Characterization of Dissolved Organic Matter in Deep Geothermal Water from Different Burial Depths Based on Three-Dimensional Fluorescence Spectra
Abstract
:1. Introduction
2. Materials and Methods
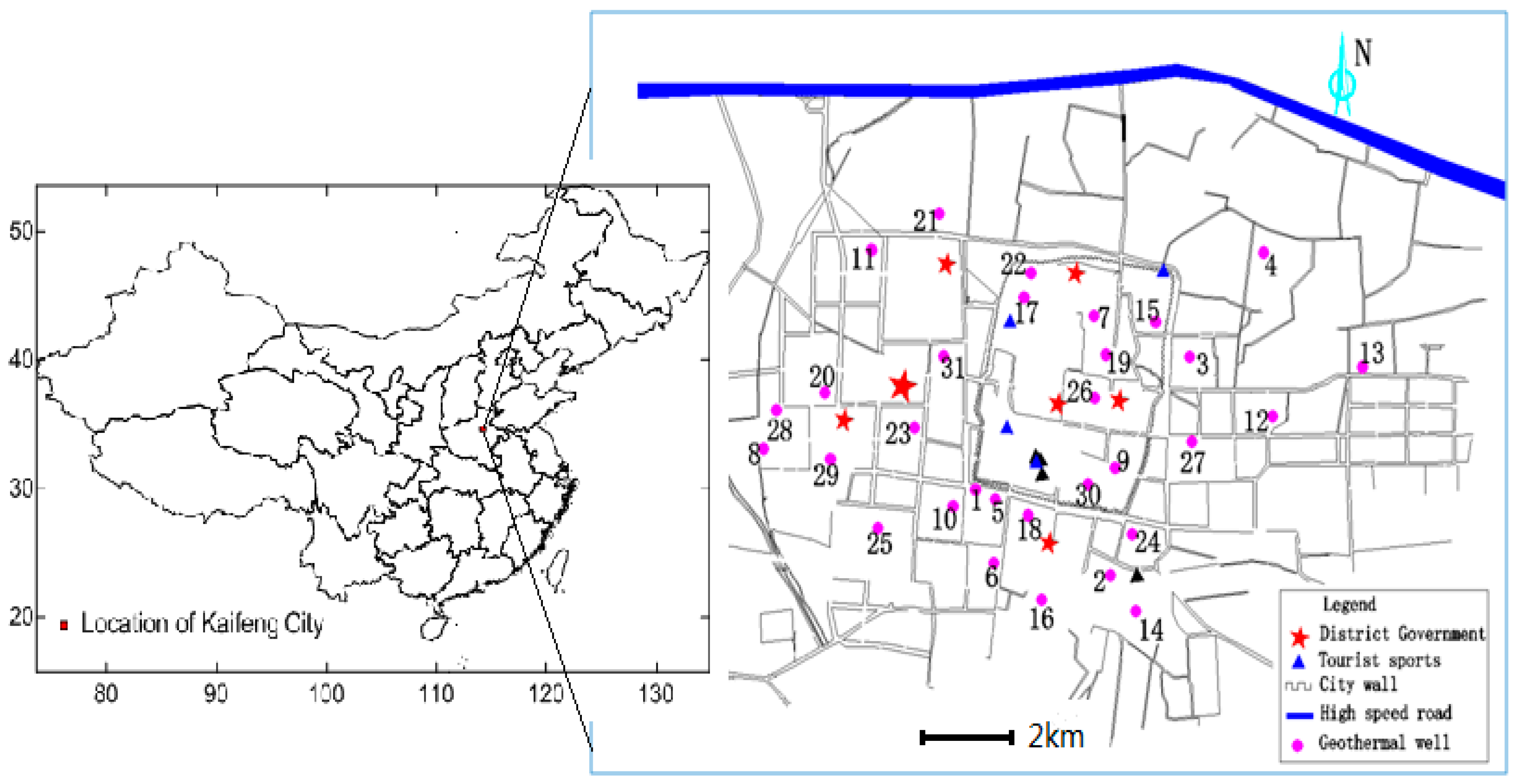
2.1. Study Area
2.2. Sample Collection and Treatment
2.3. Determination and Analysis of Fluorescence Spectra
3. Results and Discussion
3.1. Detection Index
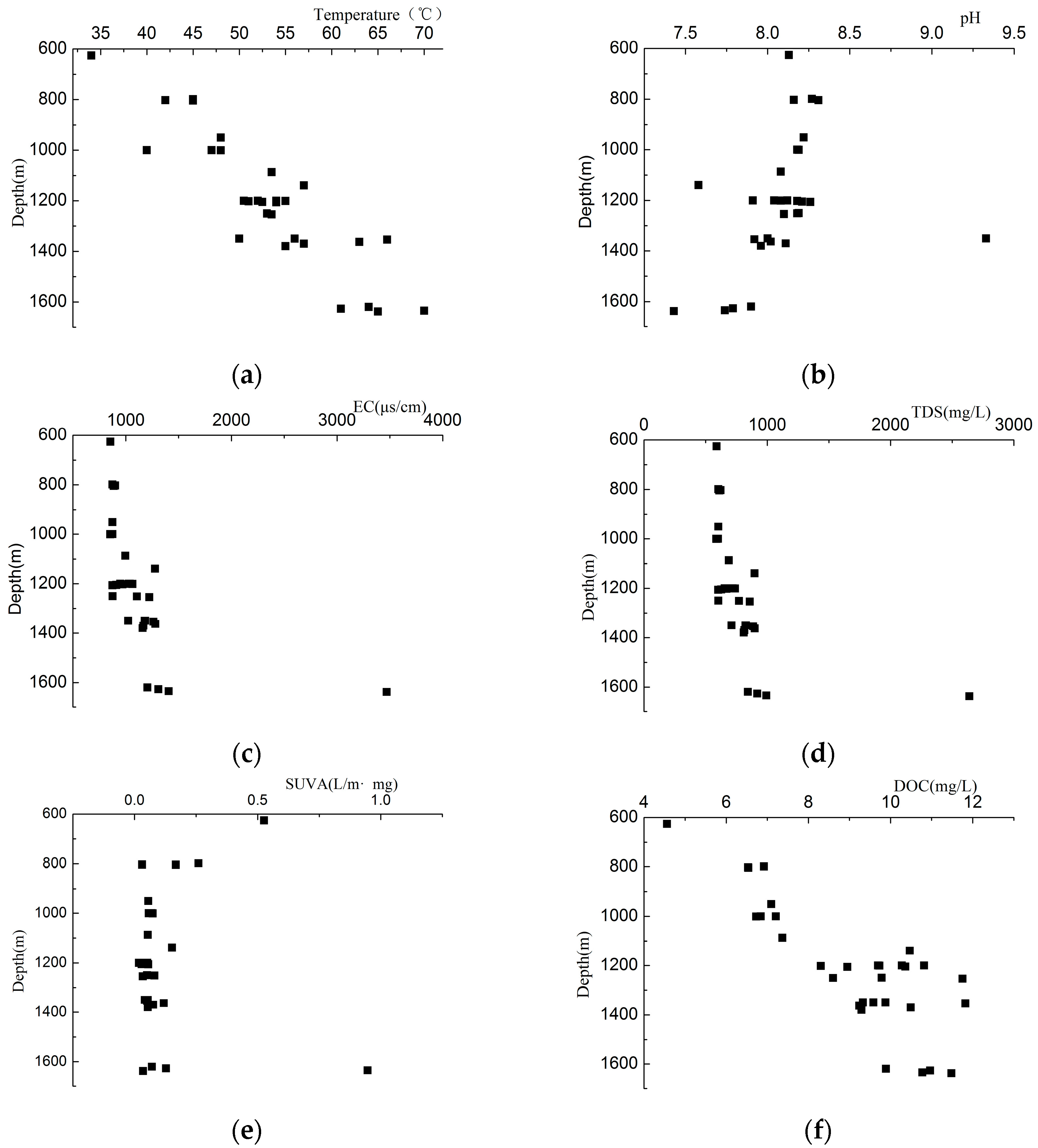
3.2. Fluorescence Peak Position
3.3. Fluorescence Peak Intensity
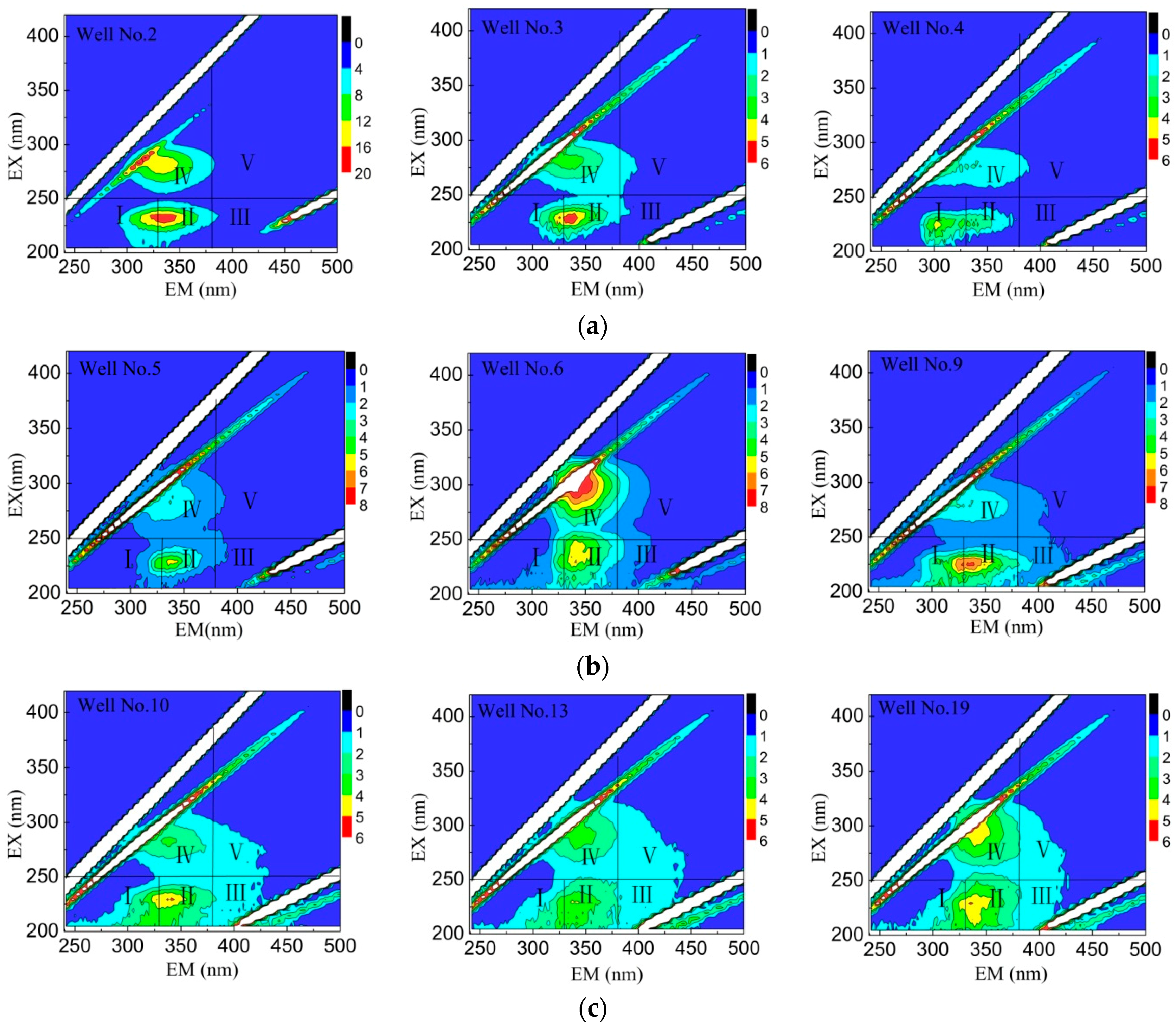
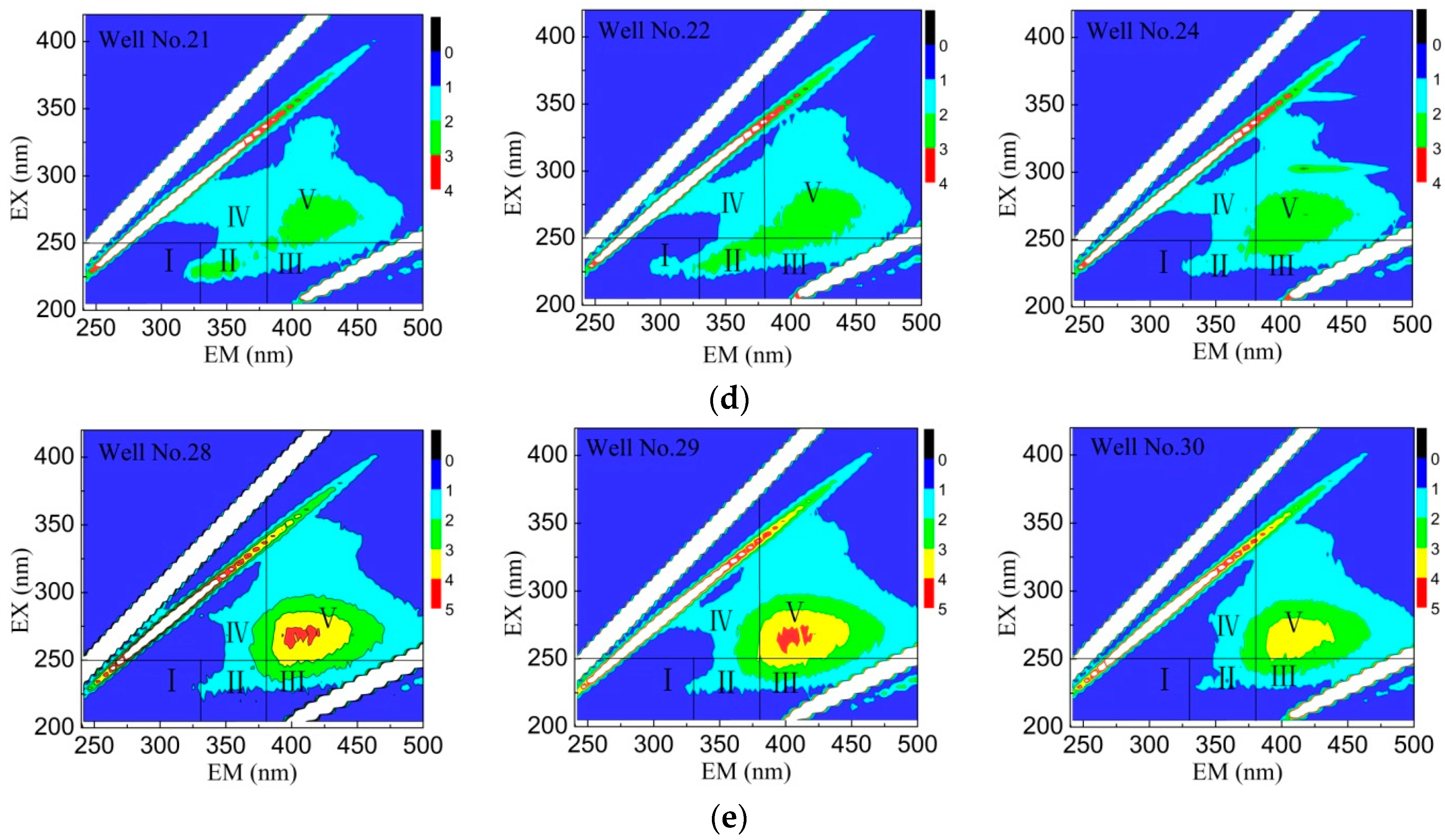
3.4. FI Characteristics of Fluorescent Clusters
3.5. DOM Composition
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coble, P.G.; Green, S.A.; Blough, N.V.; Gagosian, R.B. Characterization of dissolved organic matter in the black sea by fluorescence spectroscopy. Nature 1990, 348, 432–435. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kwon, E.; Kim, I.; Lee, S.-A.; Kim, G. Dissolved organic matter in the subterranean estuary of a volcanic island, jeju: Importance of dissolved organic nitrogen fluxes to the ocean. J. Sea Res. 2013, 78, 18–24. [Google Scholar] [CrossRef]
- Ritson, J.; Graham, N.; Templeton, M.; Clark, J.; Gough, R.; Freeman, C. The impact of climate change on the treatability of dissolved organic matter (dom) in upland water supplies: A UK perspective. Sci. Total Environ. 2014, 473, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-B.; Wang, Y.-X.; Ma, T.; Tong, L.; Wang, Y.-Y.; Liu, C.-R.; Zhao, L. Linking groundwater dissolved organic matter to sedimentary organic matter from a fluvio-lacustrine aquifer at jianghan plain, China by eem-parafac and hydrochemical analyses. Sci. Total Environ. 2015, 529, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Mu, G.; Ju, H.; Wang, R.; Li, D.; Shabbir, A.H. Spatiotemporal characterization of chromophoric dissolved organic matter (cdom) and cdom-doc relationships for highly polluted rivers. Water 2016, 8, 399. [Google Scholar] [CrossRef]
- Dittmar, T.; Stubbins, A. 12.6–dissolved organic matter in aquatic systems. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 125–156. [Google Scholar]
- Zsolnay, A. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Wallis, P.; Hynes, H.; Telang, S. The importance of groundwater in the transportation of allochthonous dissolved organic matter to the streams draining a small mountain basin. Hydrobiologia 1981, 79, 77–90. [Google Scholar] [CrossRef]
- Linlin, W.; Xuan, Z.; Meng, Z. Removal of dissolved organic matter in municipal effluent with ozonation, slow sand filtration and nanofiltration as high quality pre-treatment option for artificial groundwater recharge. Chemosphere 2011, 83, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Wolthoorn, A.; Temminghoff, E.J.; Weng, L.; van Riemsdijk, W.H. Colloid formation in groundwater: Effect of phosphate, manganese, silicate and dissolved organic matter on the dynamic heterogeneous oxidation of ferrous iron. Appl. Geochem. 2004, 19, 611–622. [Google Scholar] [CrossRef]
- Dilling, J.; Kaiser, K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using uv photometry. Water Res. 2002, 36, 5037–5044. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (don) and dissolved organic carbon (doc) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Deflandre, B.; Gagné, J.-P. Estimation of dissolved organic carbon (doc) concentrations in nanoliter samples using uv spectroscopy. Water Res. 2001, 35, 3057–3062. [Google Scholar] [CrossRef]
- Zmora-Nahum, S.; Markovitch, O.; Tarchitzky, J.; Chen, Y. Dissolved organic carbon (doc) as a parameter of compost maturity. Soil Biol. Biochem. 2005, 37, 2109–2116. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.-Z.; Guo, X.-J.; Huang, T.; Gao, P.-P.; Zhang, Y.-P.; Yuan, F. Changes and characteristics of dissolved organic matter in a constructed wetland system using fluorescence spectroscopy. Environ. Sci. Pollut. Res. 2016, 23, 12237–12245. [Google Scholar] [CrossRef] [PubMed]
- Hambly, A.; Arvin, E.; Pedersen, L.-F.; Pedersen, P.B.; Seredyńska-Sobecka, B.; Stedmon, C. Characterising organic matter in recirculating aquaculture systems with fluorescence eem spectroscopy. Water Res. 2015, 83, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Characterization of marine and terrestrial dom in seawater using excitation-emission matrix spectroscopy. Mar.Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Al Lawati, W.M.; Rizoulis, A.; Eiche, E.; Boothman, C.; Polya, D.A.; Lloyd, J.R.; Berg, M.; Vasquez-Aguilar, P.; van Dongen, B.E. Characterisation of organic matter and microbial communities in contrasting arsenic-rich holocene and arsenic-poor pleistocene aquifers, red river delta, vietnam. Appl. Geochem. 2012, 27, 315–325. [Google Scholar] [CrossRef]
- Kolic, P.E.; Roy, E.D.; White, J.R.; Cook, R.L. Spectroscopic measurements of estuarine dissolved organic matter dynamics during a large-scale mississippi river flood diversion. Sci. Total Environ. 2014, 485, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, W.; Chen, N.; Hong, H.; Huang, J.; Xu, J.; Huang, S. Influence of a summer storm event on the flux and composition of dissolved organic matter in a subtropical river, china. Appl. Geochem. 2013, 28, 164–171. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, L.; Minor, E.C. Characterization of bulk and chromophoric dissolved organic matter in the laurentian great lakes during summer 2013. J. Gt. Lakes Res. 2016, 42, 789–801. [Google Scholar] [CrossRef]
- Cui, J.; Yuan, D.H.; Guo, X.J.; He, L.S.; He, J.W.; Li, H.Y.; Li, J.Q. Characterization of dissolved organic matter in lake baiyangdian using spectroscopic techniques and multivariate statistical analysis. Clean Soil Air Water 2016, 44, 1444–1452. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Gao, R.-T.; Wang, L.; Ma, Y.; Cui, D.-Y.; Tan, W.-B. Using fluorescence spectroscopy coupled with chemometric analysis to investigate the origin, composition, and dynamics of dissolved organic matter in leachate-polluted groundwater. Environ. Sci. Pollut. Res. 2015, 22, 8499–8506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yin, J.; Zhang, P.; Wang, X.; Fan, G.; Hua, B.; Ren, B.; Zheng, H.; Deng, B. Dom removal by flocculation process: Fluorescence excitation–emission matrix spectroscopy (eems) characterization. Desalination 2014, 346, 38–45. [Google Scholar] [CrossRef]
- Henderson, R.; Baker, A.; Murphy, K.; Hambly, A.; Stuetz, R.; Khan, S. Fluorescence as a potential monitoring tool for recycled water systems: A review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Tedetti, M.; Cuet, P.; Guigue, C.; Goutx, M. Characterization of dissolved organic matter in a coral reef ecosystem subjected to anthropogenic pressures (la réunion island, indian ocean) using multi-dimensional fluorescence spectroscopy. Sci. Total Environ. 2011, 409, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Price, R.M.; Yamashita, Y.; Jaffé, R. Comparative study of dissolved organic matter from groundwater and surface water in the florida coastal everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Appl. Geochem. 2010, 25, 872–880. [Google Scholar] [CrossRef]
- Wang, C.; Wei, J.; Zhang, B. Characterization of dissolved organic matter in groundwater from the coastal dagu river watershed, china using fluorescence excitation-emission matrix spectroscopy. Spectrosc. Spectr. Anal. 2013, 33, 2460–2465. [Google Scholar]
- Liu, X.-M.; Wang, X.-Y. Evaluation of exploitable yield for geothermal water in kaifeng urban district. West China Explor. Eng. 2005, 17, 80–82. [Google Scholar]
- Wang, X.-G.; Zhang, H.; Zhang, J.-J. Analysis on the hydrochemical characteristics and isotope of geothermal water in kaifeng depression. Saf. Environ. Eng. 2012, 6, 019. [Google Scholar]
- Zhu, H.; Liu, X.; Yang, F.; Yang, H.; Wang, X. Analysis and study on geothermal reinjection test of deep groundwater in kaifeng. J. Henan Polytech. Univ. (Nat. Sci.) 2011, 30, 215–219. [Google Scholar]
- Qi, Y.-F.; Wang, X.-G.; Wang, G.-J.; Ge, Y. Development and protection of geothermal resources in kaifeng depression. Ground Water 2007, 4, 027. [Google Scholar]
- Wang, X.; Zhao, L.; Liu, X.; Lili, A.; Zhang, Y. Temperature effect on the transport of nitrate and ammonium ions in a loose-pore geothermal reservoir. J. Geochem. Explor. 2013, 124, 59–66. [Google Scholar] [CrossRef]
- Huang, S.; Tian, T. Sustainable development of geothermal resource in china and future projects. In Proceedings of the World Geothermal Congress 2005, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Yu-feng, Q. Analysis on geothermal resources in kaifeng depression geothermal field of henan province. J. Southwest Univ. Sci. Technol. 2009, 3, 014. [Google Scholar]
- Wang, X.-Y.; Li, J.-H.; Lin, X.-Y.; Han, P.-F.; Liao, Z.-S. Geothermal field characteristics in the kaifeng city. Adv. Water Sci. 2002, 13, 196–199. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Egea, F.; Martínez-Sabater, E.; Jordá, J.; Moral, R.; Bustamante, M.; Paredes, C.; Pérez-Murcia, M. Dissolved organic matter fractions formed during composting of winery and distillery residues: Evaluation of the process by fluorescence excitation–emission matrix. Chemosphere 2007, 68, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Bieroza, M.; Baker, A.; Bridgeman, J. Classification and calibration of organic matter fluorescence data with multiway analysis methods and artificial neural networks: An operational tool for improved drinking water treatment. Environmetrics 2011, 22, 256–270. [Google Scholar] [CrossRef]
- Bagdonas, S.; Zurauskas, E.; Streckyte, G.; Rotomskis, R. Spectroscopic studies of the human heart conduction system ex vivo: Implication for optical visualization. J. Photochem. Photobiol. B Biol. 2008, 92, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Swietlik, J.; Sikorska, E. Characterization of natural organic matter fractions by high pressure size-exclusion chromatography, specific uv absorbance and total luminescence spectroscopy. Pol. J. Environ. Stud. 2006, 15, 145. [Google Scholar]
- Aitken, A.; Learmonth, M.P. Protein determination by UV absorption. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2002; pp. 3–6. [Google Scholar]
| Thermal Reservoir (m) | Well Location | Well Depth (m) | Stratum | Temperature (°C) | pH Value | EC (μs/cm) | TDS (mg/L) | UV254 (cm−1) | DOC (mg/L) | SUVA (L/m·mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 600–800 m | Well No. 1 | 626 | Nm | 34.0 | 8.13 | 855 | 589 | 0.024 | 4.561 | 0.526 |
| Well No.2 | 799 | Nm | 45.0 | 8.27 | 875 | 603 | 0.018 | 6.919 | 0.260 | |
| Well No.3 | 804 | Nm | 45.0 | 8.31 | 887 | 611 | 0.011 | 6.541 | 0.168 | |
| Well No.4 | 803 | Nm | 42.0 | 8.16 | 900 | 621 | 0.002 | 6.534 | 0.031 | |
| Average value | 41.5 | 8.22 | 879 | 606 | 0.014 | 6.139 | 0.228 | |||
| 800–1000 m | Well No.5 | 950 | Nm-Ng | 48.0 | 8.22 | 875 | 603 | 0.004 | 7.098 | 0.056 |
| Well No.6 | 1001 | Nm | 48.0 | 8.19 | 854 | 588 | 0.005 | 6.731 | 0.074 | |
| Well No.7 | 1000 | Nm | 47.0 | 8.18 | 874 | 601 | 0.004 | 6.841 | 0.058 | |
| Well No.8 | 1087 | Ng | 53.5 | 8.08 | 996 | 688 | 0.004 | 7.369 | 0.054 | |
| Well No.9 | 1000 | Nm | 40.0 | 8.18 | 854 | 587 | 0.005 | 7.209 | 0.069 | |
| Average value | 47.3 | 8.17 | 891 | 613 | 0.004 | 7.050 | 0.057 | |||
| 1000–1200 m | Well No.10 | 1202 | Nm | 51.0 | 8.18 | 969 | 670 | 0.004 | 8.305 | 0.048 |
| Well No.11 | 1250 | Ng | 53.0 | 8.19 | 876 | 603 | 0.005 | 9.788 | 0.051 | |
| Well No.12 | 1205 | Ng | 52.5 | 8.21 | 911 | 627 | 0.003 | 10.362 | 0.029 | |
| Well No.13 | 1254 | Nm | 53.5 | 8.10 | 1224 | 858 | 0.004 | 11.754 | 0.034 | |
| Well No.14 | 1139 | Nm-Ng | 57.0 | 7.58 | 1278 | 897 | 0.016 | 10.471 | 0.153 | |
| Well No.15 | 1201 | Nm | 55.0 | 8.08 | 1064 | 739 | 0.003 | 9.731 | 0.031 | |
| Well No.16 | 1206 | Ng | 54.0 | 8.26 | 875 | 602 | 0.005 | 8.953 | 0.056 | |
| Well No.17 | 1251 | Nm | 53.0 | 8.18 | 1107 | 771 | 0.007 | 8.602 | 0.081 | |
| Well No.18 | 1200 | Nm | 52.0 | 8.12 | 948 | 655 | 0.005 | 9.697 | 0.052 | |
| Well No.19 | 1200 | Nm | 50.5 | 8.04 | 1038 | 720 | 0.004 | 10.279 | 0.039 | |
| Well No.20 | 1200 | Nm | 54.0 | 7.91 | 1030 | 714 | 0.002 | 10.818 | 0.018 | |
| Average value | 53.2 | 8.08 | 1029 | 714 | 0.005 | 9.887 | 0.051 | |||
| 1200–1400 m | Well No.21 | 1350 | Ng | 50.0 | 9.33 | 1184 | 827 | 0.005 | 9.331 | 0.054 |
| Well No.22 | 1380 | Ng | 55.0 | 7.96 | 1160 | 809 | 0.005 | 9.296 | 0.054 | |
| Well No.23 | 1350 | Ng | 56.0 | 8.00 | 1024 | 711 | 0.005 | 9.881 | 0.051 | |
| Well No.24 | 1363 | Ng | 63.0 | 8.02 | 1281 | 899 | 0.011 | 9.241 | 0.119 | |
| Well No.25 | 1354 | Ng | 66.0 | 7.92 | 1263 | 886 | 0.006 | 11.825 | 0.051 | |
| Well No.26 | 1350 | Ng | 56.0 | 8.00 | 1180 | 824 | 0.004 | 9.585 | 0.042 | |
| Well No.27 | 1370 | Ng | 57.0 | 8.11 | 1168 | 815 | 0.008 | 10.493 | 0.076 | |
| Average value | 57.6 | 8.19 | 1180 | 824 | 0.006 | 9.950 | 0.060 | |||
| 1400–1600 m | Well No.28 | 1635 | Ng | 70.0 | 7.74 | 1407 | 993 | 0.102 | 10.776 | 0.947 |
| Well No.29 | 1627 | Ng | 61.0 | 7.79 | 1309 | 919 | 0.014 | 10.966 | 0.128 | |
| Well No.30 | 1620 | Ng | 64.0 | 7.90 | 1205 | 843 | 0.007 | 9.889 | 0.071 | |
| Well No.31 | 1638 | Ng | 65.0 | 7.43 | 3470 | 2640 | 0.004 | 11.484 | 0.035 | |
| Average value | 65.0 | 7.72 | 1848 | 1349 | 0.032 | 10.779 | 0.297 |
| FI | FI(I) | FI(II) | FI(III) | FI(IV) | FI(V) |
|---|---|---|---|---|---|
| FI(I) | 1.0000 | ||||
| FI(II) | 0.9197 ** | 1.0000 | |||
| FI(III) | 0.6084 ** | 0.7671 ** | 1.0000 | ||
| FI(IV) | 0.7750 ** | 0.8718 ** | 0.5284 * | 1.0000 | |
| FI(V) | 0.4891 * | 0.6695 ** | 0.9726 ** | 0.4706 * | 1.0000 |
| Region | 600–800 m | 800–1000 m | 1000–1200 m | 1200–1400 m | 1400–1600 m | |
|---|---|---|---|---|---|---|
| integral standard volume Фi (au·nm2) | I | 215.93 | 113.02 | 131.12 | 23.44 | 11.53 |
| II | 292.73 | 204.58 | 216.55 | 112.11 | 106.91 | |
| III | 58.98 | 80.12 | 90.98 | 86.75 | 107.16 | |
| IV | 243.03 | 152.76 | 145.68 | 81.92 | 74.23 | |
| V | 53.22 | 46.56 | 53.82 | 85.72 | 116.63 | |
| TOT | 863.89 | 597.03 | 638.15 | 389.94 | 416.47 | |
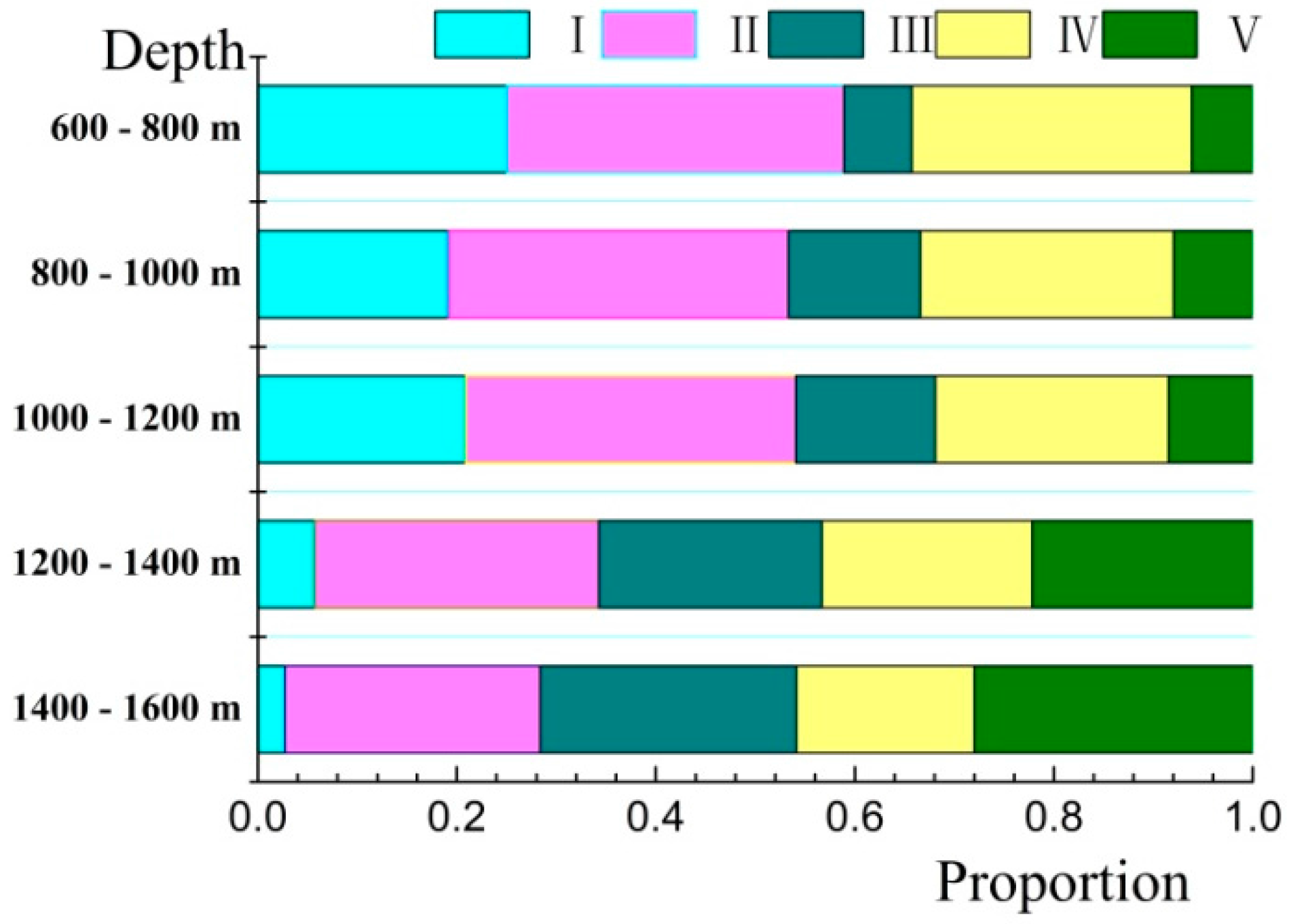
| Proportion Pi (%) | I | 25.00 | 19.20 | 20.89 | 5.72 | 2.72 |
| II | 33.89 | 34.10 | 33.17 | 28.54 | 25.65 | |
| III | 6.83 | 13.29 | 14.02 | 22.43 | 25.75 | |
| IV | 28.13 | 25.45 | 23.44 | 21.12 | 17.88 | |
| V | 6.16 | 7.97 | 8.47 | 22.18 | 28.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, W.; Wang, X.; Liu, X.; Zhen, X.; Guo, J.; Wang, S.; Yang, F.; Chen, G.; Zhang, B. Characterization of Dissolved Organic Matter in Deep Geothermal Water from Different Burial Depths Based on Three-Dimensional Fluorescence Spectra. Water 2017, 9, 266. https://doi.org/10.3390/w9040266
Qiao W, Wang X, Liu X, Zhen X, Guo J, Wang S, Yang F, Chen G, Zhang B. Characterization of Dissolved Organic Matter in Deep Geothermal Water from Different Burial Depths Based on Three-Dimensional Fluorescence Spectra. Water. 2017; 9(4):266. https://doi.org/10.3390/w9040266
Chicago/Turabian StyleQiao, Weifang, Xinyi Wang, Xiaoman Liu, Xiaoge Zhen, Jianwei Guo, Shidong Wang, Fang Yang, Guosheng Chen, and Bo Zhang. 2017. "Characterization of Dissolved Organic Matter in Deep Geothermal Water from Different Burial Depths Based on Three-Dimensional Fluorescence Spectra" Water 9, no. 4: 266. https://doi.org/10.3390/w9040266





