pore volumes (dimensionless time scale), retardation and leach factors (superimposed on the pore volumes) based on either mass balances or field observations, CaCO3 equilibrium (if relevant), redox reactions (if relevant), and expert rules on among others redox reaction kinetics.
EL was given the task to do the all-round water quality modeling during all ASR phases (infiltration, storage with bubble drift, and recovery), and to combine the output of a relatively high number of flowlines into a mixed output as generated by a well field.
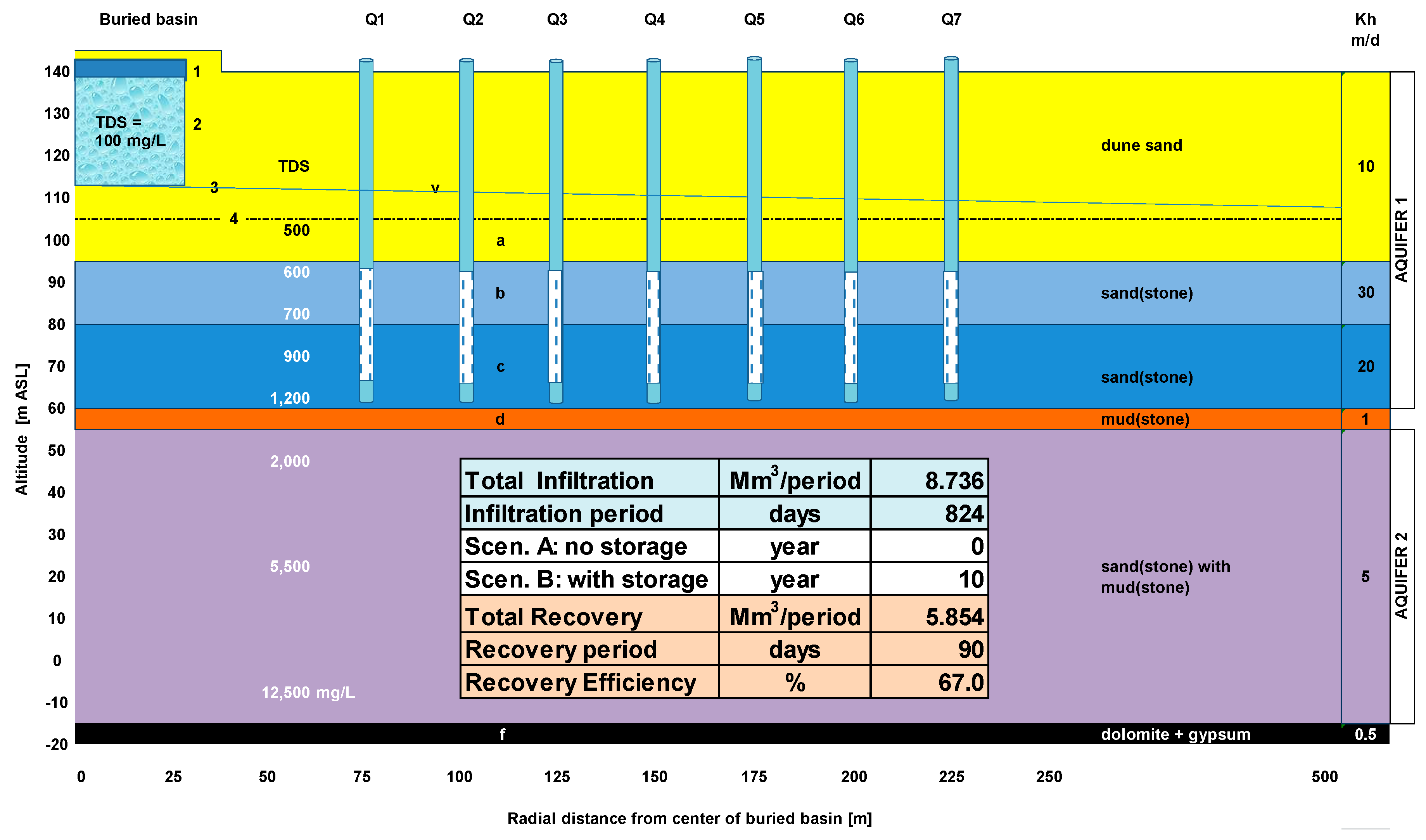
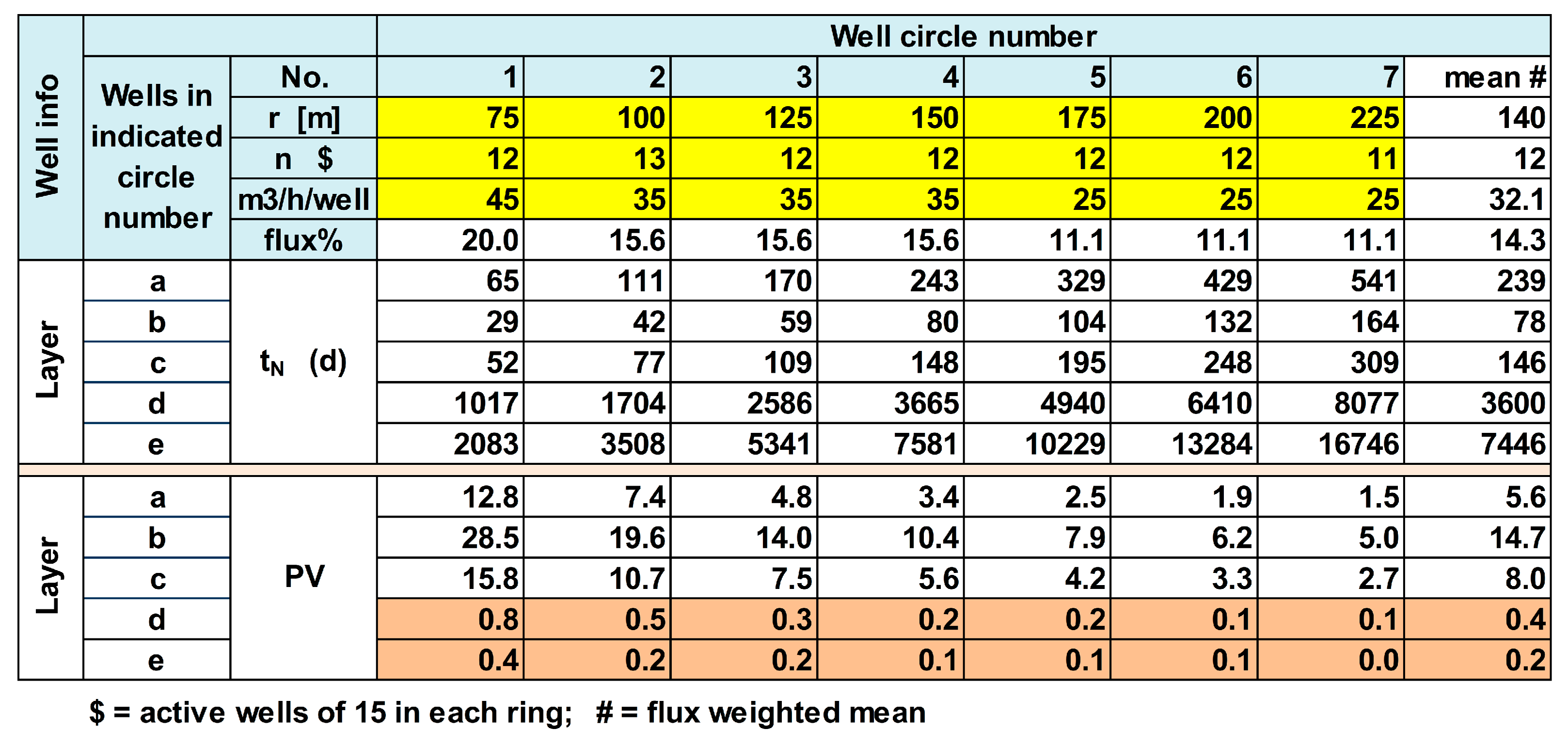
In EL, the whole ASR system was schematized by one representative recharge basin (the average of basins A–C), 5 aquifer layers (of which the upper layers a, b and c are most important) and 7 flowlines within each aquifer layer departing from the basin towards one recovery well in each of the 7 well rings at 75–225 m radius (
Figure 5).
The expansion of the DSW bubble in each aquifer layer and the travel times along each flowline were calculated by assuming first vertical flow down to each aquifer layer and then horizontal radial flow, so that:
where:
tN = 50% break-through time (
t50) in layer N [d];
nN = porosity of layer N [-];
r = radial distance from the basin center [m];
T = transmissivity of the aquifer [m
2/d];
QIN = mean infiltration rate [m
3/d];
Kh,N = horizontal hydraulic conductivity of layer N [m/d]; and
tV = vertical travel time [d] as determined by a 3D hydrological model [
8].
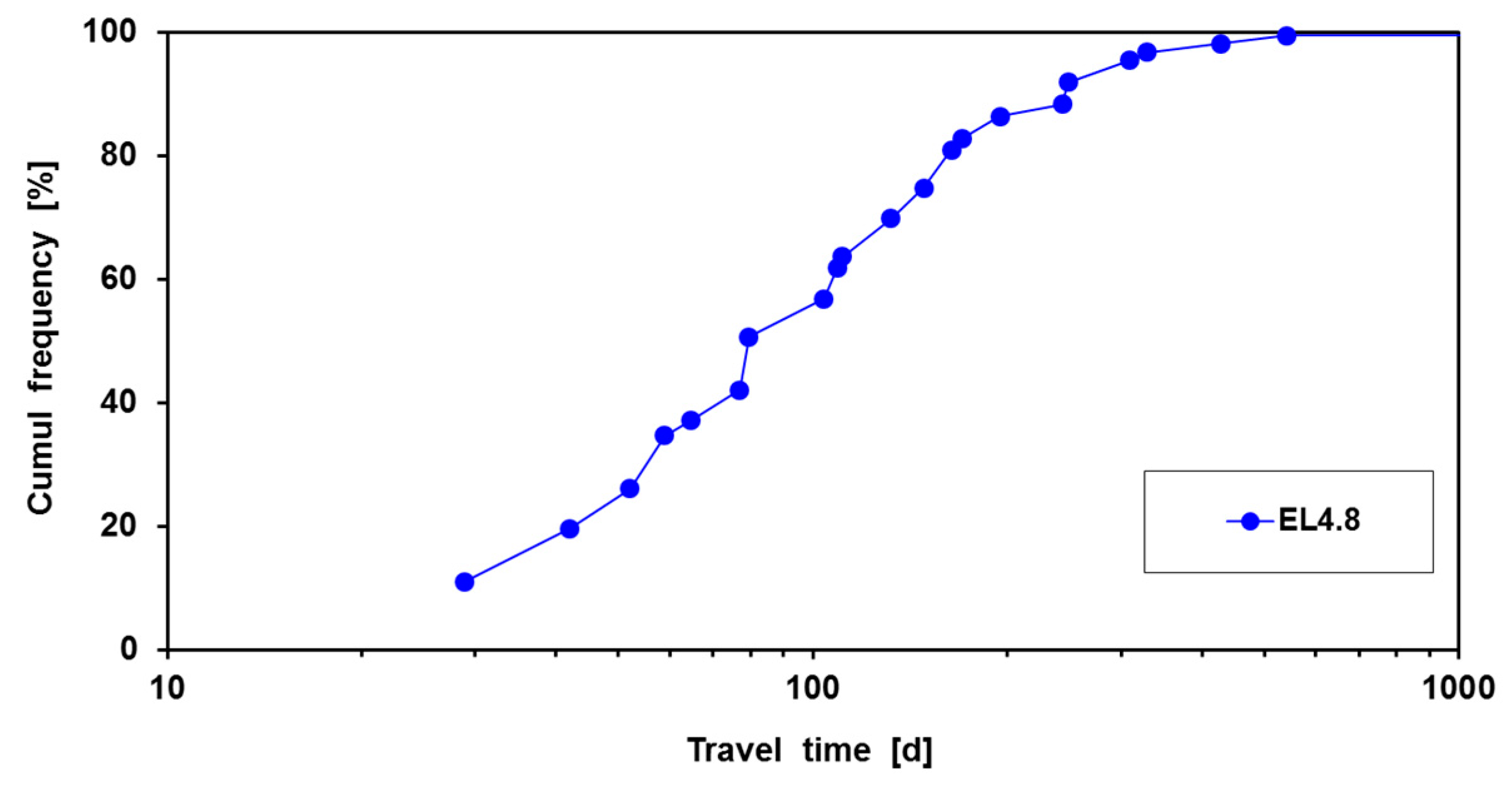
This simplification creates some distortion during the first 30 d, but these are of minor importance on the long run of 824 d of infiltration. With Equation (4), the travel time was calculated from recharge basin to its 7 surrounding rings of recovery wells at the distances specified in
Figure 6. It is deduced that in theory all wells, also those in the outermost ring will pump DSW after 824 d of infiltration. In layers D and E, probably little DSW will be present.
During 10 years of storage, the infiltrated DSW bubble is predicted to move laterally down the regional hydraulic gradient, with the following velocity (
vB,N), assuming an equal gradient in each layer:
where
ΔH/
ΔX = mean regional hydraulic gradient in the aquifer at well fields A, B and C during storage phase [m/m].
Vertical bubble drift by buoyancy has been ignored in accordance with FEFLOW model predictions [
9]. The water quality evolution during injection phase was calculated for each flowline where it crossed its destination well (node point), and also for the “fictive”, mixed sample taken from all 35 node points, in proportion to: (i) the preset pumping regime (the inner wells pumping more than the outer wells); and (ii) the transmissivity of each main contributing aquifer layer (a–c). This mixed sample thus represents the output from the whole well field, when pumping out a negligible amount of water without disturbing the continuous DSW bubble expansion.
EL in forward mode assumes the following: (1) input quality (DSW) is constant; (2) retardation factors, leach factors and the (semi)permanent concentration changes are derived from observations during the pilot; (3) redox reactions are absent as observed in the pilot; (4) reactive minerals such as calcite, dolomite and silicates are not depleted; and (5) reaction kinetics do not play an important role.
The recovery phase was modeled by moving backward in the time series that displays the quality evolution of this “imaginary”, mixed (averaged) water sample. Contrary to the infiltration phase, this “imaginary”, mixed sample now becomes the true output of the well field during recovery phase, showing after some time an increasing instead of decreasing contribution of native groundwater. This is in harmony with theory and the predictions by [
9,
11].
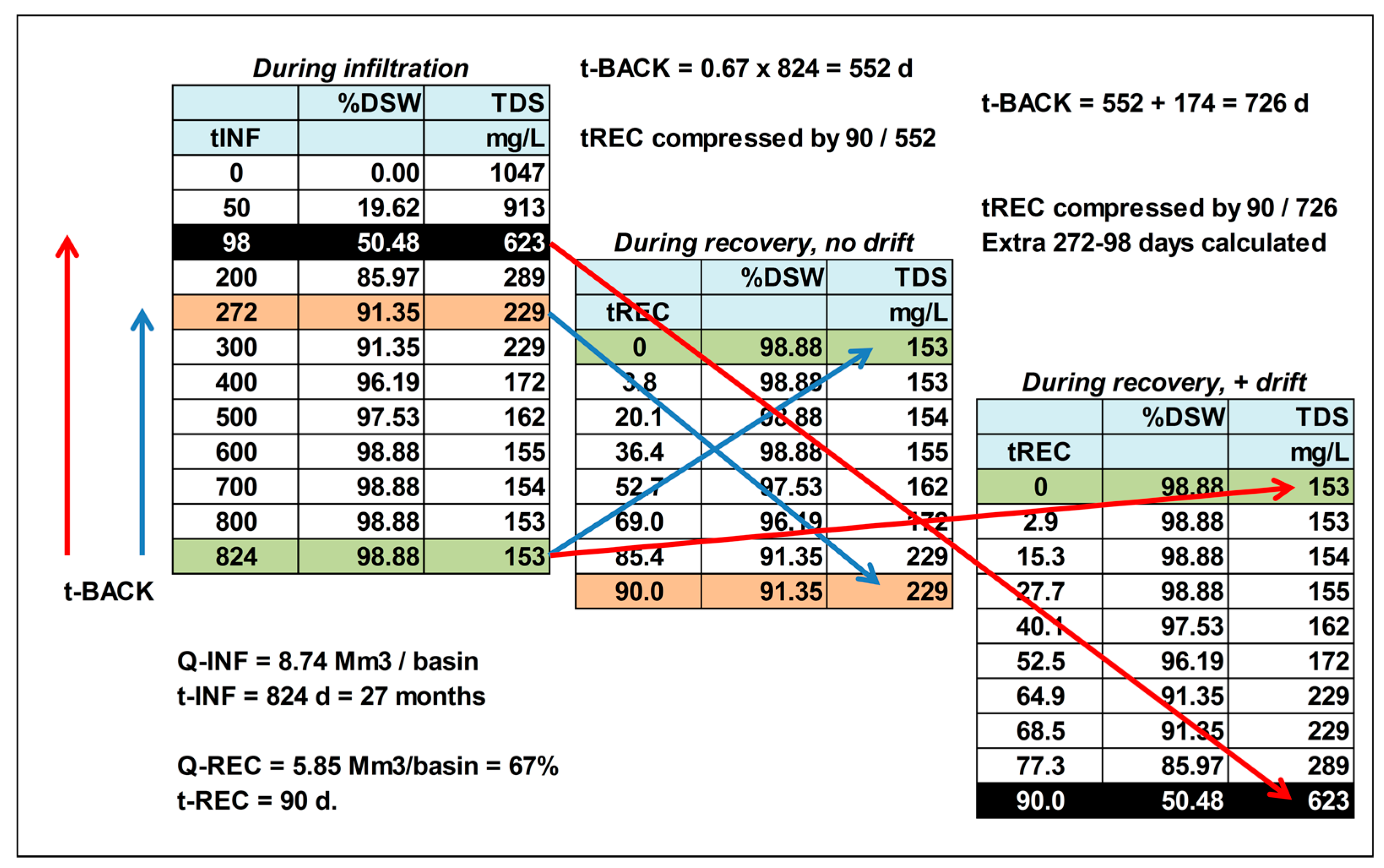
As during the 90 d of recovery 67% of the infiltrated water volume will be pumped out, the way back in the expansion time series needs to be as long as 0.67 × 824 = 552 d (= Δt
BACK-1). This way, water quality at the start of recovery, without storage phase, will be the water that flushed each of the 7 well rings prior to pumping (on day 824), and this water only needs to be mixed in proportion to the pumping rates of each well ring (
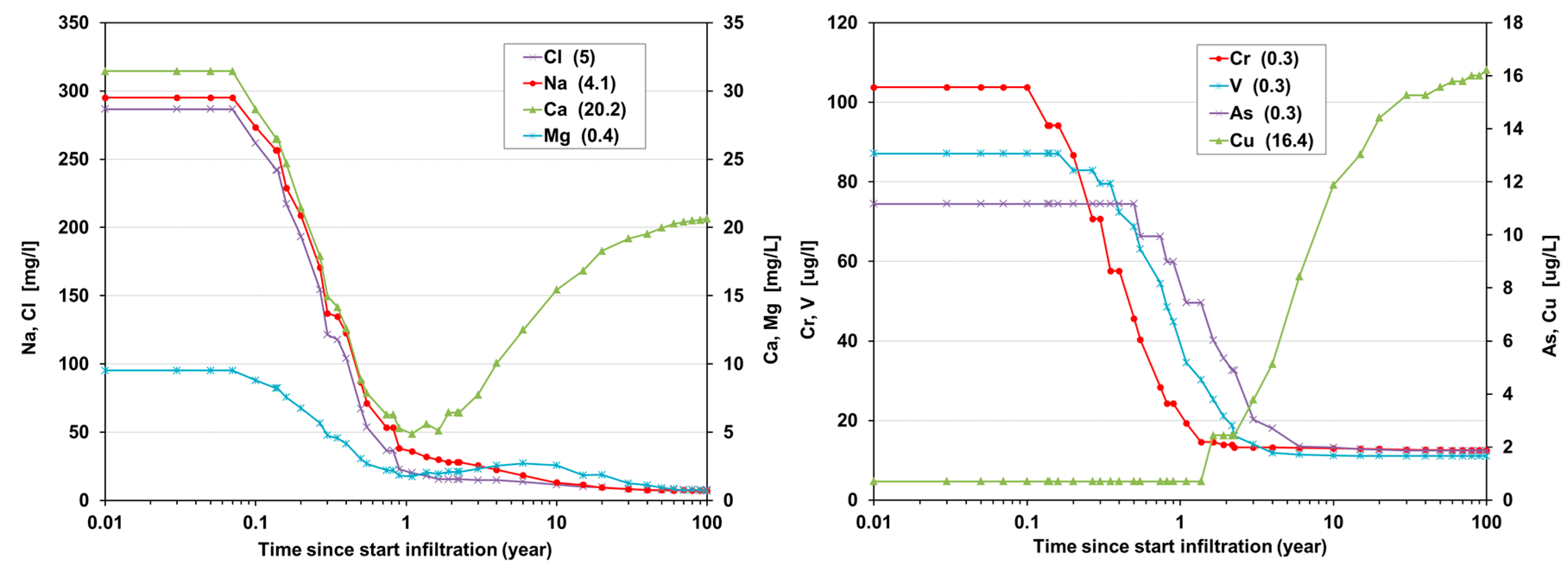
Figure 6). At the end of pumping (after 90 d) we obtain about the same water as predicted to surround the wells on day 824 − 552 = 272. Water compositions in between can be calculated by just following the predicted water quality from 824 to 272 d back in time. In order to plot this water quality evolution during 90 d of recovery, the expanded time scale (from 90 to 552 d) needs to be compressed back again to 90 d, by multiplying it with 90/552, and to mirror it from backward into forward mode (
Figure 7).
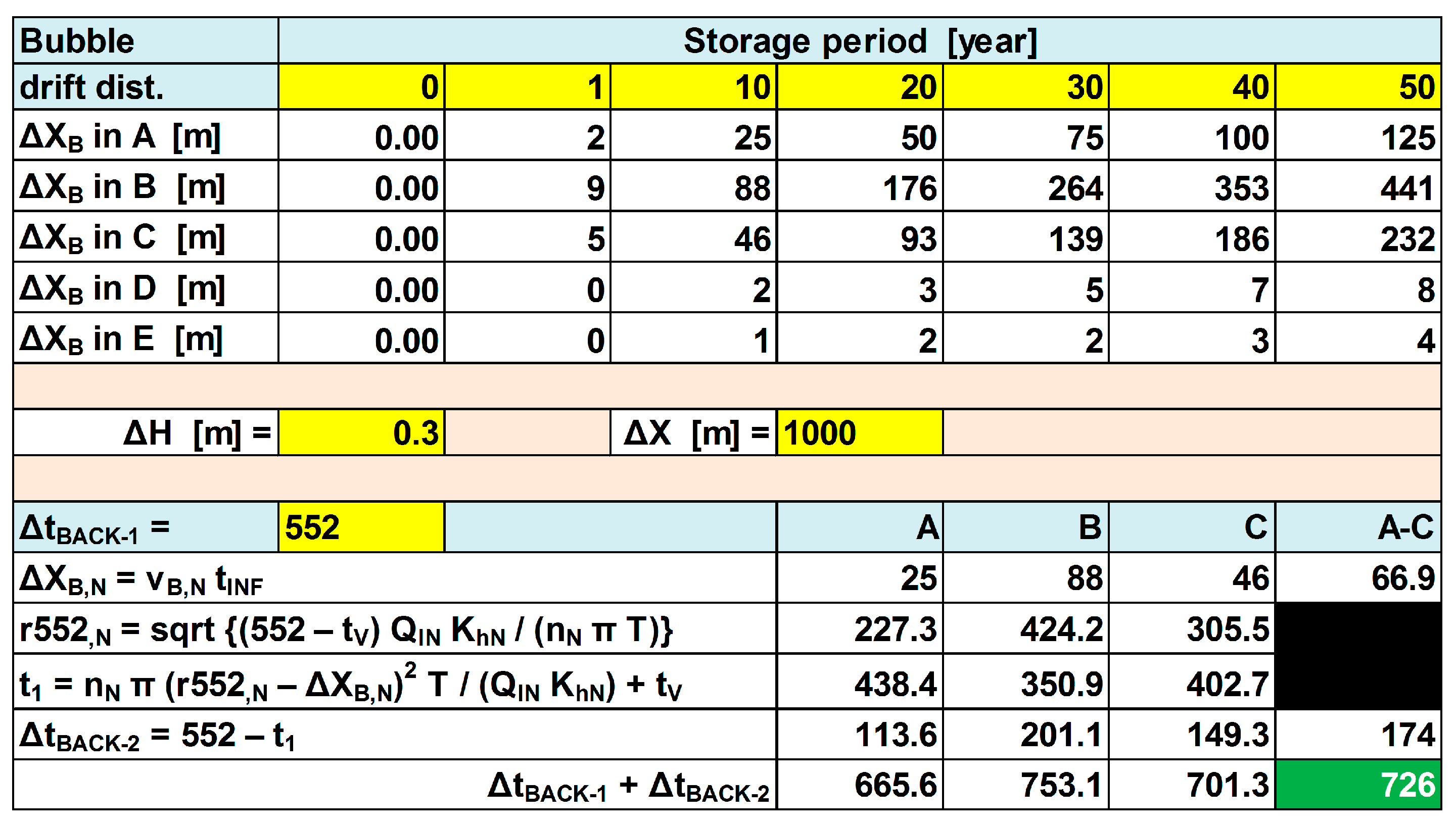
In the case of a 10-year storage phase, lateral bubble drift can be taken into account by first extending the backward period of 552 d (Δt
BACK-1) with the period (=Δt
BACK-2) that would be needed to get the retrograded bubble face back on its position at 552 d without bubble drift, and then resetting the time scale to 90 d by multiplying it with 90/(552 + Δt
BACK-2), and mirror it from backward into forward mode (
Figure 7 and
Figure 8).
The calculation of Δt
BACK-2 is then as indicated in the realistic example elaborated in
Figure 8. In order to simplify the calculations, the weighted average value of Δt
BACK-2 is taken, being 174 d in the example of
Figure 6. Addition of Δt
BACK-2 = 552 yields a total set-back time of 726 d. This means that the quality of the water recovered after 90 d of pumping, is to be looked up in the quality output list on day 824 − 726 = 98, in case of bubble drift during 10 years of storage.
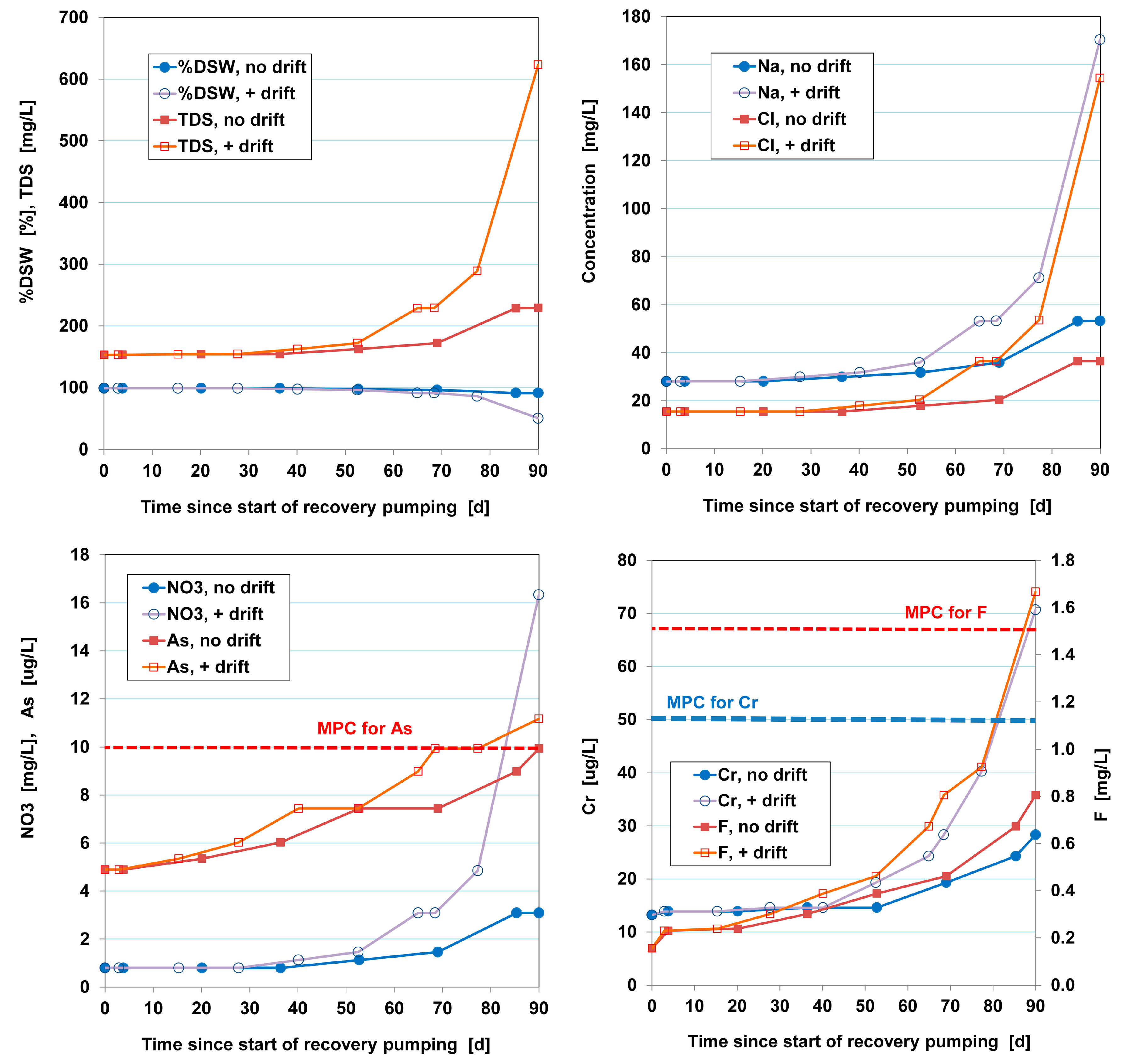
How this example with resulting time shifts works out in the %DSW and TDS concentration of the water recovered, is shown in
Figure 7. The underlying calculations were performed in EL, and match the predictions by [
9] quite well.
EL in backward mode assumes that: (a) the forward evolution is reversed at 6 times higher speed; (b) the 6 times higher recovery rate does not provoke serious upconings (as shown by 3D modeling results [
9]; and (c) during storage and recovery no further reactions with the aquifer are taking place.
Fluxes in the different aquifer layers, from the recharge basin towards the recovery wells and beyond them, were set equal to their contribution to the aquifer’s transmissivity.
Model calibration was done on: (1) available data from the pilot study in 2003–2004 when DSW was infiltrated via a recharge basin; and (2) groundwater quality as observed in the same pilot area in August 2014, after about 6 years of storage in the local aquifer system (since a second recharge run in 2008).