Global climate change is predicted to alter the rainfall patterns during the growing season. Such changes may affect soil, plant, and ecosystem properties and ultimately impact their productivity [
21]. Changes to rainfall are thought to be important in structuring arid and semi-arid plant communities. From an applied perspective, productivity responses to rainfall during the growing season are important, as they influence the capacity of the drylands to sequester carbon [
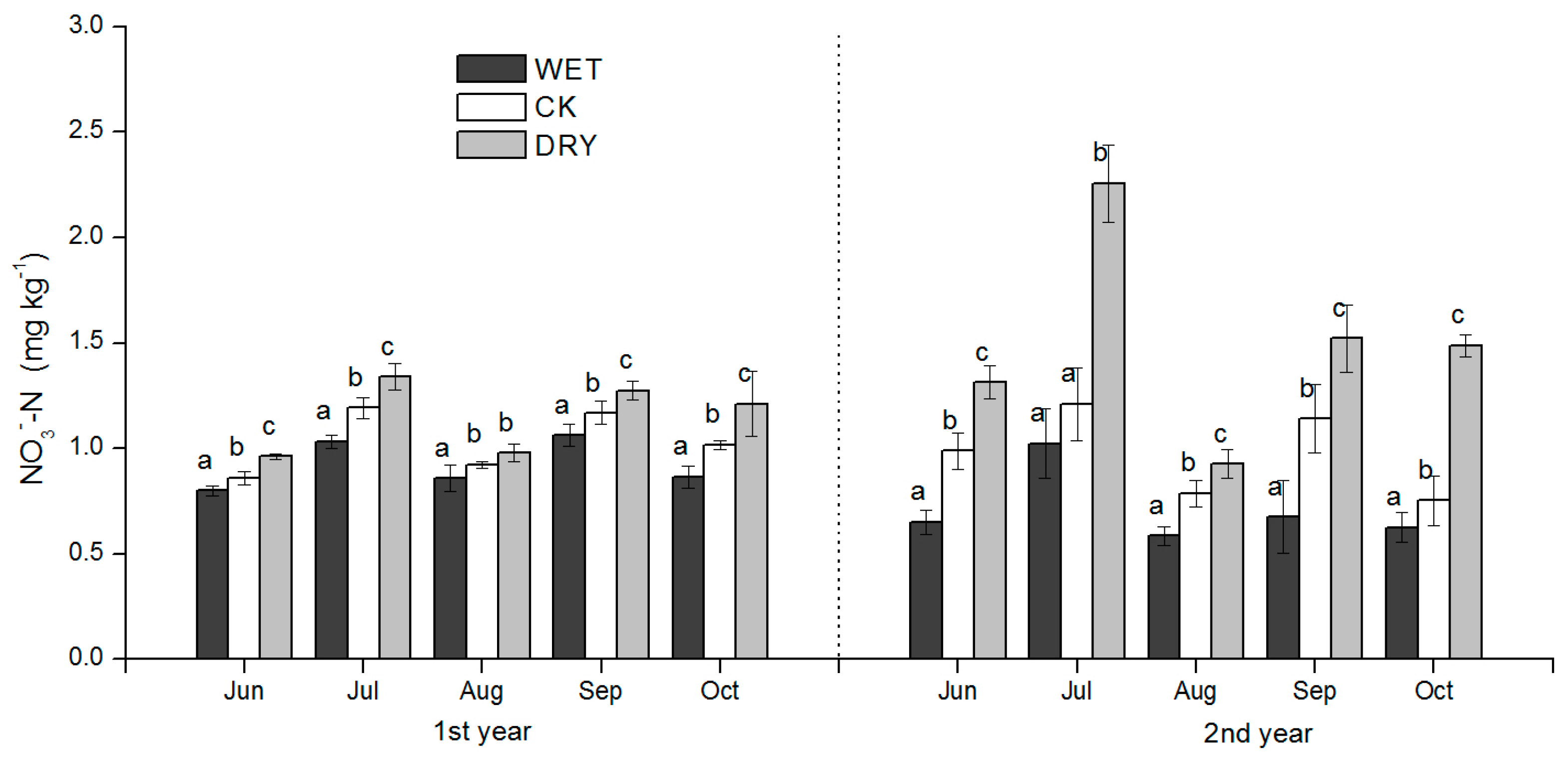
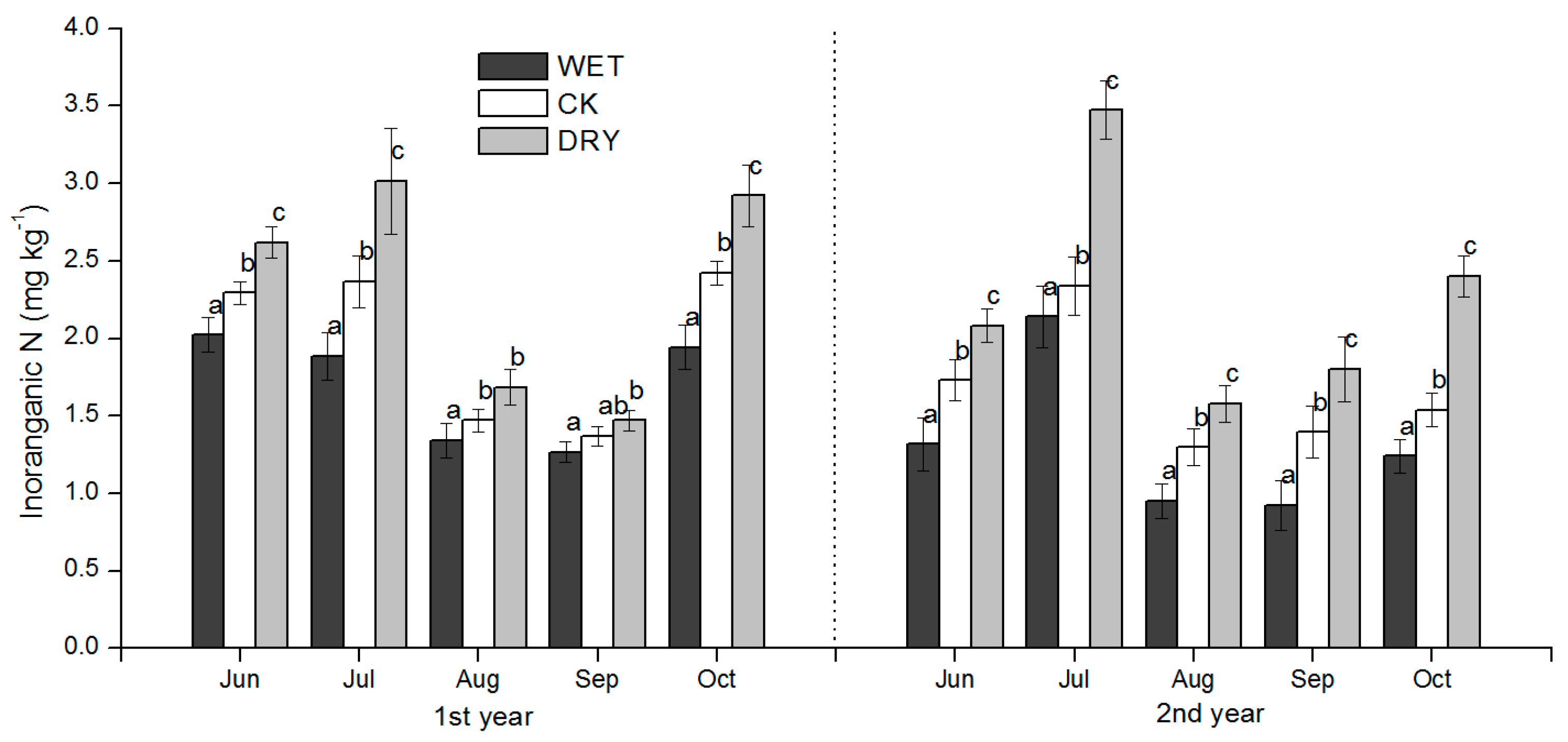
27]. Mongolian pine plantation growth is often water limited, with changes to rainfall during the growth season directly affecting plant photosynthetic characteristics (e.g., stomatal conductance) in semiarid Mongolian pine plantations. The precipitation amount in the growing season had a significant effect on the measured soil inorganic N content, and the direction of change was clear. Our results therefore confirm our hypothesis that in a semiarid Mongolian pine ecosystem: (1) increased precipitation decreases N availability; and (2) decreased precipitation restrains the microbial immobilization of N. The results are consistent with previous studies that have shown an increase of mineral N following drought, and a decrease with increased moisture [
8,
9,
15]. Soil inorganic N and component NH
4+-N and NO
3−-N concentrations in our study were all significantly responsive to WET and DRY treatments except for NH
4+-N in September after the peak rain period in July–August, but also after growth increases in microbial biomass, which was an important factor influencing soil N availability. WET and DRY treatments were also clearly reflected in monthly SWC (
Table 2) and had a significant effect on the productivity of the understory, both in the peak ANPP and root biomass. We discuss these results in the context of how variation in soil N availability may have occurred under the WET and DRY treatments and what implications they have for natural resource management and further study.
4.1. How Did the Variation in Soil N Availability Occur?
The effects of the increased precipitation amount (WET treatment) and decreased precipitation amount (DRY treatment) were significant over the growing season, and added to the background temporal variation observed under natural precipitation conditions. The effect of changes in the precipitation amount on soil N availability under Mongolian pine plantation (
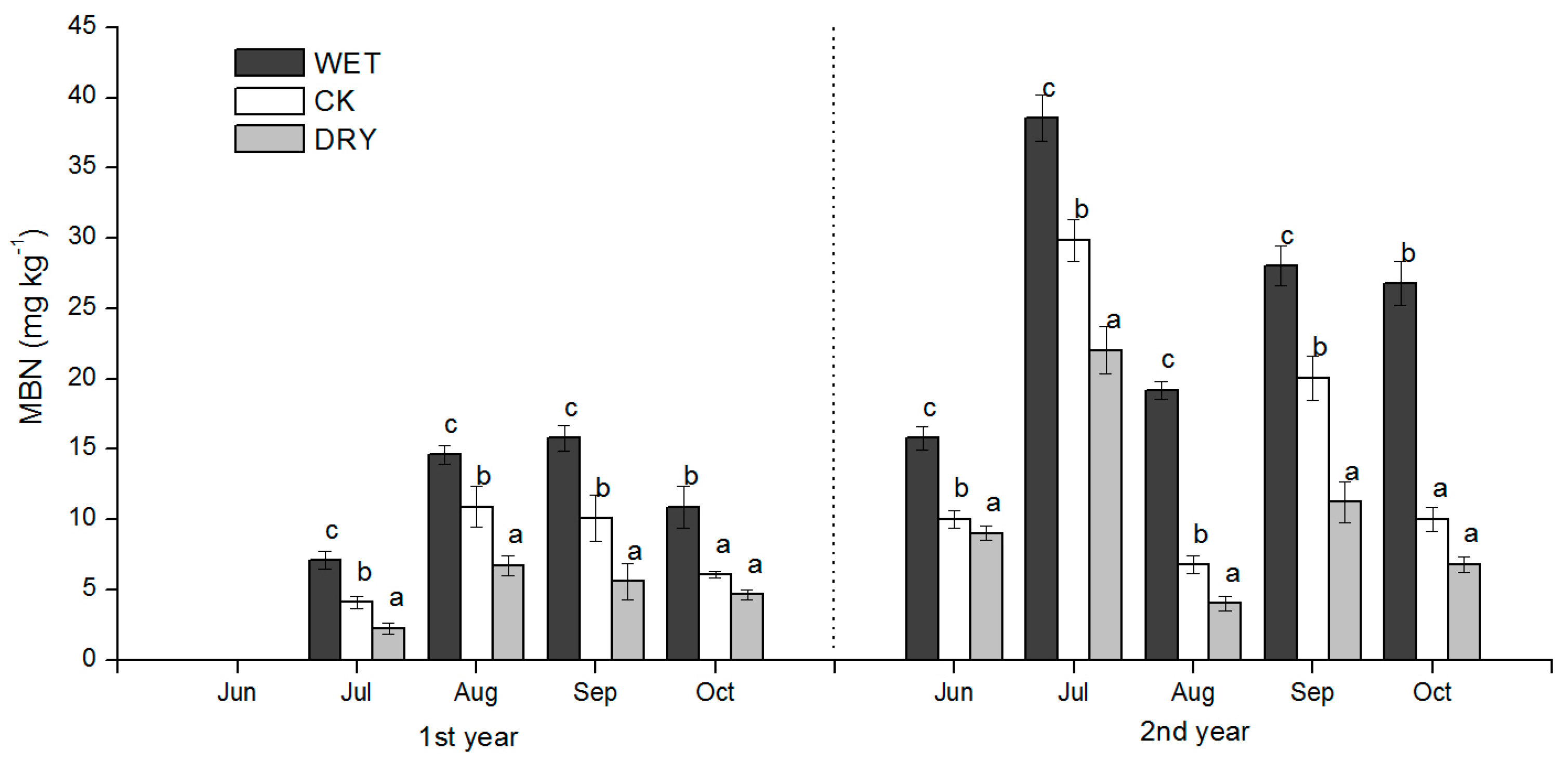
Figure 4,
Figure 5 and
Figure 6) may be explained by processes controlled by soil moisture such as the immobilization of N by microbes, plant N uptake, and N losses via leaching and volatilization.
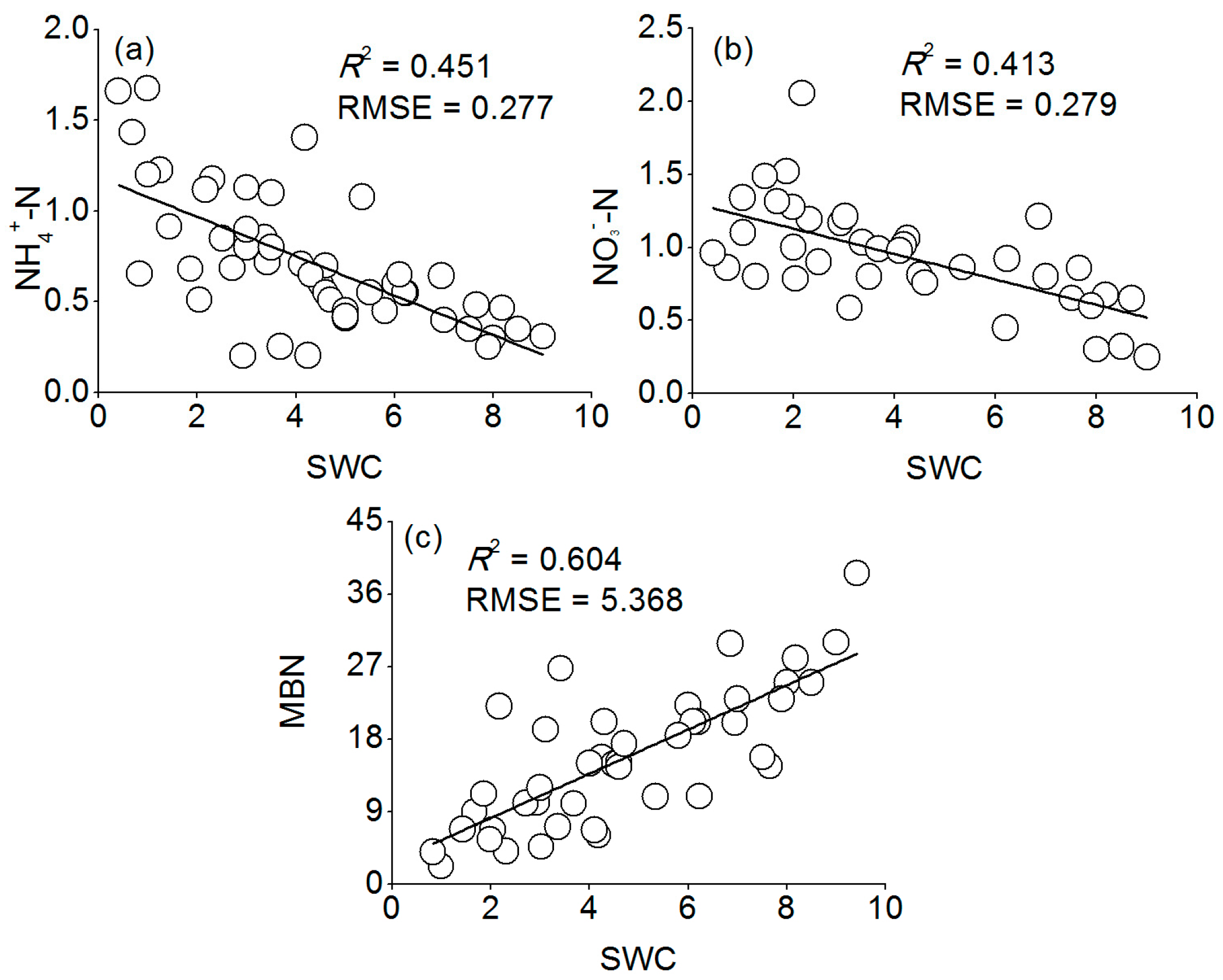
Our results showed that there was a significant negative relationship between the MBN and inorganic N (
r = −0.778,
p < 0.01) (
Table 4), and that the MBN was positively associated with soil SWC (
R2 = 0.604,
p < 0.05,
Figure 8c). Therefore, we argue that the microbial immobilization of N under Mongolian pine plantations was restrained under decreased precipitation due to slow microbial activity, which resulted in the accumulation of inorganic N in the soil. This mechanism is also supported by the findings of Fisher et al. [
15] and Reynolds et al. [
9]. Microbial biomass can thus either act as a sink or a source of nutrients [
39,
40]. As a sink, the microbial biomass is a mechanism for immobilizing N, effectively reducing N losses through leaching or associated denitrification [
41,
42]. In other words, the MBN is an indicator of the immobilization of N by microbes, and is considered a regulator of N dynamics in arid and semiarid regions [
41,
43]. Fisher et al. [
15] reported that levels of extractable N (NH
4+-N and NO
3−-N) in the Jornada Basin directly decreased after rainfall, which they attributed to increased N immobilization in the microbial biomass, as well as to increased leaching of N. Reynolds et al. [
9] concluded that extractable N (NH
4+-N and NO
3−-N) accumulation during the summer drought in the Jornada Basin could result from the effects of drought upon N immobilization in the microbial biomass and plant access to soil moisture and nutrients.
The amount of N uptake by plants could be indicated by plant biomass [
44,
45]. In our experiment, the biomass of Mongolian pine trees was insensitive to changes to the precipitation amount compared to the understory vegetation during the growing season. Our results indicated that changes in precipitation amount had an effect on the growth of Mongolian pine trees, but that the ANPP (aboveground net primary production) of the understory was significantly lowered by decreased precipitation and significantly increased with increased precipitation. Consequently, the changed amount of N uptake by plants mainly came from changes in the understory biomass. Many previous studies have reported that the ANPP increased linearly with increasing precipitation in sites below 1500 mm MAP (mean annual precipitation) [
46,
47], which is consistent with our results. With decreased precipitation, plant access to soil moisture and nutrients was reduced; therefore, N uptake was limited by water, which supports the results of the accumulation of inorganic N in soils [
9,
48]. Plant N uptake was relative to soil inorganic N levels—particularly for the understory vegetation, for which N uptake presumably increases over the growing season, and is consistent with the seasonal pattern of biomass accumulation and increased N demand by new plant tissue [
44]. The understory N uptake is likely to have influenced temporal soil inorganic N levels; therefore, the lower concentration of inorganic N during August and September may be partly explained by higher plant N uptake during the rainy season.
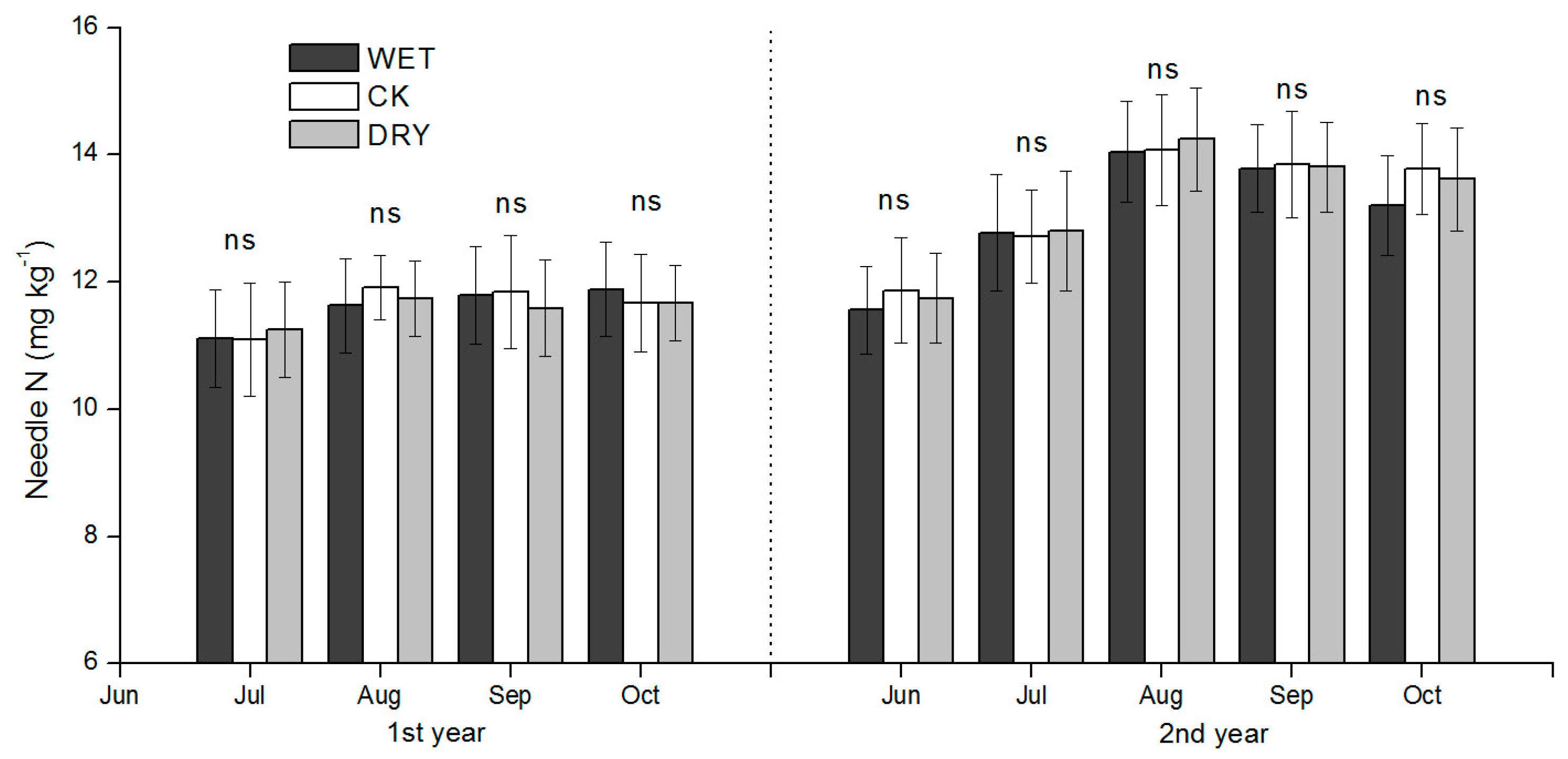
Increased leaching of N under increased precipitation amounts may have contributed to lower concentration of soil inorganic N. However, if N loss from the surface soil layer was substantial, it was not reflected in the Mongolian pine N uptake due to the lack of significant differences in needle N concentration in Mongolian pine among treatments. Nitrogen loss from the soil through leaching is generally assumed to be negligible in desert ecosystems [
49,
50]; however, Walvoord et al. [
51] found a large reservoir of available nitrogen (as NO
3−-N) in subsoil zones of arid regions that indicates important long-term leaching from desert soils. Austin and Vitousek [
8] evaluated soil nutrients on a precipitation gradient in Hawaii and concluded that decreasing concentrations of rock-derived nutrients in soils (NH
4+-N, NO
3−-N and PO
4-P) as precipitation increased indicated that the effects of increased precipitation on leaching exceeded its effects on weathering. Yahdjian et al. [
10] also found that soil NO
3−-N content significantly decreased with increasing precipitation input in the semiarid Patagonian steppe, and explained it as the increased leaching of N, as well as increased microbial growth and plant uptake.
Variation in N emissions can be positively associated with variation in soil moisture content [
50,
52]; thus, the WET treatment may have affected N emissions. Denitrification also largely depends on anoxic conditions, which are unlikely to persist for long periods in the porous sandy soils at the study site. Therefore, the loss of soil N via emissions is likely to be small in this ecosystem relative to estimates reported in other studies [
53,
54,
55].
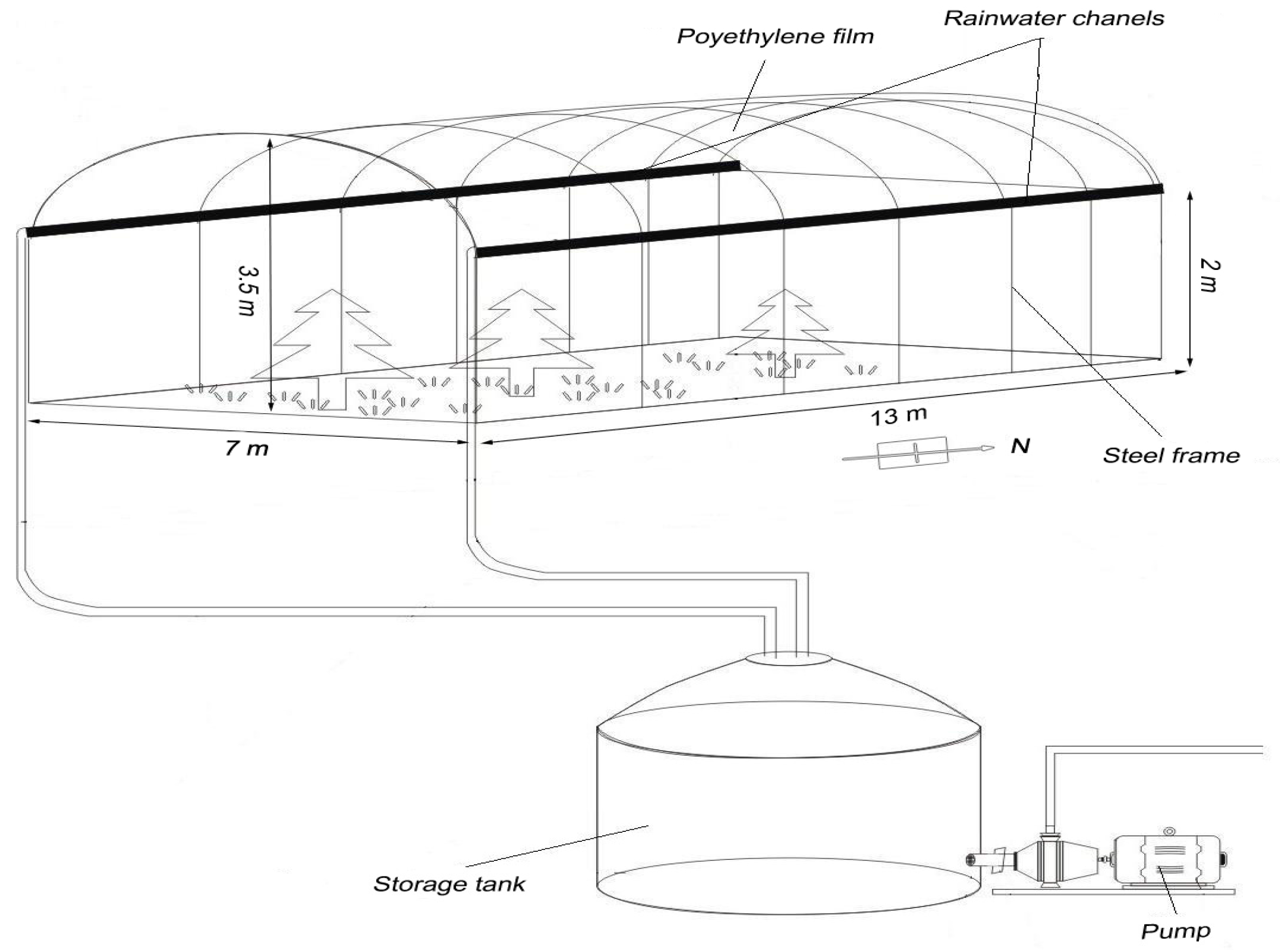
Rain water collection and storage successfully enabled the manipulation of water quantity. Compared to other rainout shelter designs, the microenvironment changes under our rainout shelters were generally small [
21,
27,
56]. Svejcar et al. [
56] found that rain shelters reduced wind speed by 28%; relative humidity by 15%; and increased soil and air temperatures by 16% and 4%, respectively. Fay et al. [
21] also found that peak daytime PFD (photosynthetic photon flux density) and net radiation influxes were reduced by approximately 22%; and that soil temperatures were 1.2–1.8 °C higher under the shelters compared with unsheltered control plots. Yahdjian et al. [
27] reported that the soil temperature was lower under the shelter than outside, with a mean difference of 1.8–2 °C and a maximum of 6–6.5 °C when the temperature was high. We used a clear polycarbonate material allowing a higher transmission of PAR; therefore, this approach was suitable for evaluating the semiarid Mongolian pine plantation responses to precipitation. The field simulation of changes in precipitation using the methodology in our study therefore proved to be effective.
4.2. Insights Relevant for Management and Further Study
The release of labile N from dead microbial biomass and plant roots could also be a contributing factor in the observed variation in soil inorganic N content [
57]. Under decreased precipitation, increased soil microbial biomass mortality is expected, as is as dying plant roots due to drought, which releases labile N into the soil and contributes to the accumulation of inorganic N [
9,
44,
58]. The understory root biomass decreased in response to the DRY treatment, and may have released some N. In arid and semiarid regions, microbial mortality during dry seasons may account for 40% or more of the gross inorganic N produced during subsequent wet seasons [
18]. Metabolic (labile) and structural fractions of litterfall above- and below-ground contribute N to plants and microbial biomass. The understory vegetation in this ecosystem represents a substantial N input to soil when they senesce at the end of every growing season [
59,
60]. Based on the results of our recent study [
60,
61], this N input will be larger after a wet growing season than after a dry growing season, which has important implications for N supply on timescales greater than one year. Moreover, when this N input is large, more N may make its way to the predominately distributed root soil layers and thus be readily available to Mongolian pine trees at the beginning of the next growing season. Considering the size of the understory N accumulation in the biomass relative to Mongolian pine N uptake (
Table 6), this could be an important mechanism in supporting the growth of Mongolian pine trees.
A consequence of the accumulation of inorganic N under decreased precipitation appears to be the asynchrony of soil moisture and soil N levels. When inorganic N concentrations are high, plants are inactive and unable to respond because of drought effects [
10,
18]. In our study, soil inorganic N concentrations significantly decrease with increasing precipitation, with consequent increases in microbial biomass and the ANPP of the understory vegetation, which suggests that water availability is the overriding limiting factor on the growth of microbial biomass and understory plants at the study site. Dry conditions cause increased soil N, which then becomes available to plants when it rains. However, N limitation became increasingly important when precipitation increased due to the asynchrony of soil moisture and soil N availability in the Mongolian pine plantation. To manage Mongolian pine plantations for potential changes in precipitation in the Horqin Sandy Lands, the pruning, thinning, and protection of litterfall has been advised to minimize evapotranspiration when precipitation decreases [
60,
62,
63]. Traditionally, the understory vegetation and litterfall are removed in managed forests to reduce resource competition between trees and the understory community; however, this may be a poor management strategy, as the understory plant community and litterfall can increase forest nutrient retention. With precipitation increases, soil N availability could feasibly be enhanced by protecting litterfall. As controls on N cycling are complicated, further study and long-term data on the effects of changes in precipitation on soil nitrogen availability in Mongolian pine plantations are highly desirable.