Algal Bioproductivity in Turbulent Water: An Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Analysis Measuring Method
2.3. Source Water
2.4. Experimental Program
3. Results and Discussion
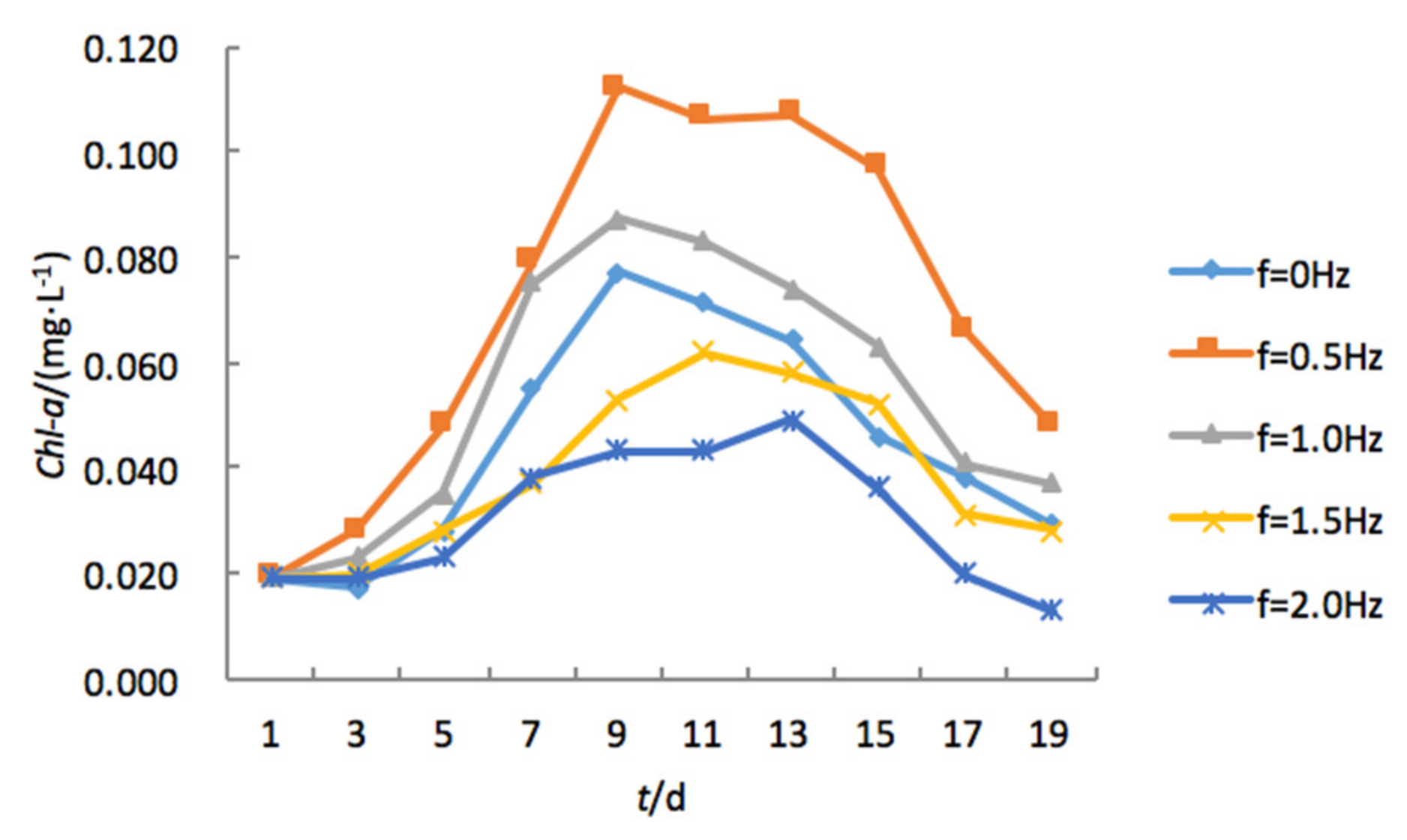
3.1. The Effects of Turbulence Intensity on Algal Growth
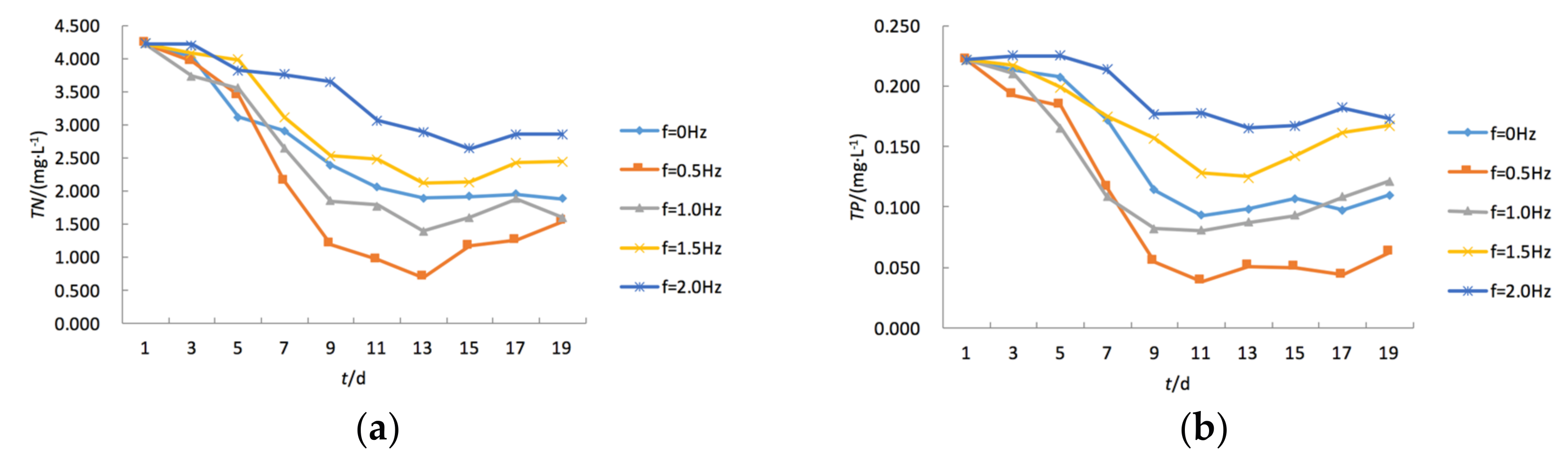
3.2. Temporal Changes of TN and TP Concentration under Varying Turbulence Intensity
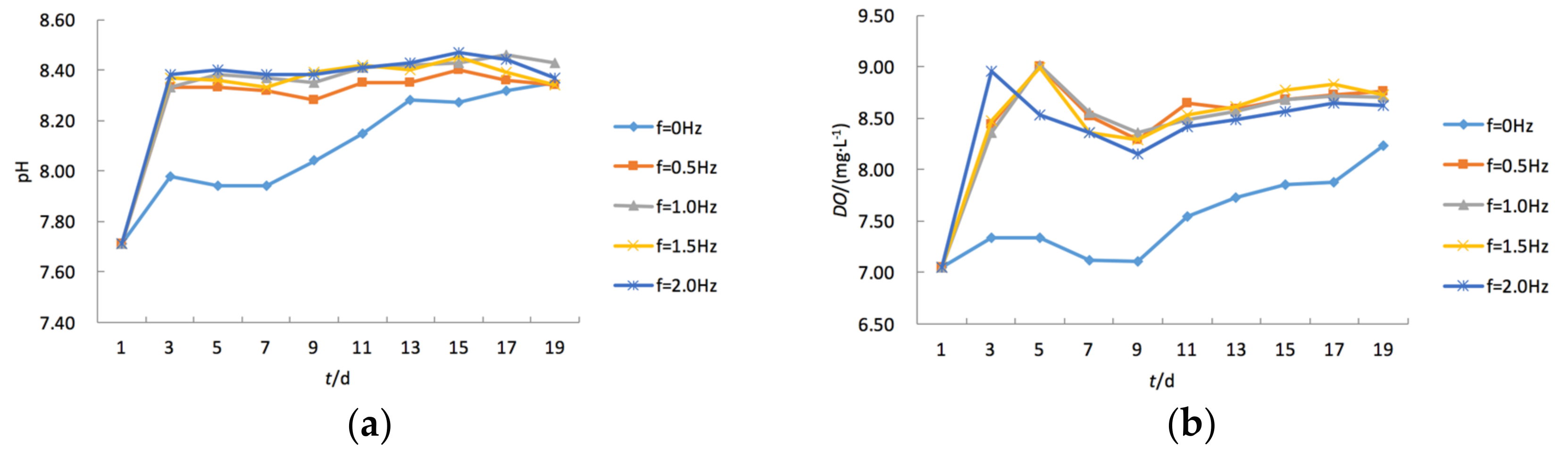
3.3. Temporal Changes of pH and DO under Varying Turbulence Intensities
4. Conclusions
- (1)
- Algal bioproductivity changed with water turbulence intensity. In moderately turbulent water, algal bioproduction increased with respect to vibration frequency. The observed peak of Chl-a concentrations in an OGT device with a vibration frequency of 0.5 Hz was 0.087 mg/L; the peak observed concentration increased to 0.112 mg/L in the OGT device with a vibration frequency of 1.0 Hz.
- (2)
- In strongly turbulent water, algal bioproduction decreased with respect to vibration frequency. The observed peak Chl-a concentrations in an OGT device with a vibration frequency of 1.5 Hz was 0.060 mg/L; the observed peak concentration was reduced to 0.049 mg/L in the OGT device with a vibration frequency of 2.0 Hz.
- (3)
- Water turbulence also had a strong influence on the concentration of TN in a water body. Experimental data indicated that when the vibration frequency in OGT reactors increased from 0.5 Hz to 2.0 Hz, the corresponding TN and TP were reduced by 27% and 19%, respectively.
- (4)
- Water turbulence adjusted the pH value to a level in the water body desirable for the growth of aquatic plants. Experimental data showed that the pH values in four OGT reactors were adjusted to their stable values in only 3 days, while the adjustment time for the OGT with stationary water was more than 19 days.
- (5)
- During this experimental investigation, the time-variation of the biodiversity of the individual algae species in the experimental OGT reactors was not evaluated. Recent literature [32,33] has reported that algal bioproductivity and biodiversity in biologically-active water are interrelated. Therefore, the effects of water turbulence on concurrent changes of bioproductivity and biodiversity—an important step toward effective control of lake eutrophication—needs to be evaluated in follow-up research.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Escartín, J.; Aubrey, D.G. Flow structure and dispersion within algal mats. Estuar. Coast. Shelf Sci. 1995, 40, 451–472. [Google Scholar] [CrossRef]
- Steinman, A.D.; Mcintire, C.D. Effects of current velocity and light energy on the structure of periphyton assemblages in laboratory streams. J. Phycol. 1986, 22, 352–361. [Google Scholar] [CrossRef]
- Wu, X.H.; Li, Q.J. Reviews of influences from hydrodynamic conditions on algae. Ecol. Environ. Sci. 2010, 19, 1732–1738. (In Chinese) [Google Scholar]
- Marshall, J.S.; Sala, K. A stochastic Lagrangian approach for simulating the effect of turbulent mixing on algae growth rate in a photobioreactor. Chem. Eng. Sci. 2011, 6, 384–392. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.Y.; Xia, K.; Yang, R.; Liu, X.D. Flow-Disturbance considered simulation for algae growth in a river-lake system. Ecohydrology 2016, 9, 601–609. [Google Scholar] [CrossRef]
- Zhong, C.H. A Study on the Eutrophication of the Three Gorges Reservoir. Ph.D. Thesis, School of Water Resources and Hydropower, Sichuan University, Chengdu, China, March 2004. (In Chinese). [Google Scholar]
- Hondzo, M.; Al-Homoud, A. Model development and verification for mass transport to Escherichia coli cells in a turbulent flow. Water Resour. Res. 2007, 43, W08413. [Google Scholar] [CrossRef]
- Zou, R.; Zhou, J.; Sun, Y.J.; Ji, X.Y.; Yue, J.; Liu, Y. Numerical experiment study on the algae suppression effect of vertical hydrodynamic mixers. Environ. Sci. 2012, 33, 1540–1549. (In Chinese) [Google Scholar]
- Hondzo, M.; Lyn, D. Quantified small-scale turbulence inhibits the growth of a green alga. Freshw. Biol. 1999, 41, 51–61. [Google Scholar] [CrossRef]
- Shy, S.S.; Tang, C.Y.; Fann, S.Y. A nearly isotropic turbulence generated by a pair of vibrating grid. Exp. Therm. Fluid Sci. 1997, 14, 251–262. [Google Scholar] [CrossRef]
- Cheng, N.S.; Law, A.W.K. Measurement of turbulence generated by oscillating grid. Can. Metall. Q. 2001, 127, 201–208. [Google Scholar] [CrossRef]
- Rouse, H. Experiments on the mechanics of sediment suspension. In Proceedings of the Fifth International Congress for Applied Mechanics, Cambridge, MA, USA, 1938; John Wiley & Sons: New York, NY, USA, 1939; pp. 550–554. [Google Scholar]
- Xuequan, E.; Hopfinger, E.J. On mixing across an interface in stably stratified fluid. J. Fluid Mech. 1986, 166, 227–244. [Google Scholar] [CrossRef]
- Hopfinger, E.J.; Toly, J.A. Spatially decaying turbulence and its relation to mixing across density interfaces. J. Fluid Mech. 1976, 78, 155–175. [Google Scholar] [CrossRef]
- Mcdougall, T.J. Measurement of turbulence in a zero-mean-shear mixed layer. J. Fluid Mech. 1979, 94, 409–431. [Google Scholar] [CrossRef]
- Rouse, H.; Dudo, J. Turbulent diffusion across a density discontinuity. La Houille Blanche 1955, 10, 530–532. [Google Scholar]
- Thompson, S.M.; Turner, J.S. Mixing across an interface duo to turbulence generated by an oscillating grid. J. Fluid Mech. 1975, 67, 349–368. [Google Scholar] [CrossRef]
- Huppert, H.E.; Turner, J.S.; Hallworth, M.A. Sedimentation and entrainment in dense layers of suspended particles stirred by an oscillating grid. J. Fluid Mech. 1995, 289, 263–293. [Google Scholar] [CrossRef]
- Orlins, J.J.; Gulliver, J.S. Turbulence quantification and sediment resuspension in an oscillating grid chamber. Exp. Fluids 2003, 34, 662–677. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lick, W. A portable device for measuring sediment resuspension. J. Gt. Lakes Res. 1986, 12, 314–321. [Google Scholar] [CrossRef]
- Brumley, B.H.; Jirka, G.H. Near-surface turbulence in a grid-stirred tank. J. Fluid Mech. 1987, 183, 236–263. [Google Scholar] [CrossRef]
- Chu, C.R.; Jirka, G.H. Turbulent gas flux measurements below the air-water interface of a grid-stirred tank. Int. J. Heat Mass Transf. 1992, 35, 1957–1968. [Google Scholar]
- Jirka, G.H. Application of LIF to investigate gas transfer near the air-Water interface in a grid-Stirred tank. Exp. Fluids 2004, 37, 341–349. [Google Scholar]
- Connolly, J.P.; Armstrong, N.E.; Miksad, R.W. Adsorption of hydrophobic pollutants in estuaries. J. Environ. Eng. 1983, 109, 17–35. [Google Scholar] [CrossRef]
- Valsaraj, K.T.; Ravikrishna, R.; Orlins, J.J.; Smith, J.S.; Gulliver, J.S.; Reible, D.D.; Thibodeaux, L.J. Sediment-to-air mass transfer of semi-volatile contaminants due to sediment resuspension in water. Adv. Environ. Res. 1997, 1, 145–156. [Google Scholar]
- Yan, J.; Cheng, N.S.; Tang, H.W.; Tan, S.K. Oscillating-grid turbulence and its applications: A review. J. Hydraul. Res. 2007, 45, 26–32. [Google Scholar] [CrossRef]
- State Environmental Protection Administration. Water and Waste Water Monitor and Analyze Method, 4th ed.; China Environment Science Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 2, 343–346. [Google Scholar] [CrossRef]
- Zeng, T. Study on Phytoplankton Composition and Water Quality in Chongqing Reach of Jialing River and Yangtze River. Master’s Thesis, College of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China, April 2004. (In Chinese). [Google Scholar]
- Chai, X.Y. Behavior Study on the Role of Irradiance and Temperature on the Algae Bloom of Typical Algae in Three-Gorges Valley. Master’s Thesis, College of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China, April 2004. (In Chinese). [Google Scholar]
- Liu, C.C.K.; Fok, Y.S. Stream waste assimilative capacity analysis using reaeration coefficients measured by tracer techniques. J. Water Resour. Bull. 1983, 19, 439–445. [Google Scholar] [CrossRef]
- Carno-Kyker, S.R.; Swanson, A.K. Temporal and spatial patterns of eukaryotic and bacterial communities found in vernal pools. J. Appl. Environ. Microbiol. 2008, 74, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, M.A.; Thenot, A.; Lepere, T.C.; Debroas, D. Genetic diversity of small eukaryotes in lakes differing by their trophic status. J. Appl. Environ. Microbiol. 2005, 71, 5935–5942. [Google Scholar] [CrossRef] [PubMed]

| Species | Diatom | Blue Algae | Green Algae | Others |
|---|---|---|---|---|
| Percent | 43–60 | 3–20 | 8–40 | 3–16 |
| Item | TN (mg/L) | TP (mg/L) | Chl-a (mg/L) | Dominant Algae (%) |
|---|---|---|---|---|
| Parameters | 4.215 (0.209) | 0.222 (0.025) | 0.019 (0.002) | 47.32 (1.91) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, L.; Long, T.; Liu, C.C.K. Algal Bioproductivity in Turbulent Water: An Experimental Study. Water 2017, 9, 304. https://doi.org/10.3390/w9050304
San L, Long T, Liu CCK. Algal Bioproductivity in Turbulent Water: An Experimental Study. Water. 2017; 9(5):304. https://doi.org/10.3390/w9050304
Chicago/Turabian StyleSan, Lei, Tianyu Long, and Clark. C. K. Liu. 2017. "Algal Bioproductivity in Turbulent Water: An Experimental Study" Water 9, no. 5: 304. https://doi.org/10.3390/w9050304





